Abstract
Background
Branched-chain amino acid (BCAA) concentrations are elevated in response to overnutrition, and can affect both insulin sensitivity and secretion. Alterations in their metabolism may therefore play a role in the early pathogenesis of type 2 diabetes in overweight children.
Objective
To determine whether pediatric obesity is associated with elevations in fasting circulating concentrations of branched-chain amino acids (isoleucine, leucine, and valine), and whether these elevations predict future insulin resistance.
Research Design and Methods
Sixty-nine healthy subjects, ages 8 to18 years, were enrolled as a cross-sectional cohort. A subset who were pre- or early-pubertal, ages 8 to 13 years, were enrolled in a prospective longitudinal cohort for 18 months (n=17 with complete data).
Results
Elevations in the concentrations of BCAA’s were significantly associated with BMI Z-score (Spearman’s Rho 0.27, p=0.03) in the cross-sectional cohort. In the subset of subjects followed longitudinally, baseline BCAA concentrations were positively associated with HOMA-IR measured 18 months later after controlling for baseline clinical factors including BMI Z-score, sex, and pubertal stage (p=0.046).
Conclusions
Elevations in the concentrations of circulating branched-chain amino acids are significantly associated with obesity in children and adolescents, and may independently predict future insulin resistance.
Keywords: branched-chain amino acids, insulin resistance, pediatric obesity, metabolomics, type 2 diabetes
INTRODUCTION
Type 2 diabetes is a global public health crisis1 that may have its origins early in life. Insulin resistance often develops during adolescence, is exacerbated by obesity2,3 and may contribute to the progression to type 2 diabetes in youth4.
Adults with obesity, with or without diabetes, demonstrate hyperaminoacidemia. In particular, increases in the concentrations of the branched-chain amino acids (BCAA’s, leucine, isoleucine, and valine) along with tyrosine and phenylalanine, have been described5. Unlike other essential amino acids, the BCAA’s are degraded in skeletal muscle, and their circulating levels are elevated post-prandially6. Elevations in BCAA’s may influence glucose homeostasis, because oxidation of branched-chain amino acids spares glucose utilization in skeletal muscle7. Infusion studies have demonstrated that increased concentrations of plasma amino acids (AA’s) disrupt insulin signaling at the molecular level8 and inhibit glucose transport and phosphorylation, resulting in lower rates of glycogen synthesis9. These mechanisms have been posited to underlie AA-induced decreases in insulin sensitivity. An amino acid “signature”, including the BCAA’s and several downstream products of their catabolism (glutamate, and C3 and C5 acylcarnitines)10, is correlated with obesity-related insulin resistance in adults. In adults without significant abnormalities in glucose homeostasis, elevations in current levels of BCAA’s, along with tyrosine and phenylalanine, are also associated with an increased future likelihood of developing type 2 diabetes, even after accounting for established baseline clinical risk factors11.
Fewer studies have investigated the association between branched-chain amino acid concentrations and adverse metabolic profiles in children, in whom a relatively shorter duration of obesity, ongoing linear growth, and pubertal hormones may be expected to yield different results. One previous investigation showed that the concentrations of branched-chain amino acids rose after a 24-hour fast in obese children12. Another study found that a fall in branched-chain amino acid concentrations occurred after a 30-hour fast in children13. Both of these protocols employed prolonged fasting conditions that may not reflect physiology in more typical post-absorptive states. Thus, the primary objective of this study was to determine whether elevations in the fasting concentrations of branched-chain amino acids are related to obesity in children and adolescents, and whether they are independently associated with insulin resistance in a prospective, longitudinal cohort.
METHODS
Study Design
The study was approved by the Partners, Massachusetts General Hospital, and MIT Institutional Review Boards; conducted according to the Declaration of Helsinki; and registered with ClinicalTrials.gov (NCT00577174). Written informed consent and assent were obtained from all participants, as appropriate. Participants were recruited by local advertisements, from pediatricians in the community, and from the obesity programs at MGH and Children’s Hospital Boston, to target a goal of 50% obese subjects, from June 2007 through April 2009.
Study participants
In the cross-sectional cohort were children, 8 to 18 years, without chronic medical illness, a personal history of diabetes or diabetes in a first-degree relative, history of smoking, medical condition or medication related to obesity or diabetes risk status. Of 74 participants in the cross-sectional cohort, 69 had metabolomic profiling performed; the balance had incomplete samples due to difficulty with phlebotomy. By design, approximately half (52%) of the cross-sectional cohort were overweight or obese. A small number of siblings were permitted to enroll and included in the final analysis, 9 subjects (from four families) in the cross-sectional cohort, and 4 subjects (from two families) in the longitudinal cohort. Similar results were obtained when analyses were performed with data from only one participant per family (data not shown). A subset of children ages 8 to 13 with initial metabolite profiling were enrolled to participate in the longitudinal component of the study (n=25). Children were pre-pubertal and early- to mid-pubertal (Tanner stages I – III), and approximately two-thirds (64%) of the longitudinal cohort were overweight or obese. Seventeen subjects have complete data at 18 months. Initial observations from these cohorts have been published14,15,16.
All participants underwent a baseline evaluation, including a complete medical and family history; physical examination, including Tanner staging by a pediatric endocrinologist (A.F.); blood pressure by oscillometry; dietary and exercise questionnaires; and baseline laboratory testing. In addition, glucose and insulin values were obtained at baseline under fasting conditions and every 30 minutes for 120 minutes after 1.75 g/kg (up to a maximum of 75 g) of an oral glucose load. Anthropometric measurements were performed and dietary records were reviewed by nutrition staff. BMI percentile and Z-score for age and sex were based on Centers for Disease Control 2000 growth charts. For the longitudinal cohort, all baseline measures, including the OGTT, were repeated annually, with fasting laboratory studies and anthropometric measurements performed at interim visits every 6 months, for up to 18 months.
The primary outcome variable in the longitudinal cohort was future insulin resistance, as estimated by the homeostasis model assessment for insulin resistance (HOMA-IR)17. HOMA-IR measures basal insulin resistance and in children correlates with the gold standard hyperinsulinemic euglycemic clamp18. The whole-body index of insulin sensitivity (WBISI) was also measured utilizing data from the annual OGTT. This index is defined as (10,000/square root of [fasting glucose x fasting insulin] x [mean glucose x mean insulin during OGTT] and is associated with the rate of glucose disposal as assessed by euglycemic clamp19.
Questionnaires
Three-day food records were collected at the baseline evaluation and reviewed by nutrition staff with analysis by Nutrition Data Systems, Minneapolis, MN. Self-reported levels of physical activity were assessed with pediatric-specific modifications made to the Modifiable Activity Questionnaire20 to quantify the daily amount of sedentary time, as well as moderate and vigorous physical activity.
Laboratory analyses
The serum insulin was measured using one of two RIA’s (Diagnostic Products Corp., Los Angeles, CA or Access Immunoassay System, Beckman Coulter, Chaska, MN) due to NIH-required CTSA laboratory site change during the study period. In same-sample comparisons, inter-assay correlation was excellent (r =0.99), using identical linear scales, without systemic differences in the results of the assays by Bland-Altman analysis21. Serum glucose and lipids were measured using standard methodologies. Insulin-like growth factor 1 was measured by enzyme immunoassay (ALPCO Diagnostics, Salem, NH).
Metabolite profiling
Targeted LC-MS/MS-based profiling was performed as previously reported for 60 metabolites, including the four specific amino acids of interest (leucine, isoleucine, valine, and glutamate), with detectable levels in more than 90% of subjects22. In order to generate a combined index of the relative magnitude of the concentrations of the three BCAA’s, Z-scores were calculated for each subject, i.e., for each of leucine, isoleucine, and valine, the subject’s Z score reflects the number of standard deviations above or below the mean. Metabolite LC-MS/MS output for each metabolite was not normally distributed, and was therefore first log-transformed prior to calculating Z-scores. The log-transformed metabolite concentrations were used to generate within-cohort Z-scores for each of the 3 BCAA’s; the simple sum of these Z scores was defined a priori and assessed with respect to its relationship to obesity and as a candidate biomarker of future insulin resistance. A within-cohort Z-score was also generated for glutamate, to explore the possible role of BCAA catabolism in producing insulin resistance. A within-cohort summed Z-score was similarly generated for the balance of the amino acids in the metabolite profile, to account for any effect of overall hyperaminoacidemia.
Statistics
Statistical analyses were performed using JMP SAS-based software and R version 2.11.1. Non-parametric (Spearman’s) or parametric (Pearson’s) correlations were used as appropriate to evaluate the relationship between variables of interest and the summed Z scores of these three amino acids. In unbiased, exploratory analyses to determine whether other metabolites exhibited correlations with the outcome variables of interest in the cross-sectional cohort, raw concentrations of analytes were used to generate individual Spearman’s correlation coefficients and p-values for each metabolite. A Bonferonni-corrected p-value threshold of 0.05/60 = 0.00083 (where 60 is the number of metabolites analyzed) was used to account for multiple comparisons.
Paired-sample Wilcoxon signed-rank tests were used to evaluate differences in subject characteristics obtained at baseline and after 18 months in the longitudinal cohort. The association of the BCAA’s with HOMA-IR at 18 months was further assessed using multivariate regression analysis controlling for demographic, anthropometric and metabolic factors. A two-sided p-value of 0.05 was used as a threshold for statistical significance. This was supplemented by an unbiased, exploratory analysis to investigate whether other metabolites, measured at baseline, exhibited correlations with HOMA-IR, measured at 18 months. Raw concentrations of analytes were used to generate individual Spearman’s correlation coefficients and p-values for each metabolite, and a Bonferonni-corrected p-value threshold of 0.00083 (=0.05/60) was again used.
RESULTS
Cross-sectional Cohort
Sixty-nine children, ages 8 – 18 years, had laboratory testing following an overnight fast. Clinical and laboratory characteristics are summarized in Table 1 and dietary intake in Table 2. No individuals in the baseline cohort met diagnostic criteria for diabetes or had impaired fasting glucose; two had impaired glucose tolerance.
Table 1.
Clinical characteristics of cross-sectional cohort and longitudinal cohort (at baseline and at 18 months).
| Cross-sectional Cohort (n = 69) |
Longitudinal Cohort (n = 17), baseline |
Longitudinal Cohort (n = 17), 18 months |
|
|---|---|---|---|
| Age (years) | 13.3 ± 2.9 | 11.0 ± 1.6 | 12.5 ± 1.6 |
| Sex (% female, n) | 42% (29) | 41% (7) | |
| Race (%, n) | |||
| African-American | 30% (21) | 24% (4) | |
| White | 52% (36) | 53% (9) | |
| More than one race/Other | 17% (12) | 24% (4) | |
| Ethnicity (%, n) | |||
| Hispanic | 22% (15) | 29% (5) | |
| Non-Hispanic | 78% (54) | 71% (12) | |
| Tanner Stage | |||
| I (%, n) | 16% (11) | 29% (5) | |
| II (%, n) | 16% (11) | 47% (8) | 12% (2) |
| III (%, n) | 16% (11) | 24% (4) | 24% (4) |
| IV (%, n) | 25% (17) | 53% (9) | |
| V (%, n) | 28% (19) | 12% (2) | |
|
Episodes of vigorous
exercise per week |
3.7 ± 2.2 | 3.0 ± 2.1 | 3.2 ± 1.7 |
| Hours of screen time daily | 4.0 ±2.5 | 4.0 ± 2 | 3.5 ± 2 |
| BMI (kg/m2) | 24.9 ± 7.4 | 26.0 ± 7.1 | 27.9 ± 7.6* |
| BMI Z-score | 1.04 ± 1.23 | 2.88 ± 2.17 | 2.95 ± 2.14 |
| Cholesterol (mmol/L) | 3.9 ± 0.8 | 4.2 ± 0.8 | 4.1 ± 0.8 |
| LDL (mmol/L) | 2.4 ± 0.6 | 2.5 ± 0.7 | 2.4 ± 0.5 |
| HDL (mmol/L) | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Triglycerides (mmol/L) | 0.8 ± 0.5 | 0.8 ± 0.4 | 1.1 ± 0.5* |
| Fasting insulin (uIU/mL) | 9 ± 10 | 8 ± 5 | 10 ± 6 |
| Fasting glucose (mmol/L) | 4.3 ± 0.4 | 4.4 ± 0.2 | 4.4 ± 0.5 |
| 2-hour glucose (mmol/L) | 5.8 ± 1.2 | 6.2 ± 1 | |
| HOMA-IR | 1.84 ± 2.04 | 1.50 ± 0.87 | 2.11 ± 1.41 |
| WBISI‡ | 7.99 ± 5.98 | 7.00 ± 4.02 | |
| IGF-1, U/L | 186 ± 88 | 156 ± 45 |
Means are presented ± standard deviations. HOMA-IR, homeostatic model of insulin resistance. WBISI, whole-body insulin sensitivity index.
indicates significant difference between the baseline and 18-month visits, by within-subject matched pair Wilcoxon signed rank test.
OGTT and IGF-1 were not performed at the 18-month visit. To convert cholesterol, HDL, and LDL (mmol/L) to mg/dL, multiply by 38.6. To convert triglycerides (mmol/L) to mg/dL, multiply by 88.5. To convert glucose (mmol/L) to mg/dL, multiply by 18.
Table 2.
Dietary Information for baseline cohort and longitudinal cohort (at baseline and 18 months).
| Baseline Cohort (n = 58) |
Longitudinal Cohort (n = 13), baseline |
Longitudinal Cohort (n = 12), 18 months |
|
|---|---|---|---|
| Calories (kcal/day) | 1,877 ± 508 | 1,815 ± 360 | 1,761 ± 440 |
| Protein (g/day) | 76 ± 20 | 71 ± 15 | 68 ± 19 |
| Protein (% of energy) |
16% ± 3 | 16% ± 3 | 15% ± 2 |
| Isoleucine (g/day) | 3.4 ± 0.9 | 3.2 ± 0.7 | 3.1 ± 0.9 |
| Leucine (g/day) | 5.9 ± 1.6 | 5.5 ± 1.2 | 5.4 ± 1.6 |
| Valine (g/day) | 3.8 ± 1.0 | 3.6 ± 0.8 | 3.5 ± 1.1 |
| Glutamate (g/day) | 15.3 ± 4.2 | 14.2 ± 2.7 | 14.3 ± 3.8 |
| Fat (g/day) | 69 ± 22 | 68 ± 16 | 66 ± 26 |
| Fat (% energy) | 33% ± 5 | 33% ± 4 | 33% ± 7 |
Means are presented ± standard deviations. Complete dietary history information is available for 58 subjects in the baseline cohort, and on 13 of the longitudinal cohort at the baseline visit, and 12 at the 18-month visit.
Obese children (i.e., those whose BMI was greater than or equal to the 95%ile for age and sex) had higher concentrations of the BCAA’s (p = 0.008, two-sample t-test). Elevations in the BCAA’s were also positively associated with BMI Z-score (Spearman’s Rho 0.27, p=0.03) in all subjects. This result also holds in the subset of children who were pre- or early-pubertal (Tanner stages I or II, Spearman’s Rho 0.59, p=0.004). Elevations in glutamate were also positively associated with BMI Z-score (Spearman’s Rho 0.5, p < 0.0001). Elevations in the BCAA’s were also positively associated with waist circumference percentile (Spearman’s Rho 0.25, p=0.04) and waist-to-hip ratio (Spearman’s Rho 0.28, p=0.02).
There was not a significant association between BCAA concentrations and baseline insulin resistance, expressed either as HOMA-IR or WBISI, in the cross-sectional cohort. There was no relationship between dietary intake of the BCAA’s and their plasma concentrations after an overnight fast. BCAA concentrations were not related to race, ethnicity, daily caloric intake, self-reported physical activity or inactivity (expressed either as number of episodes of vigorous exercise per week, or amount of screen time), family history of Type 2 diabetes, Tanner stage, or IGF-1 level.
In exploratory, unbiased analyses using all 60 metabolites, glutamate was positively related (Spearman’s Rho, 0.50, unadjusted p<0.0001) and citrulline negative related (-0.41, 0.0004) with BMI Z-score. Valine (0.34, 0.005), leucine (0.21, 0.07), and isoleucine (0.22, 0.07) were individually each positively associated with BMI Z-score, but these relationships did not remain statistically significant after Bonferonni correction. (Complete data are shown in Appendix 1A.) Using the same unbiased approach to investigate all 60 metabolites, glutamate (Spearman’s Rho 0.55, unadjusted p<0.0001) and 3-hydroxyanthranilic acid (0.43, 0.0002) were positively associated and citrulline negatively associated (-0.47, p=0.0001) with HOMA-IR. (Complete data are shown in Appendix 1B.)
Longitudinal Cohort
Of the individuals followed in the longitudinal cohort, none had impaired fasting glucose or impaired glucose tolerance (IGT) at baseline. None developed diabetes mellitus and five developed impaired fasting glucose and/or impaired glucose tolerance at the final measurement.
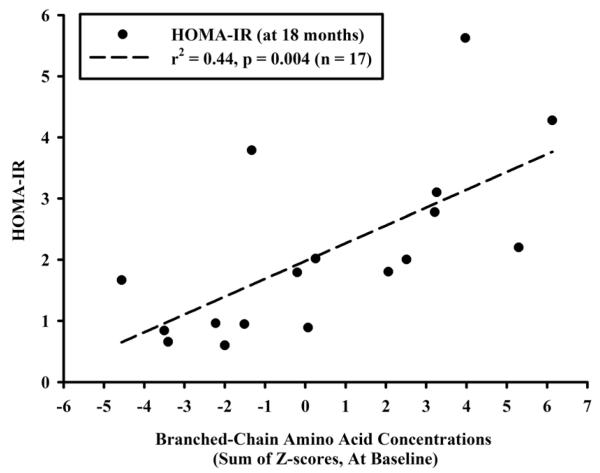
The fasting concentrations of BCAA’s, measured at baseline, were strongly associated with HOMA-IR at 18 months (r2=0.44, p=0.004, Figure 1). Elevations in the concentrations of BCAA’s were also associated with reduced insulin sensitivity, as estimated by the WBISI at 12 months (r2=0.3, p=0.03), as OGTT’s were only performed annually. The concentration of glutamate, measured at baseline, was also positively associated with HOMA-IR at 18 months (r2=0.4, p=0.007). Multivariate modeling demonstrated that absolute HOMA-IR at the 18-month visit was independently associated with the concentrations of BCAA’s measured at baseline after controlling for clinical factors assessed at baseline, including sex, BMI Z-score, and Tanner stage (Table 3). The relationship also remained significant (p=0.01) after baseline HOMA-IR was added to the model. Finally, the relationship between BCAA’s measured at baseline and HOMA-IR at 18 months was also statistically significant (p=0.003) after adjusting for a summary measure reflecting the concentrations of all other amino acids at baseline (i.e., excluding BCAA’s and glutamate). Baseline concentrations of BCAA’s predicted a worsening in HOMA-IR over time in those subjects whose initial HOMA-IR was normal, i.e., less than 2 (r2=0.40, p=0.02, n=13), but not when subjects with already elevated HOMA-IR were included (p=0.33).
Figure 1.
Concentrations of branched-chain amino acids measured at baseline (expressed as the sum of the cohort-specific Z-scores for each of leucine, isoleucine, and valine) and HOMA-IR measured at 18 months.
Table 3.
Multivariate Modeling for HOMA-IR at 18 months, Longitudinal Cohort (n=17). Relationship of BCAA’s and clinical covariates at baseline to absolute HOMA-IR at 18 months. Adjusted R2 for model is 0.48, p = 0.03.
| Covariates | Coefficient | P value |
|---|---|---|
| BCAA’s (baseline) | 0.27 | 0.046 |
| BMI Z-score (baseline) | 0.18 | 0.29 |
| Sex (female versus male) | −0.22 | 0.48 |
| Tanner Stage (baseline, mid-pubertal, Tanner III, versus pre- or early- pubertal, Tanner I or II) |
0.32 | 0.38 |
In exploratory analyses including all 60 metabolites, phenylalanine (Spearman’s Rho 0.79, unadjusted p=0.0002), valine (0.76, p=0.0004), leucine (0.75, p=0.0005) and glutamate (0.75, 0.0005) measured at baseline were positively associated with HOMA-IR measured at 18 months. (Complete data are shown in Appendix 1C.)
DISCUSSION
We have demonstrated in a cross-sectional cohort of children and adolescents that obesity is associated with elevations in the fasting circulating concentrations of three BCAA’s as well as glutamate, one of the downstream products of BCAA catabolism. In a subset of these children followed longitudinally, these elevations are positively associated with insulin resistance measured 18 months later, even after accounting for baseline clinical characteristics. These findings suggest that disordered BCAA catabolism may be an early manifestation of the adverse metabolic consequences of overnutrition. In addition, our findings extend prior mechanistic studies that implicate persistently elevated concentrations of BCAA’s in the development of future insulin resistance e.g.,8,9.
The etiology of obesity-related increases in the concentrations of BCAA’s remains incompletely understood. One possibility is that obese individuals have a higher dietary content of BCAA’s. Intake of leucine, isoleucine, and valine, particularly in the context of a high-fat diet characteristic of diet-induced obesity, could also be a cause of insulin resistance10. Insulin resistance, in turn, could lead to the failure of insulin’s physiologic capacity to suppress BCAA levels23. In the present study, however, self-reported dietary intake of BCAA’s did not appear related to circulating BCAA levels in the fasted state. As reviewed in Matthews et al.6 , a role for the BCAA’s as peripheral “nutrient signals” has been posited. The current study did not explore the potential role of post-prandial levels of BCAA’s as indicators of overall energy intake.
The relationship between disrupted BCAA metabolism and future diabetes risk is also the subject of ongoing investigation. One hypothesis is that global resistance to the many dimensions of insulin action, including suppression of proteolysis, leads to increased release of BCAA’s from muscle and increased circulating levels. However, recent work suggests individuals vary with respect to their responsiveness to insulin’s many unique effects. For example, Shaham et al.,24 have used metabolite profiling technology to show that some individuals display differential sensitivity to insulin-induced suppression of proteolysis as compared to lipolysis. Also, as noted above, elevated BCAA levels may appear long before other indices of insulin resistance become abnormal11. Taken together, these findings reflect the many different, interacting ways in which insulin action affects energy utilization.
Obesity or other, as of yet unidentified, factors could also underlie the association between alterations in BCAA metabolism and insulin resistance. She et al.,25 report that surgically-induced weight loss brought down BCAA concentrations while restoring activity of enzymes involved in BCAA catabolism. Shah et al.,26 found that elevated baseline concentrations of BCAA’s identified the subjects who would go on to experience improvements in insulin sensitivity associated with weight loss from a lifestyle intervention. These results may indicate that BCAA’s are related to insulin resistance primarily as a reflection of obesity. However, another recent study suggests that other factors may also modulate the relationship between BCAA’s and insulin sensitivity. Laferrere et al.,27 showed that BCAA levels declined more in obese subjects after gastric bypass surgery as compared to obese subjects who lost similar amounts of weight through lifestyle modification alone. Indeed, in the present study we found that although elevated concentrations of BCAA’s were related to BMI at baseline, they appeared to contribute independently to future insulin resistance. Changes in BCAA catabolism may be one way in which this occurs. Newgard et al.,10 interpret an elevation of glutamate levels in obese, insulin-resistant individuals as potentially reflecting increased BCAA catabolic flux. (The first step in the catabolism of BCAA’s is their reversible transamination, producing glutamate and branched-chain ketoacids.) We also observed elevations in glutamate in association with both obesity and insulin resistance in our pediatric subjects. Finally, glutamate, leucine and isoleucine in particular are insulin secretagogues28,29; if chronic elevations in glutamate and BCAA’s potentiate insulin secretion they could contribute both to hyperinsulinism and ultimately to beta-cell failure. Glutamate may also have direct toxicity to pancreatic beta-cells30.
The relationship between circulating BCAA’s and insulin sensitivity is complex, as illustrated by experiments demonstrating an improvement in insulin action and signaling in mice fed a high-fat diet supplemented with leucine31, as compared to a worsening of insulin resistance in rats fed a high-fat diet supplemented with all three BCAA’s10. The many potentially important interactions between BCAA’s and fatty acid metabolism are the subject of ongoing investigation, s reviewed in32.
We performed additional hypothesis-generating unbiased analyses of metabolomics data. In our study, citrulline was negatively associated with both obesity and insulin resistance, and 3-hydroxyanthranilic acid was positively associated with insulin resistance in the cross-sectional cohort. Citrulline is a part of the urea cycle. It is also a component of the arginine-nitric oxide pathway that may reflect NO availability. One study found that obese children had lower levels of both citrulline and circulating nitric oxide33. 3-hydroxyanthranilic acid (3-HAA) is a tryptophan metabolite (via the kyurenine pathway) and may serve as an endogenous NO scavenger34 and affect mitochondrial respiration35. In the present study, exploratory metabolite profiling analysis also showed statistically significant positive associations between baseline concentrations of glutamate, leucine, valine, and phenylalanine and HOMA-IR measured at the 18-month visit. Isoleucine exhibited a similar association that did not reach statistical significance. With respect to the relationship between the aromatic amino acid phenylalanine and HOMA-IR, competition with BCAA’s for uptake into tissues via their common large neutral amino acid transporter (LAT1) is a possible etiology36. These exploratory data show that two BCAA’s, leucine and valine, as well as glutamate, a product of BCAA catabolism, each exhibited independent, statistically significant relationships with future insulin resistance. In fact, these amino acids demonstrated the strongest associations among the 60 metabolites tested, supporting our initial hypothesis about their potential role in the pathogenesis of insulin resistance.
The possible effects of pubertal growth in our sample warrant consideration, because growth hormone secretion and protein turnover might be expected to affect the dynamics of BCAA uptake into or release from muscle. One study in lean children and adolescents demonstrated that proteolysis and protein oxidation appear to be reduced during puberty relative to pre-puberty, which may be the result of growth-hormone-induced increases in IGF-137. Despite reduced proteolysis and protein oxidation, no differences in the circulating concentrations of BCAA’s between pubertal and pre-pubertal children were noted in that study37. Another investigation in lean children and adolescents also failed to report an association between BCAA concentrations and pubertal status38. In addition, growth hormone seems to cause insulin resistance when insulin resistance is evaluated with respect to glucose homeostasis38. Controversy exists, however, with respect to the relative sensitivity of proteolysis (as compared to glycolysis or gluconeogenesis) to suppression by insulin during puberty. In our study of obese and normal-weight subjects, neither pubertal status nor IGF-1 levels were significantly associated with the fasting concentrations of BCAA’s, suggesting that the effect of overnutrition on BCAA’s might be primary.
Obesity itself has been associated with a higher rate of whole-body protein turnover (as compared to lean control subjects) in adolescents without associated changes in circulating levels of leucine39. This is explained by higher rates of both leucine appearance and non-oxidative disposal, suggesting greater protein turnover, without a different in leucine oxidation. Regional adiposity40,41, as well as exercise and lifestyle modification39,42 may affect protein turnover in overweight adolescents and adults. Conclusions with respect to the insulin sensitivity of proteolysis in obese individuals vary across studies, perhaps in part related to differences in subjects and methodology, e.g.,40,43,42,41, highlighting the need for additional investigations in this important area. In the present study, we found circulating levels of leucine and the other BCAA’s were positively related to BMI Z-score, which could be due to the larger sample size and greater subject heterogeneity; differences in dietary intake of either leucine and/or other macronutrients44,45 not captured by self-report; larger amounts of fat-free mass in obese subjects43 or decreased BCAA catabolism in adipose tissue or skeletal muscle32. These factors, as well as the potential role of growth hormone, were not studied directly but could be the focus of future investigation.
There are several additional limitations to the present study. The sample size was relatively small, but despite this we were able to identify statistically significant relationships between BCAA concentrations and future insulin resistance in a pediatric population. The relatively low incidence of type 2 diabetes mellitus in this age group required us to use insulin resistance, a proxy measure, instead of diabetes mellitus as the primary outcome of interest. HOMA-IR, a surrogate index of insulin sensitivity, was used for its feasibility and comparability to other pediatric studies. Similar results were obtained when fasting insulin was used (data not shown). Finally, dietary information is self-reported and may not reflect actual intake.
In conclusion, we have showed that obesity is related to elevations in BCAA concentrations in children and adolescents, and that these elevations may be independently associated with future insulin resistance, as estimated by HOMA-IR. Increased BCAA catabolic flux might be one consequence of overnutrition apparent early in life that can lead to changes in insulin action. Further studies are needed to determine the mechanisms by which altered branched chain amino acid metabolism is related to insulin sensitivity in overweight children and adolescents and how these findings might illuminate the pathogenesis of type 2 diabetes.
Supplementary Material
What is already known about this subject
Circulating concentrations of branched-chain amino acids (BCAA’s) can affect carbohydrate metabolism in skeletal muscle, and therefore may alter insulin sensitivity.
BCAA’s are elevated in adults with diet-induced obesity, and are associated with their future risk of type 2 diabetes even after accounting for baseline clinical risk factors.
What this study adds
Increased concentrations of BCAA’s are already present in young obese children and their metabolomic profiles are consistent with increased BCAA catabolism.
Elevations in BCAA’s in children are positively associated with insulin resistance measured 18 months later, independent of their initial BMI.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health [K23 DK080658 (A.F.), K24 DK064545 (S.K.G.), 5R01 DK081572-03 (R.E.G. and T.J.W), and R01 DK081457 (V.K.M.)] and a gift from from Nestle Research Center to the Broad Institute. The project was also supported by grant numbers 1 UL1 RR025758-03 and M01-RR-01066, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.) In addition, the study was supported by a Harvard Clinical Nutrition Research Center Pilot/Feasibility Project grant (5P30DK040561-15) (A.F.), a Genentech Clinical Scholars Award from the Lawson Wilkins Endocrinology Society (A.F.), a gift from the Henry T. Zarrow Foundation (A.F.), and the Career Development Award (A.F.) from the Children’s Hospital Boston.
We are indebted to the nursing and bionutrition staff of the MGH and MIT Clinical Research Centers for their excellent care of our research subjects. In addition, we thank the MIT and Harvard Catalyst core laboratories for performing the laboratory assays.
Abbreviations
- (BCAA’s)
Branched-chain amino acids
- (IR)
insulin resistance
- (HOMA-IR)
homeostasis model assessment for insulin resistance
Footnotes
Author Contributions: S.E.M. was involved in conducting the study, performed data analysis, and wrote the manuscript. O.S. performed sample processing and data analysis, contributed to discussion, and reviewed/edited the manuscript. M.A.M. was involved in conducting the study, data analysis, and reviewed/edited the manuscript. A.D. performed sample processing and data analysis. T.J.W. and R.E.G. contributed to discussion and reviewed/edited the manuscript. C.C. and V.K.M. were involved in designing sample analysis techniques, contributed to discussion, and reviewed/edited the manuscript. S.K.G. and A.F. were involved in study design, conducting the study, data analysis, contributed to discussion and manuscript writing, and reviewed/edited the manuscript.
CONFLICTS OF INTEREST STATEMENT The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89(6):2526–39. doi: 10.1210/jc.2004-0276. [DOI] [PubMed] [Google Scholar]
- 3.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315(4):215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–6. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 6.Matthews DE. Observations of branched-chain amino acid administration in humans. J Nutr. 2005;135(6 Suppl):1580S–4S. doi: 10.1093/jn/135.6.1580S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse MG, Biggers JF, Friderici KH, Buse JF. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972;247(24):8085–96. [PubMed] [Google Scholar]
- 8.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101(7):1519–29. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51(3):599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 10.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011 doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaussain JL, Georges P, Olive G, Job JC. Effect of 24 hour fast in obese children. Biomedicine. 1976;25(8):299–302. [PubMed] [Google Scholar]
- 13.Molnar D, Soltesz G. Metabolic and hormonal effects of fasting in obese children. Acta Paediatr Acad Sci Hung. 1982;23(1):45–50. [PubMed] [Google Scholar]
- 14.Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab. 2009;94(12):4923–30. doi: 10.1210/jc.2009-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischman A, Makimura H, Stanley TL, et al. Skeletal muscle phosphocreatine recovery after submaximal exercise in children and young and middle-aged adults. J Clin Endocrinol Metab. 2010;95(9):E69–74. doi: 10.1210/jc.2010-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack SE, McCarthy MA, Farilla L, et al. Skeletal muscle mitochondrial function is associated with longitudinal growth velocity in children and adolescents. J Clin Endocrinol Metab. 2011;96(10):E1612–8. doi: 10.1210/jc.2011-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 20.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 22.Shaham O, Slate NG, Goldberger O, et al. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci U S A. 2010;107(4):1571–5. doi: 10.1073/pnas.0906039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozefsky T, Felig P, Tobin JD, Soeldner JS, Cahill GF., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969;48(12):2273–82. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293(6):E1552–63. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–30. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972;51(9):2346–51. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135(6 Suppl):1547S–52S. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- 30.Di Cairano ES, Davalli AM, Perego L, et al. The Glial Glutamate Transporter 1 (GLT1) Is Expressed by Pancreatic {beta}-Cells and Prevents Glutamate-induced {beta}-Cell Death. J Biol Chem. 2011;286(16):14007–18. doi: 10.1074/jbc.M110.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macotela Y, Emanuelli B, Bang AM, et al. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6(6):e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newgard CB. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012;15(5):606–14. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber HJ, Mayer C, Mangge H, Fauler G, Grandits N, Wilders-Truschnig M. Obesity reduces the bioavailability of nitric oxide in juveniles. Int J Obes (Lond) 2008;32(5):826–31. doi: 10.1038/sj.ijo.0803795. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SR, Witting PK, Stocker R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for alpha-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. J Biol Chem. 1996;271(51):32714–21. doi: 10.1074/jbc.271.51.32714. [DOI] [PubMed] [Google Scholar]
- 35.Baran H, Staniek K, Kepplinger B, Stur J, Draxler M, Nohl H. Kynurenines and the respiratory parameters on rat heart mitochondria. Life Sci. 2003;72(10):1103–15. doi: 10.1016/s0024-3205(02)02365-2. [DOI] [PubMed] [Google Scholar]
- 36.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135(6 Suppl):1539S–46S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 37.Arslanian SA, Kalhan SC. Protein turnover during puberty in normal children. Am J Physiol. 1996;270(1 Pt 1):E79–84. doi: 10.1152/ajpendo.1996.270.1.E79. [DOI] [PubMed] [Google Scholar]
- 38.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab. 1991;72(2):277–82. doi: 10.1210/jcem-72-2-277. [DOI] [PubMed] [Google Scholar]
- 39.Balagopal P, Bayne E, Sager B, Russell L, Patton N, George D. Effect of lifestyle changes on whole-body protein turnover in obese adolescents. Int J Obes Relat Metab Disord. 2003;27(10):1250–7. doi: 10.1038/sj.ijo.0802388. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MD, Haymond MW. Protein metabolism in obesity: effects of body fat distribution and hyperinsulinemia on leucine turnover. Am J Clin Nutr. 1991;53(1):172–6. doi: 10.1093/ajcn/53.1.172. [DOI] [PubMed] [Google Scholar]
- 41.Solini A, Bonora E, Bonadonna R, Castellino P, DeFronzo RA. Protein metabolism in human obesity: relationship with glucose and lipid metabolism and with visceral adipose tissue. J Clin Endocrinol Metab. 1997;82(8):2552–8. doi: 10.1210/jcem.82.8.4182. [DOI] [PubMed] [Google Scholar]
- 42.Kanaley JA, Haymond MW, Jensen MD. Effects of exercise and weight loss on leucine turnover in different types of obesity. Am J Physiol. 1993;264(5 Pt 1):E687–92. doi: 10.1152/ajpendo.1993.264.5.E687. [DOI] [PubMed] [Google Scholar]
- 43.Caballero B, Wurtman RJ. Differential effects of insulin resistance on leucine and glucose kinetics in obesity. Metabolism. 1991;40(1):51–8. doi: 10.1016/0026-0495(91)90192-y. [DOI] [PubMed] [Google Scholar]
- 44.Motil KJ, Bier DM, Matthews DE, Burke JF, Young VR. Whole body leucine and lysine metabolism studied with [1-13C]leucine and [alpha-15N]lysine: response in healthy young men given excess energy intake. Metabolism. 1981;30(8):783–91. doi: 10.1016/0026-0495(81)90024-x. [DOI] [PubMed] [Google Scholar]
- 45.Goulet O, DePotter S, Salas J, Robert JJ, Rongier M, Ben Hariz M, et al. Leucine metabolism at graded amino acid intakes in children receiving parenteral nutrition. Am J Physiol. 1993;265(4 Pt 1):E540–6. doi: 10.1152/ajpendo.1993.265.4.E540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.