Abstract
TLR4 plays a key role in the initiation of innate immunity and in the regulation of adaptive immune responses. Using microarray analysis and PCR, TLR4 expression was observed to increase in murine skin wounds at the early stages. The cellular location of TLR4 was primarily in keratinocytes at the wound edges. Closure of excisional wounds was significantly delayed in TLR4 deficient (C3H/HeJ) as compared to wild type mice, and both IL-1β and IL-6 production were significantly lower in the wounds of TLR4 deficient mice. EGF also markedly decreased in the wound edge of epidermis in TLR4 deficient mice. In vitro studies confirmed that a wound stimulus induces TLR4 mRNA expression in primary normal human epidermal keratinocytes (NHEK). In vitro injury also induced the phosphorylation of p38 and JNK MAPK and the expression of IL-1β and TNF-α by NHEK. Blockade of TLR4 delayed NHEK migration and abolished the phosphorylation of p38 and JNK MAPK, and blockade of TLR4 and/or p38/JNK abolished IL-1β production. The results suggest that inflammatory cytokine production by injured NHEK is stimulated via the TLR4-p38 and JNK MAPK signaling pathway. Together, the results provide evidence for a role of TLR4 at sites of injury, and suggest that TLR4 is an important regulator of wound inflammation.
Keywords: TLR4, MAPK, wound healing, cytokine
Introduction
Skin wound healing is a dynamic pathophysiological process orchestrated by complicated interactions of extracellular matrix molecules, growth factors/cytokines, and various resident cells including keratinocytes, fibroblasts, and infiltrating leukocytes. The innate immune response in the skin serves not only to eliminate infections following injury but also to maintain homeostasis and functional integrity, and may be active in restoring structure to damaged tissues (Frantz et al., 2005; Martin, 1997; Saltzman, 1999).
TLRs have a key role in host defense by regulating both innate and adaptive immune responses (Takeda and Akira, 2005). They recognize multiple pathogen-associated molecular patterns (PAMPs) such as LPS via TLR4, and lipopeptides and lipoproteins via TLR2 (Miller and Modlin, 2007; Takeda and Akira, 2005). Once a TLR is activated by its corresponding ligand, downstream signaling molecules are activated leading to the nuclear translocation of transcription factor NF-kB and/or activation of the mitogen-activated protein kinase (MAPK). The MAPK family includes p38, and Jun N-terminal kinase (JNK), which leads to the transcription of target inflammatory cytokine genes (Akira and Takeda, 2004; Miller and Modlin, 2007; Takeda and Akira, 2005). Ultimately, TLR signaling pathways regulate gene expression profiles including the production of cytokines, upregulation of costimulatory molecules, and adhesion molecules (Akira and Takeda, 2004; Miller and Modlin, 2007; Takeda and Akira, 2005).
Skin keratinocytes have been demonstrated to express TLR1-6 and 9 (Baker et al., 2003; Kollisch et al., 2005; Lebre et al., 2007; Song et al., 2002). Various TLRs have also been identified to play a role in skin diseases such as psoriasis, leprosy, and atopic dermatitis (Miller and Modlin, 2007). Studies also suggest that TLR4 is involved in the response to a variety of injuries. In an incisional wound repair model, TLR4 deficient mice demonstrated a significant decrease in TNF-α in the wound and increased wound breaking strength (Bettinger et al., 1994). In addition, TLR4 deficient mice exposed to burn injury exhibited increased immunosuppression (Jobin et al., 2000). Furthermore, enhanced TLR2 and TLR4 reactivity is important to the production of IL-1β, IL-6 and TNF-α in the spleen following severe burn injury in mice (Maung et al., 2005). While a role for TLR4 in the immune response to burn injury is well-studied, the role of TLR4 in the inflammatory response to excisional wounds has not been well investigated.
In the present study, we investigated changes in the expression of TLR4 and its downstream signaling molecules in response to injury both in vitro and in vivo. The results suggest that TLR4 plays an important role in the early inflammatory response in wound healing and regulates inflammatory cytokine production in injured keratinocytes via the TLR4/p38 and JNK MAPK signaling pathways.
Results
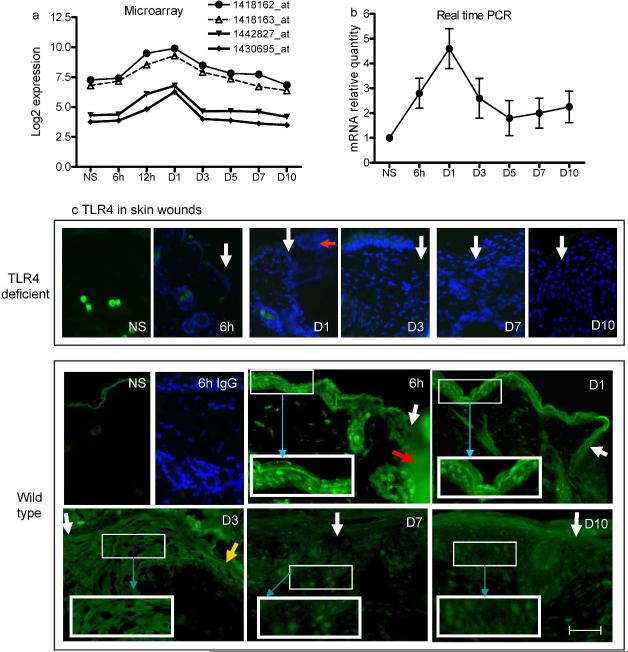
TLR4 is upregulated in the early phase of skin wound healing
To examine if TLR4 expression is modulated by injury, we analyzed data from a previous microarray study (Chen et al., 2010) which delineated the transcriptome of a 1-mm excisional skin wound in BALB/c mice. The data showed that TLR4 gene expression was significantly increased at 12 and 24h pot-wounding, and then gradually returned to baseline by day 10 (Fig.1a). In addition, TLR4 mRNA expression examined by PCR in 3-mm skin wounds of TLR4 wild type mice had a pattern the same as the microarray study (Fig.1b). These results demonstrate that TLR4 gene expression is significantly increased at sites of skin injury. To determine the cellular location of TLR4 expression in the wounds, wound sections of wild type mice were examined using indirect immunofluorescence. As shown in Fig. 1c, normal skin exhibited non-specific staining of the keratinized layer. In wounds, including time points of 6h, 1d and 3d after wounding, TLR4 was clearly observed in all layers of keratinocytes at the wound edge including those at the migrating tip (day 3). In addition, the expression was more evident in the epidermal area slightly distant from the wound edges than the areas immediately next to the edges in both 6h and day 1 wounds. In contrast, very few cells within the wounded dermis stained positively for TLR4. The intensity of TLR4 expression in the keratinocytes at wound edges at days 7 and 10 significantly decreased as the wound healing progressed. As expected, no TLR4 positive staining was seen in skin from TLR4 deficient mice (Fig.1c). The results strongly suggest that TLR4 expression in skin wounds localizes primarily to the epidermal keratinocyte compartment in wild type mice.
Figure 1.
TLR4 gene and protein expression is upregulated in early skin wounds. (a) Microarray analysis shows that all four TLR4 probes in the array demonstrate a significant increase in expression, especially from 12h to 1 day (p<1.2×10-7, one-way ANOVA). (b) Real time PCR confirms that TLR4 mRNA is markedly increased in early stage wounds in TLR4 wild type mice (p=0.0003, one-way ANOVA). N=3 at each time point. (c) Photomicrographs of indirect immunofluorescence detection of TLR4 in skin wounds of TLR4 deficient and wild type mice. The control (rabbit IgG staining) histologic sections of wounds from TLR4 deficient mice, as well as the the 6h wound sample from wild type mice, were also stained with DAPI (blue). White arrows indicate wound edges; red arrow indicates fibrin clot, yellow arrow indicates advancing epidermal tip, and blue arrows show the higher magnification of the outlined areas. Bar=200μm.
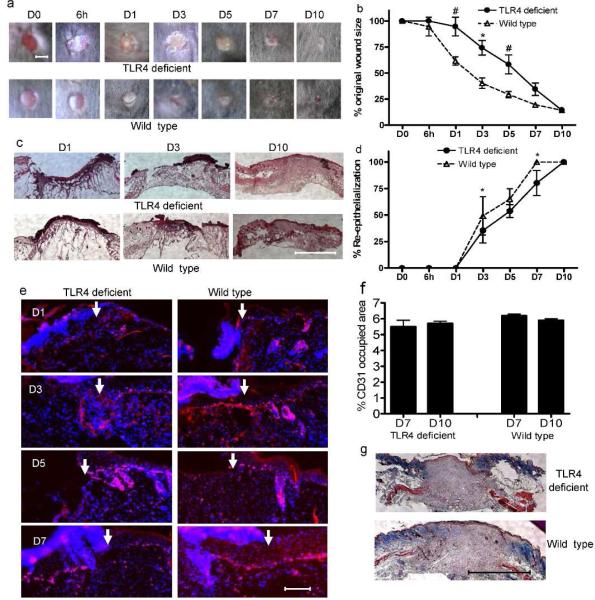
TLR4 deficient mice exhibit impaired early stage skin wound closure
From days 1-5 post-wounding the wound areas of TLR4 deficient mice were significantly larger than wild type (p<0.05, Fig. 2a&b). However, by 10 days post-wounding, the wound sizes of the two groups of mice showed no statistical difference (Fig. 2a&b). Concordant with the external analysis of wound healing, histologic analysis showed that the percent re-epithelialization in TLR4 deficient mice was lower than that of wild type mice with statistically significant differences observed at days 3 and 7 (Fig. 2c&d). Furthermore, less Ki67 positive proliferating keratinocytes were seen at the edges of wounds in TLR4 deficient mice at days 1 and 3 than in wounds of wild type mice (Fig. 2e). These data suggest that TLR4 plays a prominent role in the early stage of wound healing and specifically influences keratinocyte function. Interestingly, neither capillary density (Fig. 2f) nor collagen staining (Fig. 2g) showed any significant difference between these two groups of mice.
Figure 2.
Mice with a TLR4 mutation exhibit impaired early wound healing without vascularity changes. (a) Representative photomicrographs of wounds at 0 and 6h as well as 1, 3, 5, 7 and 10 days after injury. Bar=3mm. (b) Percent of original wound size, N=5, * p<0.01, # p<0.05 VS wild type. (c) Photomicrographs of HE stained histologic sections of wounds. Bar=500μm. (d) Rate of wound re-epithelialization measured by histomorphometric analysis of tissue sections. N=5, * p<0.01 VS wild type. (e) Photomicrograpahs of ki67+ (red) proliferating keratinocytes with nuclear DAPI counterstaining (blue). Arrows indicate wound edges. Bar=200μm. (f) Percent area occupied by CD31 stained vessels at day 7 and 10 after wounding. N=5 at each time point. (g) Photomicrographs of Masson's trichrome stained histologic sections of day 10 wounds. Bar=500μm.
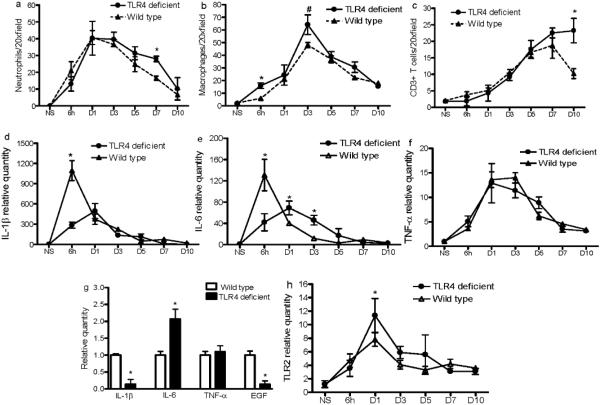
TLR4 mutation alters inflammatory cell infiltration and cytokine expression in skin wounds
At day 7 post-wounding, the number of neutrophils was significantly higher in wounds of TLR4 deficient mice versus those of wild type mice (p<0.01, Fig. 3a). Although a slight increase in neutrophils was observed in the wounds of the deficient mice at multiple other time points, the difference did not reach significance (p>0.05, Fig. 3a). Macrophage content was significantly higher in wounds of deficient mice than in wild type mice at 6h and 3 days post-wounding (p<0.01 and <0.05 respectively, Fig. 3b). The pattern of T cell infiltration also differed in deficient and wild type mice. Whereas the number of CD3+ T cells in the wounds of wild type mice peaked at day 7 and started to decline at day 10, the number of CD3+ cells continued to increase from day 7 to day 10 post-wounding in the deficient mice. By day 10, the wounds of deficient mice contained significantly more T cells than wild type mice (p<0.01, Fig. 3c). Taken together, the results suggest that the loss of a functional TLR4 resulted in increased inflammatory cell content in wounds at discrete time points during the healing process.
Figure 3.
Wounds of TLR4 deficient and wild type mice show differences in inflammatory cell and cytokine content. (a, b, and c) The number of neutrophils, macrophages, and CD3+ T cells, respectively, in the wounds, determined by immunohistochemistry. (d, e, f, and h) mRNA levels of IL1β, IL-6, TNF-α and TLR2 in wounds, as determined by real time PCR. * p<0.01, # P<0.05, N=5 at each time point. Expression in the normal skin of wild type mice was used as baseline. (g) mRNA levels of IL1β, IL-6, TNF-α and EGF in epithelial cells at the 6h wound edges determined by LCM and RT-PCR. Data are averages of triplicate wells, and are representative of two independent experiments. * p<0.01 VS wild type.
Changes in the expression of several proinflammatory cytokines were also observed in the TLR4 deficient mice. IL-1β expression levels were significantly reduced in the wounds of deficient versus wild type mice 6h post-wounding (Fig 3d). The expression of IL-6 in the wounds of deficient mice exhibited both decreased levels at 6h after wounding as well as a shift in the time of peak expression from 6h to day 1 compared to wild type mice (Fig 3e). A significantly higher level of expression of IL-6 in the wounds of TLR4 deficient mice persisted from day 1 to day 3 (Fig 3e). No significant changes were observed between these two groups of mice for TNF-α expression (Fig. 3f). These results suggest that engagement of TLR4 is involved in the modulation of the inflammatory cytokines IL-1β and IL-6 in early skin wounds.
Using laser capture microdissection (LCM) and RT-PCR, we further examined the mRNA expression of these cytokines specifically in epithelial cells isolated from the edge of 6h wound. Compared to wild type mice, IL-1β was downregulated 7 fold (p<0.01) while IL-6 was upregulated 2 fold in TLR4 deficient mice (p<0.01) (Fig. 3g). Since IL-6 expression in the total wound tissue was less in TLR4 deficient than wild type mice, the data from isolated epithelium suggests that TLR4 associated expression patterns of IL-6 within the wound bed itself differ from that of isolated wound edge epithelium. Interestingly the LCM analysis also showed a 7-fold decrease in EGF expression in the wound edge epithelium from TLR4 deficient mice (Fig. 3g) as compared to control (p<0.01). No difference was observed in TNF-α levels (Fig. 3g). Interestingly, TLR2 expression was significantly higher in wounds of TLR4 deficient mice than wild type mice at day 1 (Fig. 3h), suggesting TLR4 deficiency results in compensatory expression of TLR2 post-wounding.
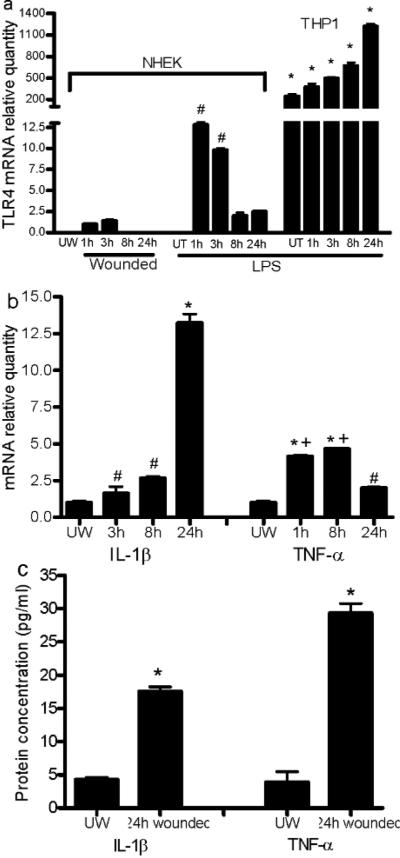
Injury induces keratinocyte expression of IL-1β and TNF-α through activation of TLR4 via p38/JNK MAP kinase
TLR4 mRNA was not detectable in NHEK under standard conditions. However, after in vitro scratch injury, NHEK expressed detectable and significant TLR4 mRNA from 1 to 3h post-wounding (Fig. 4a). The level of TLR4 induced by wounding, while demonstrable, was significantly less than levels induced by LPS treatment, a known robust stimulator of TLR4 in keratinocytes (P<0.01). NHEK showed a temporal response to in vitro injury with TLR4 mRNA levels returning to undetectable by 8h post-wounding (Fig. 4a). To evaluate the levels of TLR 4 expression in NHEK, these results were compared to TLR4 expression in LPS treated or untreated THP1, a cell type known to exhibit high levels of TLR4. Although TLR4 expression in NHEK was several fold lower than THP1 (Fig 4a, p<0.0001), the relative increase upon LPS stimulation was much greater in NHEK as compared to THP1 cells. This result suggests that TLR4 expression by wound keratinocytes may include responses to stimuli beyond bacterial molecular patterns. mRNA expression of IL-1β and TNF-α was dramatically elevated at 3, 8 and 24h post-wounding (p<0.05-0.01 VS unwounded cells) (Fig. 4b). In vitro wounding also led to substantial production of IL-1β and TNF-α protein by injured keratinocytes (Fig. 4c).
Figure 4.
Scratch wound injury induces mRNA expression of TLR4 and production of inflammatory cytokines in NHEK cells. (a) LPS and injury-induced expression of TLR4 mRNA. # p<0.01 VS 1 and 3h expression in injured cells, and 8 and 24h LPS treated cells. THP1 treated with or without LPS were used as positive control. * p<0.0001 VS wounded and LPS treated NHEK. (b) Increased IL-1β and TNF-α mRNA expression in NHEK from 3 to 24h after wounding. # p<0.05 VS unwounded (IL-1β), * p<0.01 VS unwounded, 3h, and 8h (IL-1β), * p<0.01 and # p<0.05 VS unwounded (TNF-α), + p<0.01 VS 1h and 8h. Data in a&b represent the average of triplicate wells and are representative of two independent experiments. (c) Protein levels of IL-1β and TNF-α in the culture media 24h after injury. N=3, *p<0.01 VS unwounded cells. UW, unwounded; UT, LPS untreated.
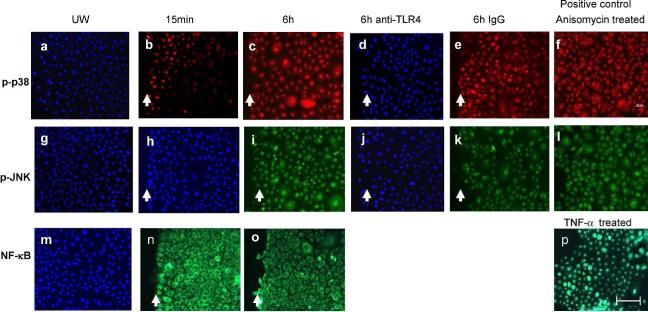
To investigate the role of TLR4 in the production of IL-1β and TNF-α following injury, the activation of the TLR4 signaling pathway was examined. Nuclear translocation of NF-κB was not observed at either 15 min or 6h post-injury (Fig. 5n&o). However, NHEK at the wound edge exhibited phosphorylated p38 (p-p38) (Fig. 5b), but not p-JNK (Fig. 5h) at 15 min post-injury. By six hours post-wounding, a significant increase in p-p38 and p-JNK was observed (Fig. 5c&i). Levels of p-p38 and p-JNK diminished to undetectable by 24h (data not shown). Unwounded cells did not show any positive staining (Fig. 5a&g). A direct link between TLR4 and p38/JNK phosphorylation was also shown, as antibody neutralization of TLR4 completely inhibited the phosphorylation of p38 and JNK (Fig. 5d&j) while non-specific IgG did not have such an effect (Fig. 5e&k). NHEK also displayed p38 phosphorylation in response to LPS treatment. Following LPS treatment, p-p38 was observed by 15-min and and reached peak levels at 30-min. Levels of p-p38 decreased dramatically with longer treatment times (1-24h) (Data not shown). LPS treatment did not result in phosphorylation of JNK or nuclear translocation of NF-kB (data not shown).
Figure 5.
Scratch wound injury induces expression of p-p38 and p-JNK MAPK, but not NF-κB nuclear translocation in NHEK. Nuclear NF-κB, p-p38, and p-JNK were detected using immunofluorescence 15 min and 6h after wounding. To test the effects of anti-TLR4 antibody on induction of p-p38 (red) and p-JNK (green), NHEK were treated with anti-TLR4 or mouse IgG prior to scratch injury. (a, g, and m) Unwounded NHEK; (b, h, and n) 15 min after injury; (c, d, e, i, j, and k) 6h after injury; (p) TNF-α treated NHEK as NF-κB positive control; (f and l) Anisomycin treated NHEK as positive control for p-p38 and p-JNK; (a, d, g, h, j, and m) were also stained by DAPI (blue). UW: unwounded. Arrows indicate wound edges. Bar=100μm.
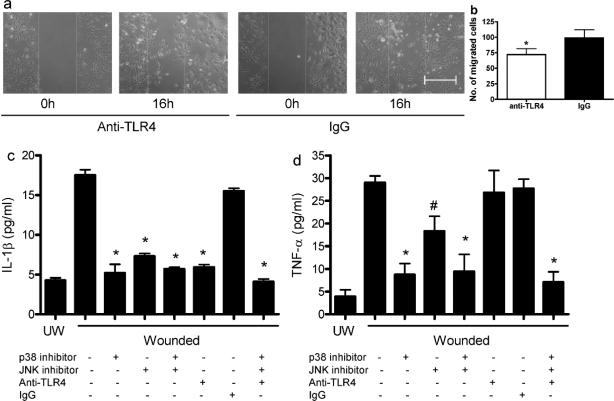
The importance of TLR4 for keratinocyte migration and in the production of IL-1β and TNF-α following in vitro injury was explored using an antibody blockade strategy. When compared with control, treatment with neutralizing anti-TLR4 antibody before injury resulted in a significant decrease in the number of migrating cells (Fig. 6 a&b, p<0.05). Blockade of TLR4 by anti-TLR4 antibody also abolished IL-1β production (Fig. 6c), suggesting that TLR4 activation is requisite for IL-1β expression. Inhibition of p38 or JNK inhibitor also resulted in a significant decrease in IL-1β production (Fig. 6c). Use of both p38 and JNK inhibitors, or anti-TLR4, p38 and JNK inhibitors combined resulted in IL-1β at levels similar to p38 inhibition alone (Fig 6c). Overall, the induction of IL-1β production appeared to occur through the TLR4 pathway via phosphorylation of p38 and JNK, but not via NF-κB. In contrast to IL-β, anti-TLR4 treatment did not affect TNF-α production following in vitro injury (Fig. 6d). However, both p38 and JNK inhibitors caused a significant reduction in TNF-α (p<0.01 and <0.05 respectively), with the effect of the p38 inhibitor being considerably greater (p<0.05) (Fig. 6d). The results suggest that for the induction of TNF-α, molecules other than TLR4 lie upstream of p38 and JNK.
Figure 6.
Neutralization of TLR4 delays NHEK migration and abolishes IL-1β production in injured NHEK. (a and b) NHEK monolayers were pretreated with anti-TLR4 or normal mouse IgG (20μg/ml) followed by mitomycin C (10μg/ml) treatment, and then wounded by scratches. The defined areas were photographed at 0 and 16h after wounding. The numbers of cells migrated out of the initial areas were counted. N=4. * p<0.05 compared to IgG control. (c and d) Cultured NHEK were incubated with anti-TLR4 and/or p38 inhibitor SB203580, and/or JNK inhibitor SP600125 and then cells were injured by scratching. The supernatants were collected 24h later for IL-1β and TNF-α ELISA analyses. N=3,*p<0.01, # p<0.05 compared to wounded untreated cells. UW, unwounded. Bar=100μm.
Discussion
The current investigation demonstrates that TLR4 gene expression is modulated over the time course of healing. A functional role for TLR4 in wound healing was shown as wound closure was significantly impaired in TLR4 deficient mice. TLR4 deficient mice also exhibited changes in the levels of IL-1β, IL-6, and EGF as well as altered infiltration of neutrophils, macrophages, and T cells in the wounds. Together with the current evidence that TLR4 expression localizes primarily to keratinocytes at the wound edge rather than cells in the dermis, our results suggest that TLR4 engagement by keratinocytes is critical to the inflammatory response in skin wounds and that TLR4 positive keratinocytes play a significant role in the healing response.
Keratinocytes provide an important physical barrier between the external and internal environments. The production of cytokines by keratinocytes influences keratinocyte proliferation and differentiation processes, enhances the recruitment of inflammatory cells, and may have systemic effects on the immune system (Grone, 2002; Uchi et al., 2000). Keratinocytes at the edge of the wound of TLR4 deficient mice exhibited a noticeably lower level of EGF, a growth factor which can substantially affect proliferation and migration of keratinocytes (Chen et al., 1993; Dvir et al., 1991; Rheinwald and Green, 1977). This result may provide an explanation for the reduction in proliferating keratinocytes in the wounds of TLR4 deficient mice. A decrease in epithelial proliferation in TLR4 deficient mice is consistent with the findings that TLR4 deficient mice had larger wounds and delayed re-epithelialization compared to wild type mice.
The current studies describe a particular signaling pathway in keratinocytes by which TLR4 promotes the initial inflammatory response to injury in vitro. Our studies suggest that the expression and activation of TLR4 in injured keratinocytes leads to phosphorylation of p38 and JNK, followed by the production of IL-1β. Our studies demonstrate that the production of IL-1β by injured keratinocytes occurs primarily through the TLR4-p38/JNK pathway rather than the TLR4-NF-κB pathway. Interestingly, the activation of TLR4 did not appear to be linked to injury-induced TNF-α production, suggesting that other pathways induce TNF-α production by keratinocytes following wounding. One receptor complex which might also play a role in the post-injury response is the inflammasome, a protein complex whose major components consist of NLRs [NACHT (domain present in neuronal apoptosis inhibitory protein/NAIP, CIITA, HET-E and TP-1)-LRRs (leucine rich receptor)]. Activation of the inflammsome, which is a recently identified complex of intracellular pathogen-recognition receptors (Becker and O'Neill, 2007; Martinon and Tschopp, 2005) results in activation of caspase-1, which cleaves pro-IL to produce mature IL-1β (Becker and O'Neill, 2007; Martinon and Tschopp, 2005). Interactions between TLRs and NLRs have also been reported (Becker and O'Neill, 2007; Martinon and Tschopp, 2005). Since our study suggests that injured keratinocytes produce IL-1β through TLR4 activation, and the inflammasome has previously been detected in keratinocytes (Watanabe et al., 2007), it seems likely that the inflammasome is also involved in responses described here. A role for the inflammasome in inflammation associated with injury has been proposed (McDonald et al.), and this topic will require additional investigation.
TLRs not only recognize PAMPs, they also detect endogenous danger signals, or damage associated molecular patterns (DAMPs), that are released by stressed and injured tissues or cells. Numerous recent reports have demonstrated that DAMPs, such as hyaluronanic acid (Taylor et al., 2004; Termeer et al., 2002), heparan sulfate (Johnson et al., 2002), fibrinogen (Smiley et al., 2001), surfactant protein-A (Okamura et al., 2001), high-mobility group box 1 (Park et al., 2006; Park et al., 2004; Wang et al., 1999), β-defensin (Biragyn et al., 2002) and heat shock proteins (Asea et al., 2002; Dybdahl et al., 2002; Ohashi et al., 2000; Vabulas et al., 2002), interact with TLR4 and induce the production of inflammatory cytokines. While we have not yet investigated the specific ligands that interact with TLR4 in the context of the wound, the identification of these endogenous ligands from stressed or injured tissues by TLR4 may have important pathophysiological implications in wound healing. The current study would suggest that following injury, local mechanical damage leads to a rapid and significant increase in TLR4 expression. One important caveat is that inflammatory patterns in wounds are complex, and most certainly modulated by many ligands beyond TLR4. Such complexity may explain the observation of decreased levels of IL-1β and IL-6 in the early wounds of TLR4 deficient mice yet increased inflammatory cell infiltration at discrete time points. One possible explanation relates to alternative activation of other TLRs such as TLR2 when TLR4 does not function. Indeed, TLR2 expression was significantly increased post-wounding in TLR4 deficient mice. Complicated patterns of cytokine expression also appear to be involved. More specifically, IL-6 expression in the wounds of TLR4 deficient mice exhibited a pattern that included an early decrease but sustained levels above that of wild type at several time points beyond day 1. This sustained level of IL-6 may contribute to altered healing outcomes, as prior studies suggest that IL-6 is important to normal wound closure yet may promote scar formation (Dasu et al., 2004; Gallucci et al., 2000; Ghazizadeh et al., 2007). Overall, the changes in IL-6 expression seen in the wounds of TLR4 deficient mice might impact healing in many ways.
Our findings show that the wounds of TLR4 deficient mice heal more slowly, and that in the absence of functional TLR4, the number of inflammatory cells in the wound was slightly increased. While the increase was subtle, this finding is in keeping with the general notion that excessive cellular inflammation can be detrimental to repair (Martin and Leibovich, 2005). Indeed, some wounds that heal extremely well, including fetal skin and mucosa, exhibit little or no inflammation (Longaker et al., 1990). However, in adult skin the situation is complex and there appears to be a fine line between “good” inflammation and “bad” inflammation as it pertains to repair. TLR4 may play a role in the innate immune system of skin which subtly harnesses inflammation so that wounds heal favorably with resistance to infection, and with appropriate restoration of structure.
Pertinent to the current study is the finding that incisional wounds of TLR4 deficient mice show a significant increase in wound breaking strength (Bettinger et al., 1994). A recent study also suggests that TLR4 may play an role in the fibrotic response in skin wounds (Wang et al., 2011). Although we did not see significant changes in collagen content in TLR4 deficient mice, we did not quantify the wound collagen architecture for characteristics that are important to wound strength or that signify wound fibrosis. Thus the question of whether TLR4 signaling influences the fibrotic response in skin wounds remains open.
In summary, our results demonstrate that 1) TLR4 is upregulated and activated in early skin wound healing; 2) a functional mutation of TLR4 results in altered inflammatory cell infiltration, differential cytokine production, and impaired wound closure; and 3) IL-1β production by injured keratinocytes is induced through the TLR4-p38/JNK pathway. The results provide previously unreported evidence that TLR4 is a critical regulator in wound inflammation, and that this receptor supports the creation of an optimal early inflammatory environment in healing skin.
Materials and Methods
Wound model
Six-8 weeks old C3H/Hej TLR4 deficient and wild type C3H/HeOuj mice (Jackson Laboratory, Bar Harbor, ME) were used in the study. C3H/Hej mice have a mutation in the third exon of the TLR4 gene. A C to A substitution at nucleotide position 2342 results in an amino acid substitution that replaces proline with histidine at position 712; this mutation renders the molecule non-functional (Hoshino et al., 1999; Poltorak et al., 1998). Six full-thickness dermal excisional wounds were placed on opposite sides of the midline using a 3-mm punch biopsy instrument. Wounds and surrounding tissues were harvested using 5-mm and 3-mm biopsy punches for samples at 6h and day 1 and samples at days 3, 5, 7 and 10 respectively. The wounds were also photographed at the time points indicated above and the wound sizes were determined by software AxioVision (ZEISS, Oberkochen, Germany). The microarray analysis was performed as previously described (Chen et al., 2010). The procedures for making and harvesting wounds were the same as 3-mm wound model described above. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Examination of re-epithelialization and collagen
Hematoxylin/eosin (HE) and Masson's Trichrome staining were performed according to the manufacturer's instructions (Sigma, St Louis, MO). The extent of re-epithelialization was measured by histomorphometric analysis of the HE stained wounds as described previously (Schrementi et al., 2008).
Cell culture and in vitro wound injury
NHEK (ATCC, Manassas, VA) were cultured in 2 chamber slides to 80% confluence. Injury was produced by the application of 9 horizontal and 9 vertical scratch wounds using 1ml pipette tips. In some experiments, cells were treated with mouse anti-human TLR4 or mouse IgG (eBioscience, San Diego, CA) for 90 min before injury. NHEK and THP1 (Human acute monocytic leukemia cell line, 1×105, ATCC) treated with LPS (100ng/ml, Sigma) for indicated times were used as positive controls for TLR4 expression after injury. At 1, 3, 8, and 24h after injury or LPS treatment, total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). At 15 min and 6h after injury, samples were examined for the nuclear translocation of NF-kB and p-p38/p-JNK using immunofluorescence. Anisomycin (25μg/ml, Sigma), a kinase activator, was used to treat NHEK for 30 min, which served as a positive control to detect p-p38/JNK. To examine the role of TLR4 and its signaling pathway in the production of IL1β and TNF-α, cells were incubated with anti-TLR4 antibody (20μg/ml) and/or p38 inhibitor SB203580 (5μM), and/or JNK inhibitor SP600125 (0.15μM) (both from LC Laboratories, Woburn, MA) for 90 min prior to injury. 24h after injury, supernatants were collected for detection of protein levels of cytokines by ELISA. All in vitro cell culture experiments were performed in the presence of 10μg/ml polymyxin B (Sigma) to exclude the effects of any LPS contamination (Horton et al., 1998; Jiang et al., 2005). For in vitro migration assay, confluent NHEK were treated with mitomycin C (10μg/ml, Sigma) for 2h to prevent cell proliferation and then incubated with anti-TLR4 or IgG (20μg/ml) for 90 min followed by scratch wounding. The defined areas were photographed at 0 and 16h after wounding and the number of cells that had migrated out of the initial wound area were counted.
Real-time PCR, laser capture microdissection, cytokine ELISA, detection of TLR4, CD3+ T cells, neutrophils, macrophages, proliferating cells (Ki67), NF-κB, p-p38, and p-JNK by immunohistochemistry
See details in Supplementary Materials and Methods.
Statistical analyses
Data are expressed as mean+SD. The Student t test was used to compare the difference between TLR4 deficient and wild type mice at each time point. One –way ANOVA test was used to identify significance in some experiments. p values less than 0.05 were considered significant.
Supplementary Material
Acknowledgement
This publication was supported by NIH Grants RO1-GM50875, P20-GM078426 (LAD), and S10RR026493 (Xiaofeng Zhou). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Abbreviations
- JNK
Jun N-terminal kinase
- LCM
laser capture microdissection
- MAPK
Mitogen-activated protein kinase
- NHEK
normal human epidermal keratinocytes
- PAMP
pathogen-associated molecular patterns
- TLR
Toll-like receptor
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- Becker CE, O'Neill LA. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin Immunopathol. 2007;29:239–48. doi: 10.1007/s00281-007-0081-4. [DOI] [PubMed] [Google Scholar]
- Bettinger DA, Pellicane JV, Tarry WC, Yager DR, Diegelmann RF, Lee R, et al. The role of inflammatory cytokines in wound healing: accelerated healing in endotoxin-resistant mice. J Trauma. 1994;36:810–3. doi: 10.1097/00005373-199406000-00010. discussion 3-4. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Chen JD, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer RH, et al. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993;209:216–23. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics. 2010;11:471. doi: 10.1186/1471-2164-11-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol. 2004;202:476–85. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- Dvir A, Milner Y, Chomsky O, Gilon C, Gazit A, Levitzki A. The inhibition of EGF-dependent proliferation of keratinocytes by tyrphostin tyrosine kinase blockers. J Cell Biol. 1991;113:857–65. doi: 10.1083/jcb.113.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–90. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Frantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res. 2005;96:15–26. doi: 10.1161/01.RES.0000153188.68898.ac. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. Faseb J. 2000;14:2525–31. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh M, Tosa M, Shimizu H, Hyakusoku H, Kawanami O. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi: 10.1038/sj.jid.5700564. [DOI] [PubMed] [Google Scholar]
- Grone A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol. 1998;160:3023–30. [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jobin N, Garrel DR, Bernier J. Increased burn-induced immunosuppression in lipopolysaccharide-resistant mice. Cell Immunol. 2000;200:65–75. doi: 10.1006/cimm.2000.1619. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–9. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–41. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–41. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–8. doi: 10.1016/s0022-3468(05)80165-4. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–73. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 330:362–6. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–4. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Saltzman WM. Delivering tissue regeneration. Nat Biotechnol. 1999;17:534–5. doi: 10.1038/9835. [DOI] [PubMed] [Google Scholar]
- Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16:80–6. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119:424–32. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(Suppl 1):S29–38. doi: 10.1016/s0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–12. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol. 2011;226:1265–73. doi: 10.1002/jcp.22454. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–63. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.