Abstract
Objective
Insulin resistance (IR) is associated with increased cardiovascular risk in multiple patient populations, including ones on chronic hemodialysis (CHD). Active vitamin D deficiency is postulated to play a role in the extent of IR observed in CHD patients. We postulated that administration of Paracalcitol, an active vitamin D medication, influences IR in CHD patients.
Design
Pilot randomized-controlled trial
Setting
10 prevalent chronic hemodialysis patients on stable Paracalcitol.
Methods
Paracalcitol was withheld for 8 weeks in all patients (phase I). Parathyroid hormone levels were managed by calcium sensing receptor agonist, Cinacalcet. At week 8, patients were randomized to continue Cinacalcet or to restart Paracalcitol for 8 weeks (phase II). Primary outcome was the change in IR measured by glucose disposal rate (GDR) by hyperinsulinemic euglycemic clamp (HEGC). Secondary outcomes included changes in IR between groups in indirect indices of IR, biomarkers of inflammation, and adipokines.
Results
Mean age was 49 years (range 46–57) and 40% were female. Compared to baseline, there was no detectable change in the GDR at the end of phase I (p=0.7). There was also no statistically significant difference in GDR between groups at the end of phase II (p=0.9). No changes were observed in indirect indices of IR, adipokines or biomarkers of inflammation in either phase.
Conclusion
The results of this pilot study suggest that withdrawal of Paracalcitol over 8–16 weeks and replacement for 8 weeks after withdrawal does not influence IR measured by HEGC in CHD patients.
Keywords: insulin resistance, HOMA, chronic kidney disease, metabolism
Introduction
Insulin resistance is highly prevalent in patients with chronic kidney disease (CKD), especially in ESRD patients1 and has been proposed as a potential mediator for the accelerated CVD that afflicts this patient population2. A complex relationship between uremia, glucose dispersion, and insulin function has been long recognized3. Investigators have demonstrated alterations in glucose metabolism in chronic hemodialysis (CHD) patients, including hyperinsulinemia and diminished tissue sensitivity to insulin which is only partially correctable by initiation of maintenance hemodialysis4. Studies have also demonstrated that altered insulin sensitivity is mostly due to a post-receptor defect primarily affecting skeletal muscle glucose uptake5.
The etiology of insulin resistance in ESRD is multifactorial and includes adipose tissue dysregulation6,7, inflammation7–10, metabolic acidosis11, and secondary hyperparathyroidism (SHPT) 12–14. Epidemiological studies have consistently shown that the administration of active vitamin D (Vit D) in CHD patients confers a survival advantage15–18,19. The mechanisms behind the beneficial effects of the administration of active Vit D in CHD patients are unknown, but there is an increasing body of evidence highlighting the pleiotropic actions of Vit D that go beyond bone health20. Several small studies showed that the administration of active Vitamin D improves insulin sensitivity in CHD patients 21–31. However in most of the studies, the treatment was accompanied by a significant reduction in intact parathyroid hormone (iPTH) levels creating a confounder of whether the effect seen on IR was due to the administration of active Vit D or the correction of SHPT 23,24,26,29,31.
In this prospective, consecutive phase study, we aimed to evaluate 1) whether withdrawal of active Vit D over 8 – 16 weeks would lead to worsening of insulin sensitivity measured using the gold standard hyperinsulinemic euglycemic clamp (HEGC) in otherwise stable prevalent CHD patients, and 2) to examine whether reinitiating active Vit D following 8 weeks of withdrawal would have an impact on insulin sensitivity in the same patient population.
Methods
Study population
The study was conducted at the Vanderbilt University Outpatient Dialysis Units and Clinical Research Center (CRC) between April 2008 and January 2010. Prevalent CHD patients 18 to 75 years old on CHD three times a week, Kt/V >1.2, and body mass index (BMI) between 25 and 45 and on stable dose of active Vit D (Paracalcitol) were eligible to participate. Exclusion criteria included hospitalizations within the last 3 months, any acute or chronic inflammatory process, uncontrolled diabetes mellitus (HbA1c >10), being on an insulin sensitizer (metformin or TZDs), iPTH >1500 pg/ml, serum phosphorus >10 mg/dl, or serum calcium >10.5 mg/dl. The study was approved by the Institutional Review Board and signed informed consent was obtained from all patients.
Study Design
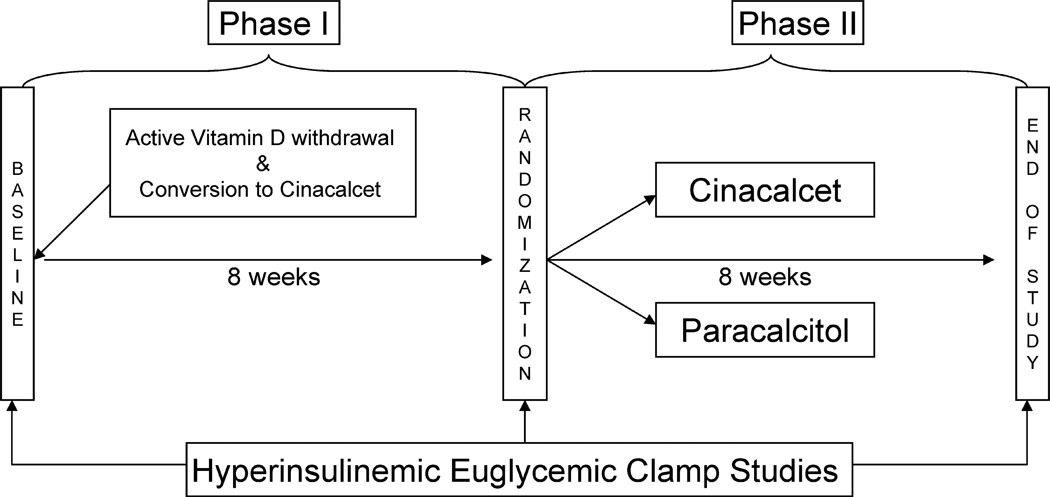
This was a single center, double-blinded, randomized, parallel-design pilot study (ClinicalTrials.gov number NCT00656032). Figure 1 depicts the study design. Prior to baseline, all subjects were on stable dose of paracalcitol for at least 4 weeks. Following the initial metabolic study assessment, paracalcitol was stopped in all subjects for 8 weeks (Phase 1). Patients were started on a calcium-sensing receptor agonist (Cinacalcet) for control of iPTH levels with dose adjustment based on calcium levels, with the goal of preventing a drop of iPTH of more than 10% of the baseline value. Serum calcium, phosphorus and iPTH levels were assessed every 2 weeks and medications were adjusted accordingly. At 8 weeks, subjects underwent another clamp study. Following this second assessment, 10 subjects were randomized to receive either Vitamin D or continue on Cinacalcet for another 8 weeks (Phase 2). 25-hydroxy-vitamin D was measured in all individuals at baseline and replaced with oral ergocalciferol with doses equivalents to those recommended for CKD stages 3 & 4 in the KDOQI guidelines. Randomization was performed with a computer generated sequence.
Figure 1.
Study schematic for phase I (washout period) and phase 2 (intervention period).
Study endpoints
All outcomes of interest were measured at baseline, 8 weeks and 16 weeks. The primary outcome was insulin sensitivity measured by glucose disposal rate (GDR, mg/kg/min) obtained by hyperinsulinemic euglycemic clamp. The basic clamp-derived IR index was the average value of the glucose infusion rate during the final 30 min of the study (steady-state), known as the M-value. We chose to normalize the M value to total body weight as this has been validated across individuals with different weights including obesity32. Secondary outcomes included biomarkers of inflammation (high sensitivity C-reactive protein [hsCRP] and interleukin 6 [IL-6]), adipokines (adiponectin and leptin), and indirect indices of insulin resistance, including homeostatic model assessment of insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), homeostatic model assessment of insulin resistance corrected by adiponectin (HOMA-AD), and leptin adiponectin ratio (LAR).
Procedures
Hyperinsulinemic euglycemic glucose clamp study
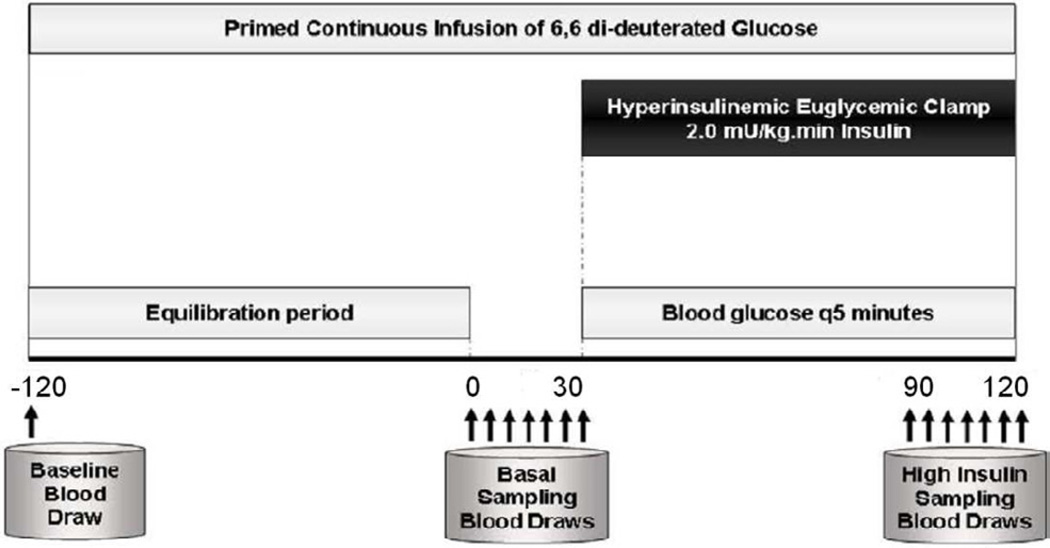
All metabolic studies were performed at the VUMC CRC facilities. On the morning of the clamp study (Figure 2), fasting blood samples were obtained for glucose, inflammatory markers and adipokines. The dialysis shunt was accessed and the venous needle was used for the infusions of glucose, insulin and dextrose. All blood samples were drawn from the arterial side of the dialysis access. Blood samples were drawn at 5-minute intervals for thirty minutes to assess basal levels of glucose at steady state. A primed continuous infusion of human regular insulin (50 units/50 ml of normal saline) was started at a rate of 2.0 mU/kg/min and maintained at that level through 120 minutes. After insulin initiation, the plasma glucose levels were allowed to drop to ± 5 mg/dL of the patient’s baseline glucose value, and were maintained at that level throughout the study by adjusting a variable infusion of 20% dextrose. Constant monitoring of plasma glucose concentration was done every 5 minutes. Once steady state was reached and confirmed at 90 minutes, the average value of the glucose infusion rate was calculated over the last 30 minutes (M-value) and then normalized to total body weight to estimate the glucose disposal rate (GDR, mg/kg/min), which was calculated as an index of in vivo insulin resistance.
Figure 2.
Schematic diagram of the Hyperinsulinemic Euglycemic Clamp (HEGC) Procedure. Arrows denote time points for blood draws. A primed continuous infusion of human regular insulin (50 units/50 ml of normal saline) was started at a rate of 2.0 mU/kg/min and maintained at that level through 120 minutes. Glucose was maintained at baseline (+/− 5 mg/dl) throughout the clamp procedure (−120 to +120 minutes). The glucose disposal rate during steady state was calculated over the last 30 minutes (M-value).
Indirect Insulin Resistance Indices
Indirect insulin sensitivity indices were calculated as follows: homeostasis model assessment (HOMA-IR), [insulin (µU/ml) X glucose (mg/dl)/405] 33,34; quantitative insulin sensitivity check index (QUICKI), 1/(log glucose [mg/dl] + log insulin[µU/ml])35; homeostasis model assessment corrected by adiponectin (HOMA-AD; insulin (µU/mL) X glucose (mg/dl)/adiponectin[µg/ml])36; and LAR, leptin (ng/ml)/adiponectin (µg/ml)7,37.
Blood samples
All blood sampling was performed at the GCRC and processed at Vanderbilt Cytokine and Hormonal Core facilities. Blood samples were stored in aliquots at −80°C. Glucose concentrations were measured by the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter, Brea, CA). Insulin was measured by double-antibody radioimmunoassay (DA RIA; Millipore, St Charles, MO). Total adiponectin and resistin were measured by MILLIPLEX MAP Panel A kit (Millipore, Billerica, MA). Interleukin 6 concentrations were determined using cytometric bead arrays (CBAs; Becton Dickinson, San Jose, CA). High sensitivity C-reactive protein levels were measured by particle-enhanced turbidimetric UniCel DxI (Beckman Coulter, Brea, CA). All other measurements were performed using routine laboratory tests and certified methods.
Body composition by Dual-Energy X-ray Absorptiometry
In this study we performed an assessment of body composition using a Lunar iDEXA machine, encore 2007, v.11.40.004 (General Electric, Madison, WI). The Lunar system measures bone mass, fat mass and lean body mass for the whole body and by body regions (truncal and legs). The details of the procedure has been previously published 7. The Lunar iDEXA also reports by body regions and truncal fat mass percent, which has been previously reported to be closely related to visceral fat mass in CHD patients and is reported in this study38.
Statistical analyses
Our primary analysis was the change in insulin resistance as measured by GDR after 8 weeks of active vitamin D withdrawal. For this purpose we used Wilcoxon signed-rank test to compare (this is a paired analysis) the changes in the primary and secondary outcomes from baseline to week 8. Our secondary analysis was the change from 8 to 16 weeks between the treatment groups (Paracalcitol versus Cinacalcet). Multivariable linear regression was used to adjust for values at week 8; whereas at week 16, values were used as dependent variables—thus regression coefficient of treatment group reflects the effect in change from week 8 to 16 (baseline adjusted model). By recognizing the observed imbalance between the groups due to a small sample size, propensity score analysis was conducted to control for selection bias. Propensity score adjustment preserved statistical power by reducing confounders into a single variable. Propensity scores were estimated through binary logistic regression providing the predicted probability of being assigned into treatment as a function of other risk factors including age, truncal fat, IL-6, iPTH and leptin. Propensity score was then added as a covariate in the adjusted model to further evaluate the adjusted effect of intervention on outcomes (propensity score adjusted model). The indices of insulin resistance and other biomarkers were natural logarithmically transformed when appropriate. Regression diagnostics were performed to verify assumptions of normality of residuals. All analyses used a 5% 2-sided significance level and were performed using R version 2.10.0 (www.r-project.org).
Results
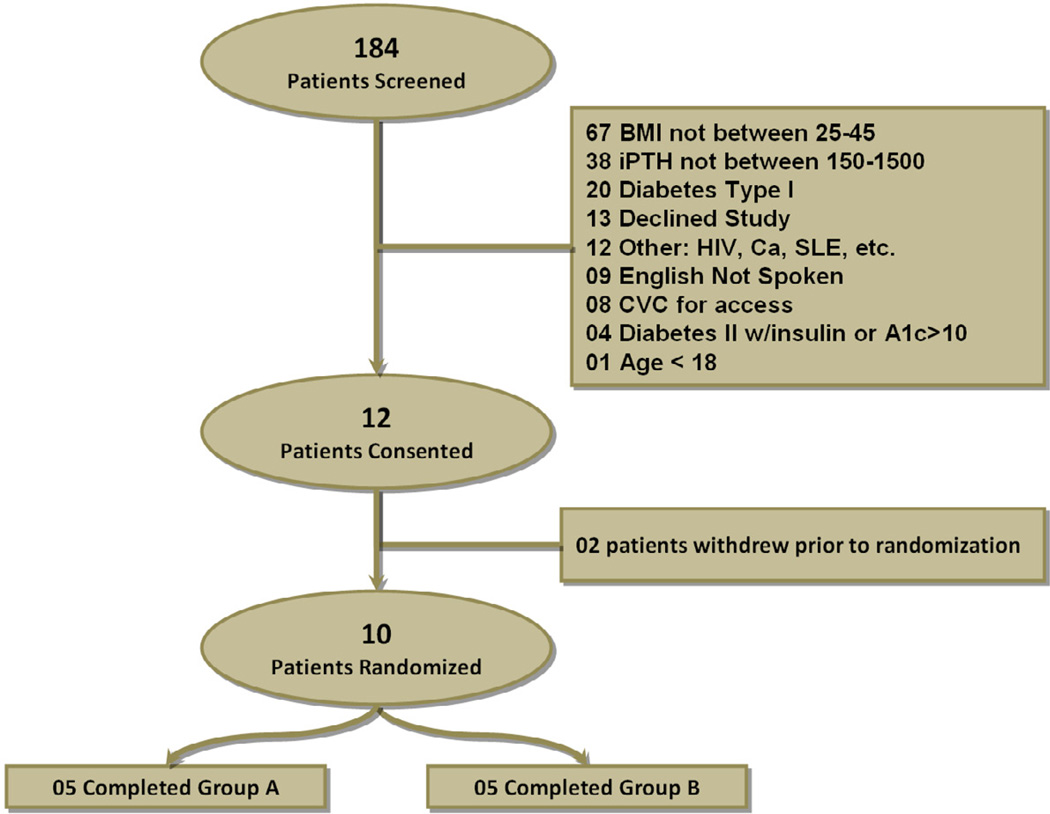
We screened 182 prevalent CHD patients from two outpatient dialysis units and 12 of which were enrolled in the study. Two of these patients dropped from the study prior to randomization due to personal reasons (Figure 3). Baseline characteristics of the study subjects are shown in Table 1. Mean age was 48.5 years (range 46 to 57), 4 subjects were female and all patients were African American. Sixty percent of patients were obese by BMI cut-off > 30 kg/m2. The median time on dialysis was 41 months (IQR 18, 115). We classified our study participants based on the American Diabetes Association (ADA) criteria as being diabetic, with impaired fasting glucose (IFG), and with normal fasting glucose. Two individuals had diabetes based on at least two fasting glucose >125 mg/dl (one individual carried the diagnosis of diabetes and one did not), four individuals had IFG based on at least two fasting glucose between 100 to 125 mg/dl, and four had normal fasting glucose <100 mg/dl. Two of the participants with IFG and 1 of the participants with normal fasting glucose had previous history of diabetes which had resolved before reaching to ESRD. Table 2 displays patient characteristics by intervention group at baseline, 8 weeks and 16 weeks.
Figure 3.
Disposition of patients in the trial. Groups are as follows: Group A: paricalcitol and Group B: Placebo. Both groups completed an 8 weeks washout period (phase I) prior to randomization.
Table 1.
Characteristics of the study subjects at baseline and at 8 weeks after vitamin D withdrawal for all participants
| Characteristics | Baseline (n=10) | 8 weeks (n=10) |
|---|---|---|
| Age, years | 48.5 [46.2, 53.8] | |
| Females, % [n] | 40% [n=4] | |
| Vintage, months | 40 [24, 107] | |
| Body composition by DEXA | ||
| Body Mass Index, kg/m2 | 34.6 [26.2, 38.5] | |
| Total fat mass, % | 39.9 [33.1, 48.7] | |
| Truncal fat, % | 44.0 [39, 53] | |
| Lean body mass, kg | 51.6 [45.9, 56.4] | |
| Mineral bone metabolism parameters | ||
| Parathyroid hormone, pg/mL | 285 [228, 474] | 711 [254, 949] |
| Calcium, mg/dL | 9.4 [8.8, 9.5] | 8.1 [7.6, 11.3] |
| Phosphorus, mg/dL | 5.1 [3.9, 6.9] | 5.2 [4.1, 6.1] |
| Glucose metabolism measurements | ||
| Fasting Glucose, mg/dL | 101 [88, 111] | 104 [100, 116] |
| Fasting Insulin, µU/ml | 11.6 [9.3, 16.8] | 18.6 [13.7, 21.7] |
| HbgA1c | 5.5 [5.0, 6.0] | 5.5 [5.2, 6.4] |
| Insulin sensitivity indices | ||
| These data numerically consistent worsening in IR. Any thoughts? | ||
| GDR, mg/kg/min, mg/kg/min | 6.1 (4, 7.3) | 5.4 (4.6, 7.0) |
| HOMA index | 2.8 [2.4, 3.9] | 4.8 [4.2, 6.0] |
| QUICKI | 0.33 [0.31, 0.33] | 0.30 [0.29, 0.31] |
| HOMA-adiponectin | 57 [42, 96] | 86 [51, 130] |
| Leptin-adiponectin-ratio | 0.99 [0.62, 3.73] | 1.24 [0.44, 3.75] |
| Inflammatory Markers: | ||
| hsCRP, mg/dL | 5.8 [1.9, 7.3] | 4.5 [2.9, 8.5] |
| Interleukin 6, pg/mL | 9.5 [5.4, 17.9] | 8.6 [7.7, 9.5] |
| Glucose metabolism measurements | ||
| Adiponectin, µg/mL | 19 [16, 33] | 22 [17, 31] |
| Leptin, ng/mL | 19 [12, 68] | 22 [12, 76] |
| Nutritional Parameters | ||
| Albumin, g/dL | 3.8 [3.5, 4.1] | 4.0 [3.7, 4.1] |
| Pre-albumin, mg/dL | 33 [26, 43] | 36 [28, 40] |
| LDL cholesterol, mg/dL | 88 [65, 100] | 89 [60. 97] |
| HDL cholesterol, mg/dL | 38 [35, 50] | 39 [34, 46] |
| Triglycerides, mg/dL | 120 [98, 192] | 134 [93, 191] |
Except where indicated otherwise, values are the median [interquartile range]
Table 2.
Clinical characteristics at baseline, 8 weeks and 16 weeks by randomization group.
| Cinacalcet | Paracalcitol | |||||
|---|---|---|---|---|---|---|
| Baseline | 8 weeks | 16 weeks | Baseline | 8 weeks | 16 weeks | |
| Age, years | 53 [48, 54] | 47 [46, 49] | ||||
| BMI, kg/m2 | 33 [28, 36] | 38.8 [26.0, 43.5] | ||||
| Truncal fat, % | 38.9 [30, 46.5]* | 53 [46, 55]* | ||||
| 25 (OH) Vit D, ng/mL | 16 [15, 22] | 35 [27, 46] | 33 [26,39] | 16 [11.5, 28.2] | 30 [28, 38] | 21 [20,28] |
| Parathyroid hormone, pg/mL | 294 (241, 475) | 846 (185, 1012) | 1073 (292, 1527) | 267 (176, 617) | 576 (311, 915) | 498 (225, 855) |
| Calcium, mg/dL | 9.4 [8.8, 9.5] | 8.1 [7.7, 8.2] | 8.2 [7.9, 8.5] | 9.1 [8.4, 9.5] | 8.1 [7.4, 8.3] | 10.0 [9.6, 10.1] |
| Phosphorus, mg/dL | 5.1 [3.9, 6.9] | 4.9 [3.8, 6.2] | 4.9 [4.1, 4.9] | 4.4 [4.2, 7.5] | 5.4 [4.9, 6.0] | 6.3 [5.8, 6.9] |
| Interleukin 6, pg/mL | 5.6 [4.2, 8.5] | 8.1 [7.7, 9.0] | 5.4 [5.1, 11.6,] | 16.2 [10.5, 18.4] | 9.0 [7.7, 9.7] | 7.2 [3.6, 7.6] |
| hsCRP, mg/dL | 2.4 [1.7, 8.6] | 3.7 [2.0, 8.9] | 2.8 [2.5, 9.5] | 6.1 [5.6, 6.2] | 5.3 [3.5, 7.1] | 2.8 [2.6, 8.8] |
| Insulin, mU/L | 11.3 [8.7, 11.6] | 17.9 [8.3, 19.3] | 10.5 [7.6, 12.2] | 13.8 [11.6, 23.2] | 19.8 [14.3, 22.3] | 24.3 [8.2, 24.6] |
| Glucose, mg/dL | 87 [84, 92) | 102 [95, 105] | 85 [84, 87] | 111 [109, 127] | 116 [102, 133] | 121 [102, 124] |
| GDR, mg/kg/min | 6.9 [5.8, 8.6] | 6.7 [5.4, 7.9] | 6.9 [5.7, 8.6] | 4.1 [3.5, 6.4] | 5.0 [3.4, 5.4] | 4.7 [4.2, 4.8] |
| HOMA | 2.4 [1.7, 2.4]* | 4.2 [2.1, 4.8] | 2.1 [1.9, 2.7] | 3.5 [3.2, 7.3]* | 4.9 [4.8, 6.4] | 7.4 [2.5, 7.7] |
| QUICKI | 0.33 [0.33, 0.35] | 0.31 [0.30, 0.34] | 0.34 [0.33, 0.35] | 0.32 [0.29, 0.32] | 0.30 [0.29, 0.30] | 0.29 [0.29, 0.33] |
| HOMA-adiponectin | 44 [41, 59] | 48 [34, 103] | 28 [23, 42] | 76 [54, 140] | 105 [70, 139] | 90 [88, 132] |
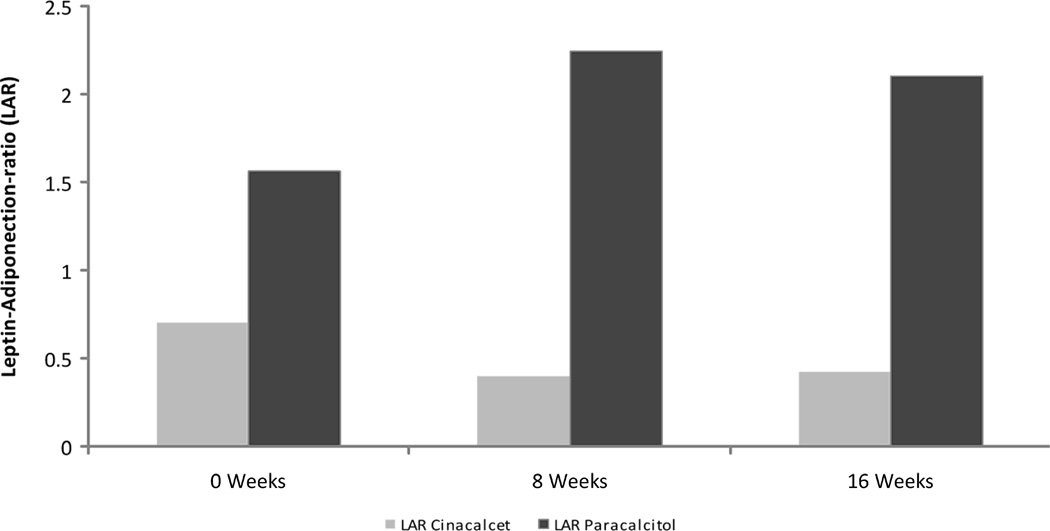
| Leptin-adiponectin-ratio | 0.70 [0.60, 0.82] | 0.71 [0.35, 1.58] | 0.42 [0.21, 4.15] | 1.56 [1.15, 4.45] | 2.24 [0.91, 4.26] | 2.10 [0.87, 3.80] |
| Adiponectin, ug/mL | 19 [16, 33] | 20 [19, 34] | 26 [26, 36] | 21 [16, 24] | 25 [17, 26] | 23 [17, 31] |
| Leptin, ng/mL | 19 [12, 68] | 14 [12, 30] | 12.0 [8, 50] | 64 [15, 70] | 74 [13, 77] | 71 [10, 77] |
Except where indicated otherwise, values are the median [interquartile range]
Truncal fat and HOMA were statistically significantly different at baseline with a *p>0.05
Changes during phase 1 (paracalcitol withdrawal)
After baseline assessment, Paracalcitol administration was stopped in all subjects (n=10) for 8 weeks and Cinacalcet was initiated to control iPTH levels, with the goal of preventing a drop of iPTH of more than 10% of the baseline value. There was no significant change in insulin sensitivity measured by clamp-derived GDR between baseline and week 8. The observed median of the difference from baseline to 8 weeks was −0.19 with an inter-quartile range of [−0.585, 0.370] (p=0.7) (Tables 1 & 3, Figure 4). There were no significant changes in any of the indirect insulin resistance indices except for QUICKI. Median QUICKI at baseline was 0.33 (inter-quartile range 0.31, 0.33) and decreased to a median of 0.30 (IQR 0.29, 0.31, p=0.04) at week 8 (Table 3, Figure 5). There were no changes in the inflammatory parameters or adipokines between the two time-points (Tables 1 & 3). In regards to mineral bone metabolism parameters, iPTH increased statistically significantly from a median of 285 pg/ml (IQR 228, 474) to a median of 711 pg/ml (IQR 254, 949; p=0.02) and serum calcium decreased from a median of 9.4 mg/dl (IQR 8.8, 9.5) to a median of 8.1mg/dl (7.6, 11.3; p= 0.008). Serum phosphorus did not significantly change during phase 1.
Table 3.
Statistical comparison of the changes for the primary and secondary endpoints between baseline vs. week 8 (Phase I: Vitamin D withdrawal period) and between week 8 vs. week 16 (Phase II: randomization to Paracalcitriol versus Cinacalcet). Data are presented as P values.
| Variables | Phase I Comparison between Baseline –to- 8 weeks |
Phase II Comparison between 8 weeks-to-16 weeks |
|
|---|---|---|---|
| Unadjusted | Adjusted | ||
| Insulin Resistance Indices | |||
| GDR | 0.7 | 0.9 | 0.86 |
| HOMA-IR | 0.1 | 0.4 | 0.74 |
| QUICKI | 0.04* | 0.4 | 0.78 |
| HOMA-adiponectin | 0.24 | 0.25 | 0.53 |
| Leptin-adiponectin-ratio | 0.9 | 0.6 | 0.74 |
| Biomarkers of Inflammation | |||
| IL-6 | 0.57 | 0.5 | 0.87 |
| hsCRP | 0.3 | 0.5 | 0.81 |
| Adipocytokines | |||
| Adiponectin | 0.5 | 0.3 | 0.08 |
| Leptin | 0.3 | 0.7 | 0.53 |
| Mineral Metabolism parameters | |||
| Parathyroid hormone, pg,mL | 0.02* | 0.06 | 0.2 |
| Calcium, mg/dL | |||
| Phosphorus, mg/dL | |||
Comparison from baseline to 8 weeks: p values were calculated using Wilcoxon Signed Rank test to compare the changes in outcomes from baseline to week 8.
Comparison from 8 weeks to 16 weeks:
Unadjusted: p values were calculated using multivariable linear regression. Independent variables included: treatment assignment and corresponding outcomes measured at week 8.
Adjusted: p values were calculated using multivariable linear regression with independent variables including: treatment assignment and corresponding outcomes measured at week 8, and propensity score [propensity scores were estimated through binary logistic regression providing the predicted probability of being assigned into treatment group A as a function of other risk factors including age, truncal fat, IL6, pth and leptin]
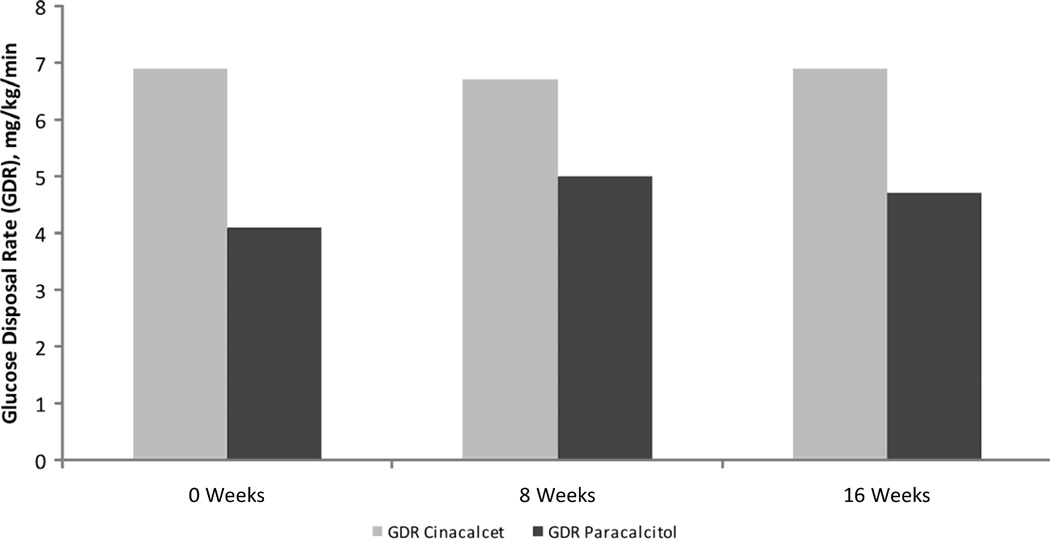
Figure 4.
Changes in Glucose Disposal Rate between baseline and weeks 8 and 16 for both the Cinacalcet and Paracalcitol groups. Changes in Glucose disposal rate (GDR) from week 0 to week 8 and from week 8 to week 16 for Cinacalcet (□) group and Paricalcitol (▪) group. There were no statistically significant differences between time points for either of the study groups, individually or combined.
Figure 5.
Changes in Leptin Adiponectin Ratio from week 0 to week 8 and from week 8 to week 16 for Cinacalcet (□) group and Paricalcitol (▪) group. There were no statistically significant differences between time points for either of the study groups, individually or combined.
Changes during phase 2 (randomization/intervention period)
At the end of week 8, 10 subjects were randomized to receive either Paracalcitol or to continue on Cinacalcet. The median and interquartile ranges for all parameters at each study point further stratified by randomization group are displayed in Table 2. Compared to Cinacalcet, GDR at week 16 in the Paracalcitol group was −0.09 lower (95% confidence interval [−1,63, 1.45] and p-value=0.9), indicating that there was no statistically significant difference in GDR at week 16 between the treatment groups after adjusting for GDR at week 8 (Table 3, Figure 4). Similarly, we did not observe any differences in groups for any of the inflammatory biomarkers, adipokines or indirect indices of insulin resistance (Table 3, Figure 5). Associations for changes in GDR between groups were tested in unadjusted and adjusted analyses using a propensity score, which showed similar results. Adjustment for a propensity score was chosen because one group had significant more truncal fat (p=0.05) and higher HOMA (p=0.05) at baseline. In addition, the Paracalcitol group was younger, had higher leptin and higher interleukin-6, although these differences were not statistically significantly different (Table 2).
25-hydroxy-vitamin D levels and nutritional VitD replacement
Levels of 25-hydroxy-vitamin D (calcidiol) were measured in all participants at baseline. Median 25 (OH) D was 16 ng/mL (interquartile range: 14.25 ng/ml, 28.75 ng/ml). Three individuals had mild deficiency (5–15 ng/ml), four were insufficient (16–30 ng/ml) and three had levels greater than 30 ng/ml, none were severely deficient. All individuals were supplemented with oral ergocalciferol following the KDOQI recommended supplementation scheme for CKD stages 3 & 4. Levels increased in all at 8 weeks but in two individuals in whom levels were less than 30 ng/ml despite supplementation (table 2).
Discussion
There is growing body of evidence indicating that patients with advanced CKD suffer from abnormalities in insulin secretion, insulin metabolism and tissue insulin sensitivity. Several earlier studies in CHD patients have suggested that active VitD administration improves insulin secretion and peripheral insulin sensitivity28,30,39. In contrast to these earlier findings, our results did not show any noticeable effect of active VitD withdrawal over 8 weeks or 16 weeks on insulin resistance measured by the gold standard hyperinsulinemic euglycemic clamp. We were also not able to show any effect of re-initiating active VitD for 8 weeks after this withdrawal period on insulin sensitivity. Similarly, there was a lack of effect of active Vit D withdrawal or reinitiation on markers of inflammation, serum concentrations of adipokines, and other indirect measurements of insulin resistance over 16 weeks of the study period.
The lack of any appreciable effect on markers of insulin sensitivity is somewhat contradictory to the previous reports and can be explained in several ways. In the majority of the previous studies, a significant suppressive effect was observed on iPTH levels after initiation of active VitD.23,24,26,29–31 In contrast, in our study iPTH levels initially increased during the withdrawal period and remained stable during re-initiation of active Vit D. This raises the question whether the earlier described effects of active Vit D administration on insulin resistance were due to the treatment of secondary hyperparathyroidism rather than the pleotropic effects of active Vit D administration. Consistent with this hypothesis, there are reports showing that insulin resistance improves following parathyroidectomy 12,14. The primary reason for the increase in iPTH concentrations in our study was the inability to effectively administer Cinacalcet due to profound decreases in serum calcium concentrations. While the inability to maintain iPTH levels closer to the baseline values might have confounded our results, theoretically this increase in iPTH should have worsened insulin resistance, especially at the presence of diminished active Vit D availability. Since we did not observe any change in the most precise measure of insulin sensitivity, i.e. GDR, during this phase, the conclusion that active Vit D does not play a significant role in IR is further supported. It should also be noted that iPTH levels were stable during phase II (week 8 to week 16) during active vitamin D re-initiation. We did not observe any change in GDR during this phase either, further supporting the lack of effect of active vitamin D administration on the GDR.
Another distinctive feature of our study was that we took advantage of the availability of calcimimetic non- Vit D intervention, i.e. cinacalcet, to control iPTH levels, at least to reasonably suppressed levels. This approach allowed us to perform a randomized, parallel-design study. While it is possible that cinacalcet administration might have counteracted any changes in GDR during active Vit D withdrawal, there are no studies indicating any effect of cinacalcet on insulin and glucose metabolism, at least to our knowledge.
In addition to its design and use of cinacalcet, there are several other important strengths of our study. Specifically, we used the gold standard hyperinsulinemic euglycemic clamp to measure insulin resistance. We also measured practical measures of IR, which were consistent with our clamp findings, strengthening the validity of our results. Inflammatory markers and certain adipokines were examined as secondary outcome measures in our study. Our results did not show any appreciable changes in any of these variables during the two phases of the study. While our study was not powered to examine these outcomes meaningfully, these observations raise the question whether some of the “non-classical” actions of active Vit D are independent of the circulating levels of 1,25 vitamin D and potentially more dependent on the local “intra-cellular” production of active vitamin D at the tissue level. For example, there is preliminary data to indicate that the administration of nutritional vitamin D increases 1,25(OH)2D levels intra-cellularly via monocyte 1-alpha hydroxylase which results in down-regulation of inflammation40. In order to minimize its potential confounding effects, all subjects were supplemented with nutritional vitamin D prior to start of the study as recommended by the KDQI guidelines.
Recent in vitro animal and small human studies have suggested that in ESRD there is a potential role for non-renal 1-alpha hydroxylase to be up-regulated resulting in generation of high tissue levels of 1,25(OH)2D from nutritional D 40. If this hypothesis is correct, 25(OH) D repletion could potentially have had an impact on the “non-classical” actions of vitamin D and could explain some of the published findings. In order to overcome this issue, we repleted our study subjects with nutritional vitamin D as needed. This supplementation occurred at the beginning of the study and potentially could have blunted our expected changes following withdrawal of Paricalcitol. Nevertheless, future research is needed to understand the role of nutritional vitamin D in ESRD, especially in relation to nutritional vitamin D supplementation 41.
Our study has certain limitations, which might have influenced the results. Most importantly, our sample size was relatively small, especially during Phase II. In support of the reliability of our findings, the point estimate showed no meaningful clinical change in value. Furher, the use of the most precise and sensitive measure of insulin resistance allowed us to interpret our results with more accuracy as a true lack of effect and not due to a small sample size. Nevertheless, this study should be considered as pilot. Another limitation is that the study is performed exclusively in African American subjects and results cannot be generalized to other racial groups. Additional studies in other racial and ethnic groups are necessary to confirm and generalize our results. Our study population was obese which could have affected our results; however our comparisons for phase I were intra-individual changes and a propensity score was used for phase II to adjust for between group differences. Our study duration of 8–16 weeks can be considered relatively short such that the withdrawal or the exposure period needed to be longer in order to see an effect. Notably, previous studies examining the effects of active vitamin D3 on insulin resistance were of even shorter duration27–29,39. Finally, our study protocol allows us to interpret only the peripheral insulin resistance due to high dose insulin administration during the clamp study. Accordingly, it is possible that the effects of active Vit D on insulin and glucose homeostasis could be mediated centrally, which should be tested with further studies.
In summary, our results suggest that the administration of active Vit D in the setting of elevated iPTH has no effect on peripheral insulin resistance in nutritional vitamin D repleted prevalent African-American CHD patients. Larger and longer term studies using practical markers of insulin resistance are needed to confirm our findings.
Practical Application: In addition to its role on bone and mineral metabolism, vitamin D is proposed to have much wider effects on the body, influencing many physiological processes such as blood pressure regulation, modulation of the immune response and insulin actions. Since insulin resistance is common in CHD patients, we tested the hypothesis whether active vitamin D might have a role on its variability in a pilot study. Our results indicate that short-term (i.e. 8 weeks) withdrawal or reinitiating active vitamin D3 replacement therapy in CHD patients do not substantially improve or worsen insulin sensitivity. Other strategies, including nutritional vitamin D should be tested to improve insulin action in CHD patients.
Acknowledgments
This study was supported in part by grants Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, K24 DK 62849 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Center for D-Receptor Activation Research. A. Hung is supported by Veterans Administration Career Development Award CSR&D (2-031-09S). The sponsors had no influence on the design, execution, and analysis of the results of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests:
Adriana M. HUNG: none
Mary B. SUNDELL: none
Natalia E. PLOTKINOVA: none
Aihua BIAN : none
Ayumi SHINTANI : none
Charles D. ELLIS: none
Edward D. SIEW: none
T. Alp IKIZLER: none
References
- 1.DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest. 1978;62:425–435. doi: 10.1172/JCI109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:1894–1900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Andres R, Edgar P, Walker WG. Carbohydrate metabolism in uremia: a review. Medicine (Baltimore) 1973;52:469–481. doi: 10.1097/00005792-197309000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Adrogue HJ. Glucose homeostasis and the kidney. Kidney Int. 1992;42:1266–1282. doi: 10.1038/ki.1992.414. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guebre-Egziabher F, Kalbacher E, Fouque D. [Insulin resistance and inflammation in chronic kidney diseases] Nephrol Ther. 2009;5(Suppl 5):S346–S352. doi: 10.1016/S1769-7255(09)75168-5. [DOI] [PubMed] [Google Scholar]
- 7.Hung AM, Sundell MB, Egbert P, et al. A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:767–774. doi: 10.2215/CJN.08070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanetti M, Barazzoni R, Guarnieri G. Inflammation and insulin resistance in uremia. J Ren Nutr. 2008;18:70–75. doi: 10.1053/j.jrn.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Borazan A, Binici DN. Relationship between insulin resistance and inflamation markers in hemodialysis patients. Ren Fail. 2010;32:198–202. doi: 10.3109/08860220903491232. [DOI] [PubMed] [Google Scholar]
- 10.Hung AM, Ikizler TA. Factors Determining Insulin Resistance in Chronic Hemodialysis Patients Contrib. Nephrol. 2011;171:127–134. doi: 10.1159/000327177. [DOI] [PubMed] [Google Scholar]
- 11.Mak RH. Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int. 1998;54:603–607. doi: 10.1046/j.1523-1755.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 12.Amend WJ, Jr, Steinberg SM, Lowrie EG, et al. The influence of serum calcium and parathyroid hormone upon glucose metabolism in uremia. J Lab Clin Med. 1975;86:435–444. [PubMed] [Google Scholar]
- 13.Graf H, Prager R, Kovarik J, Luger A, Schernthaner G, Pinggera WF. Glucose metabolism and insulin sensitivity in patients on chronic hemodialysis. Metabolism. 1985;34:974–977. doi: 10.1016/0026-0495(85)90148-9. [DOI] [PubMed] [Google Scholar]
- 14.Mak RH, Bettinelli A, Turner C, Haycock GB, Chantler C. The influence of hyperparathyroidism on glucose metabolism in uremia. J Clin Endocrinol Metab. 1985;60:229–233. doi: 10.1210/jcem-60-2-229. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 16.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 17.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 18.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 19.Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–1078. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- 20.de Boer IH. Vitamin D and glucose metabolism in chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:566–572. doi: 10.1097/MNH.0b013e32830fe377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allegra V, Luisetto G, Mengozzi G, Martimbianco L, Vasile A. Glucose-induced insulin secretion in uremia: role of 1 alpha,25(HO)2-vitamin D3. Nephron. 1994;68:41–47. doi: 10.1159/000188085. [DOI] [PubMed] [Google Scholar]
- 22.Bonakdaran S, Ayatollahi H, Mojahedi MJ, Sharifipoor F, Shakeri M. Impact of treatment with oral calcitriol on glucose intolerance and dyslipidemia(s) in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:942–947. [PubMed] [Google Scholar]
- 23.Gunal AI, Celiker H, Celebi H, Ustundag B, Gunal SY. Intravenous alfacalcidol improves insulin resistance in hemodialysis patients. Clin Nephrol. 1997;48:109–113. [PubMed] [Google Scholar]
- 24.Kautzky-Willer A, Pacini G, Barnas U, et al. Intravenous calcitriol normalizes insulin sensitivity in uremic patients. Kidney Int. 1995;47:200–206. doi: 10.1038/ki.1995.24. [DOI] [PubMed] [Google Scholar]
- 25.Khajehdehi P, Taheri S. Effect of oral calcitriol pulse therapy on the lipid, calcium, and glucose homeostasis of hemodialysis-patients: its safety in a combination with oral calcium carbonate. J Ren Nutr. 2003;13:78–83. doi: 10.1053/jren.2003.50026. [DOI] [PubMed] [Google Scholar]
- 26.Lin SH, Lin YF, Lu KC, et al. Effects of intravenous calcitriol on lipid profiles and glucose tolerance in uraemic patients with secondary hyperparathyroidism. Clin Sci (Lond) 1994;87:533–538. doi: 10.1042/cs0870533. [DOI] [PubMed] [Google Scholar]
- 27.Mak RH. Amelioration of hypertension and insulin resistance by 1,25-dihydroxycholecalciferol in hemodialysis patients. Pediatr Nephrol. 1992;6:345–348. doi: 10.1007/BF00869730. [DOI] [PubMed] [Google Scholar]
- 28.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–1357. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 29.Quesada JM, Martin-Malo A, Santiago J, et al. Effect of calcitriol on insulin secretion in uraemia. Nephrol Dial Transplant. 1990;5:1013–1017. doi: 10.1093/ndt/5.12.1013. [DOI] [PubMed] [Google Scholar]
- 30.Strozecki P, Kretowicz M, Odrowaz-Sypniewska G, Manitius J. The influence of intravenous 1,25(OH)2D3 therapy on glucose metabolism in hemodialyzed patients with secondary hyperparathyroidism. Ren Fail. 2004;26:345–348. doi: 10.1081/jdi-120039815. [DOI] [PubMed] [Google Scholar]
- 31.Turk S, Yeksan M, Tamer N, Gurbilek M, Erdogan Y, Erkul I. Effect of 1,25 (OH)2D3 treatment on glucose intolerance in uraemia. Nephrol Dial Transplant. 1992;7:1207–1212. doi: 10.1093/ndt/7.12.1207. [DOI] [PubMed] [Google Scholar]
- 32.Gniuli D, Castagneto-Gissey G, Iaconelli A, Leccesi L, Mingrone G. Fat mass largely contributes to insulin mediate glucose uptake in morbidly obese subjects. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.99. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Shoji T, Emoto M, Nishizawa Y. HOMA index to assess insulin resistance in renal failure patients. Nephron. 2001;89:348–349. doi: 10.1159/000046098. [DOI] [PubMed] [Google Scholar]
- 35.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 36.Matsuhisa M, Yamasaki Y, Emoto M, et al. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007;77:151–154. doi: 10.1016/j.diabres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Teta D, Maillard M, Halabi G, Burnier M. The leptin/adiponectin ratio: potential implications for peritoneal dialysis. Kidney Int Suppl. 2008:S112–S118. doi: 10.1038/sj.ki.5002611. [DOI] [PubMed] [Google Scholar]
- 38.Axelsson J, Rashid Qureshi A, Suliman ME, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr. 2004;80:1222–1229. doi: 10.1093/ajcn/80.5.1222. [DOI] [PubMed] [Google Scholar]
- 39.Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41:1049–1054. doi: 10.1038/ki.1992.159. [DOI] [PubMed] [Google Scholar]
- 40.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]