Abstract
Purpose
Studies of seizure outcome in patients undergoing serial antiepileptic drug trials have all been uncontrolled, with no account made for the spontaneous changes in disease state that could confound the elucidation of drug effects. In addition, no study has ever looked at outcome following antiepileptic drug switch in seizure-free patients, despite the fact that this is done routinely in clinical practice. We aimed to address both of these issues using a matched case-cohort design.
Methods
We followed patients taking phenytoin or carbamazepine in monotherapy for focal epilepsy who were being crossed over to a newer agent as part of studies on the metabolic effects of anticonvulsant therapy. Many had been seizure-free but were being switched nonetheless due to side effects or concerns about long-term adverse consequences. Each patient was matched with two controls of the same seizure status who were on anticonvulsant monotherapy and whose drug was not switched. Seizure freedom over the ensuing 6 months was the primary endpoint.
Key Findings
There were 43 cases and 86 matched controls. Twenty-three case patients had been seizure-free on their old drug; 5 (21.7%) had seizure recurrence after drug switch compared to 2/46 matched controls (4.3%). Twenty case patients were having seizures on their old drug; 6 (30%) entered remission after drug switch, compared to 8/40 matched controls (20%). The two groups differed at baseline in number of anticonvulsants previously failed, which was the most important factor for prognosis. After statistical adjustment to account for this, seizure-free patients had 6.53 times higher odds of seizure recurrence if switched to a new drug (95% CI 1.02 – 61.19; p=0.06). Non-seizure-free patients had 1.66 times higher odds of remission if they remained on the same drug compared to switching, though this was not significant (95% CI 0.36 – 8.42; p=0.532). Neither dose changes, nor drug mechanism, nor duration of seizure-freedom had any bearing upon the results.
Significance
While the large majority of seizure-free patients remain so when switched to another agent, about one-sixth have a recurrence attributable to the change. Conversely, our study design provides the first evidence to suggest that most improvements in drug-resistant patients are likely due to spontaneous remissions, not new drug introductions. These findings have conflicting implications for two competing models of comparative antiepileptic drug efficacy, which will require further study to elaborate.
Keywords: antiepileptic drugs, seizure recurrence, seizure remission, crossover
INTRODUCTION
Previous studies have examined the outcome of drug treatment of focal epilepsy both in new-onset patients and in patients who are resistant to medical therapy. In the former group, outcomes are overall quite similar across different drugs(Dam et al., 1989; Brodie et al., 1995; Privitera et al., 2003; Brodie et al., 2007), with few well-designed studies showing significant differences in outcome(Marson et al., 2007; Kwan et al., 2011). Furthermore, those who fail even one drug due to poor efficacy are unlikely to attain complete seizure control with future drug trials(Kwan and Brodie, 2000; Schiller and Najjar, 2008). These findings imply that outcome is likely to be the same for the majority of patients regardless of which agent is chosen.
One would therefore expect that patients who are seizure-free on a given antiepileptic drug (AED) are likely to remain seizure-free if switched to a different drug. Such alterations are performed regularly in practice for a variety of reasons, including side effects, pregnancy planning, or concerns about long-term consequences of therapy. Yet to our knowledge no study has addressed this issue despite its fundamental importance to routine epilepsy care.
At the other end of the spectrum, only a modest proportion of patients with drug-resistant epilepsy gain seizure control after multiple therapeutic failures(Kwan and Brodie, 2000; Schiller and Najjar, 2008). The most important prognostic factor appears to be the number of drugs previously tried, though studies show some chance of seizure-freedom remains even after failure of many drugs(Callaghan et al., 2007; Luciano and Shorvon, 2007). Yet all of these investigations have been uncontrolled, and are therefore unable to account for the spontaneous changes in disease state that are characteristic of epilepsy(Berg et al., 2003).
We examined the impact of changing antiepileptic agents, but unlike previous studies, we matched these cases with patients who remained on the same AED. Our chief aim was to elucidate the risks and benefits of changing therapy in seizure-free patients whose current AED is nonetheless not ideal. A second aim was to determine the extent to which improvements in seizure outcome among treatment-resistant patients can truly be ascribed to changes in therapy, or whether these instead reflect spontaneous disease fluctuations.
METHODS
This was a mixed propsective-retrospective study which involved 129 subjects: 43 case patients and 86 matched controls. The case subjects had been recruited for two other studies at the Jefferson Comprehensive Epilepsy Center to assess the effects of AEDs on serum lipids and other metabolic measures (Mintzer et al., 2009, 2012). These patients had been taking CBZ or PHT in monotherapy when their physician had decided to switch them over to monotherapy with LTG, LEV, or TPM. The decision to switch to a new drug and the choice of new AED were made purely on clinical grounds. Patients were switched due to side effects, inadequate seizure control, or concerns about the chronic effects of their existing AED. Patients were titrated onto a therapeutic dose of their new agent (≥200mg/day of LTG, ≥100mg/day of TPM, ≥1000mg/day of LEV) before being fully titrated off their old drug, and the precise time frame for this was determined by the patient’s treating physician.
Of these subjects, we included patients who had a diagnosis of focal epilepsy and who had enough data to establish a seizure outcome for six months prior to and six months following the switch in drug therapy. We defined an “established” outcome as either a) having had no seizures for 6 months on monotherapy, which classified the patient as seizure-free; or b) having had a seizure on a therapeutic dose of their AED during the 6 month period, which classified the patient as not seizure-free. Seizure outcome data was determined by retrospective review of physician notes, and, when necessary, direct discussion with the treating physician. Isolated auras were not considered in this assessment. The subjects who met the qualifications were considered case patients and the date their AED switchover began was considered their index date. The most common reasons potential cases did not qualify for inclusion into this study were insufficient duration of follow up after the drug switch and insufficient duration of drug trial in monotherapy before the drug switch (e.g. patient had been on that drug in monotherapy for only 3 months).
Two control patients were matched to each case patient using a sequential retrospective chart review of patients seen at our center within one month of the index date for that case. The first two patients reviewed who meet the matching criteria for each case were assigned as the controls for that case. For each patient, controls had to meet the following criteria: a) diagnosis of focal epilepsy; b) a visit at our center within one month of the index date of the case patient (with that visit date becoming the index date for that control subject); c) the same established seizure outcome as the case patient prior to the index date; d) taking a single AED; e) remaining on that same AED in monotherapy after the index date; and f) having an established 6 month seizure outcome (as defined previously) after the index date. Note that controls were matched to cases by seizure outcome before index date only -- no consideration was given to what the seizure outcome after index date was, only that an adequate determination of seizure outcome could be made. AED dose changes at the index date were permissible, as it would have been prohibitively difficult to find enough refractory controls who had remained on the same drug at the same dose. In addition, case patients who had achieved seizure-freedom after epilepsy surgery were matched to controls who had also attained seizure freedom surgically.
Seizure freedom 6 months after index date was then tabulated as the primary outcome measure for the study. As a secondary outcome measure, we determined 1 year seizure-free rates for the cases and controls whose monotherapy outcome could be established for both one year before and one year after the index date. We planned to exclude seizures occurring during the titration period of the new drug, but no patients experienced seizure recurrence during titration.
Data collected included age, gender, age at onset of epilepsy, duration of seizure freedom (when appropriate), seizure frequency (when appropriate), drug name and dose, duration of therapy, and number of previous drugs tried. Data regarding the number of AEDs previous failures was unavailable for 6 cases and 13 controls.
A logistic regression model was used to analyze seizure freedom and the effects of other variables on seizure freedom. The covariates considered were seizure status before index date (seizure free vs. refractory), cohort type (case vs. control), number of AEDs failed (as ordinal variable), duration of AED trial (log transformed), number of days seizure-free before index date (log transformed, for seizure-free patients only) and gender. The final parsimonious model, selected in stepwise fashion using Akaike Information Criterion(Venables and Ripley, 2002) included seizure status before index date, cohort type, and number of AEDs failed. An interaction term between number of AEDs failed and seizure status before index date was also included because a two-sample Wilcoxon test showed there was a significant association between the two variables (p = 0.002). An interaction term between cohort type and seizure status before index date was included as well because of the role of both variables in the study’s design.
RESULTS
The baseline data for all 129 subjects appear in Table 1. Cases and controls were comparable in age, age of onset, epilepsy duration, months seizure-free (for the seizure-free patients) and seizure frequency (for the non-seizure-free patients). There were significant differences between the groups in gender, duration of index AED trial (i.e. how long they had been taking their AED as of the index date), and total number of AEDs failed. Case patients had been on their present agent for a significantly longer time as of the index date than had the controls. This also held true within both refractory and seizure-free groups when the baseline data were separated (data not shown). Control patients had failed more AEDs than their case counterparts, and this too held true separately within refractory and seizure-free groups (data not shown; p<0.01 for each).
Table 1.
Baseline data for study subjects.
| Cases | Controls | p-value | |
|---|---|---|---|
| Number of Patients | 43 | 86 | --- |
| Gender | 58% M | 36% M | 0.0235 |
| Age at Index Date (years) | 38.8±12.8 | 42.8±14.6 | 0.1353 |
| Age at Seizure Onset (years) | 22.7±15.0 | 26.0±17.0 | 0.3360 |
| Duration of Epilepsy (years) | 16.5 (±13.1) | 16.9 (±14.6) | 0.9416 |
| Duration of current AED (months) | 68±78 | 33±39 | 0.0006 |
| Total # of AEDs Failed (n= 37 cases, 73 controls) | 1.7 (±1.5) | 3.5 (±2.5) | <0.0001 |
| Months Seizure-free (seizure-free pts. only) | 57 (8 to 300) (±72) (n=23) | 53 (6 to 321) (±64) (n=46) | 0.9106 |
| Seizures per Month (non-seizure-free pts. only) | 2.6 (0.2 to 12) (n=20) | 5.1 (0.9 to 90) (n=40) | 0.5458 |
| Carbamazepine | 56% | 16% | |
| Phenytoin | 44% | 9% | |
| Lamotrigine | 0% | 24% | |
| Levetiracetam | 0% | 19% | |
| Topiramate | 0% | 8% | |
| Oxcarbazepine | 0% | 11% | |
| Others | 0% | 13% |
The design of the study set up anticipated differences in AEDs used, with case patients all being initially treated with either PHT or CBZ, while control patients were being treated with a wide variety of agents. After drug switch, 38% of cases were taking LTG, 33% were taking LEV, and 26% were taking TPM. One patient developed a rash shortly after starting LTG and was then switched to oxcarbazepine; this patient’s seizure outcome on the latter drug was included.
Seizure outcome data are shown in Table 2. Among cases who were seizure free at time of AED switch, 5/23 (21.7%) had at least one recurrent seizure within six months of changing to the new drug, while 2/46 (4.3%) of their matched controls who remained on the same drug experienced spontaneous seizure recurrence within six months. Of note, there were 7 case patients who had been seizure-free for over 6 months on the first drug on which they had been tried; 2 of these 7 (29%) had a recurrent seizure after switching to another drug. Among those who were not seizure-free at the index date, 6/20 case patients (30%) attained 6-month seizure remission after drug switch, while 8/40 control patients (20%) experienced a spontaneous remission without a change in drug.
Table 2.
Seizure outcomes at 6 months after index date.
| Status at index date | Group | N | Seizure-free after index date | Recurrent seizure after index date |
|---|---|---|---|---|
| Seizure-Free | Cases | 23 | 78.3% | 21.7% |
| Controls | 46 | 95.7% | 4.3% | |
| Not Seizure-Free | Cases | 20 | 30% | 70% |
| Controls | 40 | 20% | 80% |
Table 3 shows odds ratios adjusted for covariates. Compared to patients who were not seizure free before index, patients who were seizure free before index had 3.34 times higher odds of being seizure free after index (odds ratio (OR) = 3.34; 95% CI = 0.54–22.9; p = 0.202). Among those who were not seizure-free before index, control patients who remained on the same AED had 1.66 times higher odds of attaining seizure remission after index compared to case patients whose AED was switched (OR = 1.66; 95% CI = 0.36–8.42; p = 0.523). Though it is not significant, the direction of this result contrasts with the raw data (Table 2) because the case and control groups were dissimilar in several respects (Table 1), so that adjusting for these covariates yields a different conclusion than that suggested by the raw, unadjusted numbers. The odds of attaining seizure freedom decreased significantly by 0.39 with each additional AED that the patient had failed (OR = 0.61; 95% CI = 0.37–0.91; p = 0.032).
Table 3.
Odds of seizure-freedom 6 months after index date, after controlling for covariates, among various subgroups relative to seizure status at index date (seizure-free or not seizure-free), whether or not AED was switched (case vs. control), and number of AEDs failed.
| Comparison | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| All subjects, seizure-free vs. non-seizure-free at index date | 3.34 | 0.54 – 22.90 | 0.202 |
| Non-seizure-free patients, controls vs. cases | 1.66 | 0.36 – 8.42 | 0.523 |
| Non-seizure-free patients, per previous AED failure | 0.61 | 0.37 – 0.91 | 0.032 |
| Seizure-free patients, controls vs. cases | 6.53 | 1.02 – 61.19 | 0.060 |
| Seizure-free patients, per previous AED failure | 0.85 | 0.57 – 1.39 | 0.469 |
CI = confidence interval
Among patients who were seizure free before index, control patients who remained on the same AED had 6.53 times higher odds of remaining seizure free after index compared to case patients whose AED was switched, a finding of marginal significance (OR = 6.53; 95% CI = 1.02–61.19; p = 0.06). The odds of remaining seizure free after index in this group decreased by only 0.15 with each additional AED that the patient previously failed (OR = 0.85; 95% CI = 0.57–1.39; p = 0.469). Of note, duration of seizure-freedom was not associated with likelihood of remaining seizure-free after drug switch (OR = 0.73; 95% CI = 0.31 – 1.69; p = 0.448).
Though controls were (by definition) those who did not have a new drug introduced, 18 of 40 refractory control patients (45%) had their AED dose changed at their index visit. Four of these 18 (22%) became seizure-free. Of the 22 who remained on the same drug and dose, 4 (18%) became seizure free (p = NS).
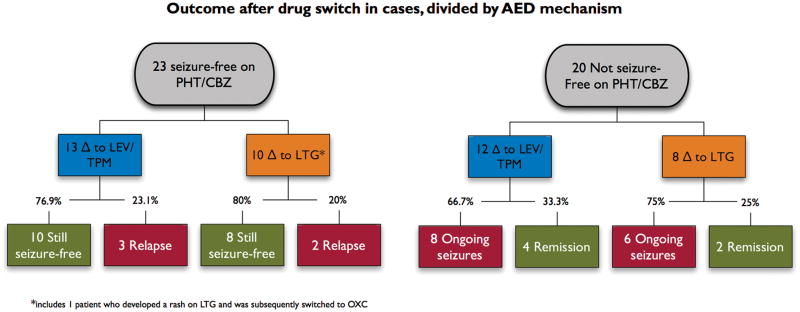
Case patients were also subdivided based on the type of drug to which they were switched. They were changed to either LTG, a drug with sodium channel blocking properties similar to those of the two older agents, or to LEV or TPM, which lack sodium channel blocking properties of this kind(McLean et al., 2000; Zona et al., 2001). When these two cohorts were compared in the statistical model, type of target drug was not significantly associated with outcome (Fig. 1).
Figure 1.
Seizure outcomes at six months in case patients, divided by index status and mechanism of action of target drug.
*Includes one patient who as switched to LTG, developed a rash, and was then converted to oxcarbazepine. PHT = phenytoin; CBZ = carbamazepine; LEV = levetiracetam; TPM = topiramate; LTG = lamotrigine.
Ninety-six patients had sufficient data to compare outcomes at 1 year before and after index. Nine of these 96 patients had different outcomes at 1 year than they had had at 6 months. The logistic regression model was applied to the data at 1 year producing results that were fairly similar to those seen at 6 months, albeit not achieving significance, presumably due to reduced statistical power (data not shown).
DISCUSSION
This study has two novel features: first, it is the only study to date to examine the outcome of switching AEDs using a control group; and second, it is the only study to systematically assess the outcome of switching AEDs in seizure-free patients.
With regard to the latter point, we found that the odds of seizure recurrence in seizure-free patients were about 6.5 times higher if the AED was switched than if the existing therapy was maintained. This difference was marginally significant, but from a practical perspective clinicians may nonetheless be interested in the absolute difference to which this corresponds. We found the rate of spontaneous recurrence in seizure-free patients without medication change was 4.3% over 6 months; this number is similar to that seen in the landmark Medical Research Council study, which showed a spontaneous recurrence rate of 11% at one year(Medical Research Council Antiepileptic Drug Withdrawal Study Group, 1991). Assuming this, the corresponding recurrence rate after changing to a new drug is 22.7% (derived from the formula for the odds ratio, in this case (p1/1−p1)*6.53 = (p2/1−p2)), meaning that changing the AED in a seizure-free patient would come with roughly an 18% incremental additional risk of seizure recurrence. In seizure-free patients, an aggressive approach to side effect reduction has always been at odds with fears of “rocking the boat”; our data would suggest that the latter is a moderate but legitimate concern.
The other major feature of the study is also of practical import, since all previous studies of AED switch in untreated or refractory populations have essentially been large, uncontrolled case series, a design which cannot account for spontaneous fluctuations in disease course(Kwan and Brodie, 2000; Callaghan et al., 2007; Luciano and Shorvon, 2007; Schiller and Najjar, 2008) We found that, for patients who were not seizure-free, the odds of seizure freedom did not differ after switching drugs compared to remaining on the same drug; seizure remission was, if anything, more likely (though not significantly so) when patients remained on the same drug. The number of AEDs failed was a far more important predictor of future seizure-freedom, in keeping with previous findings (Schiller and Najjar, 2008). Thus, while the aforementioned studies have shown some appreciable incidence of seizure-freedom even in patients who have failed previous therapies, our data imply that such studies have largely documented spontaneous improvements in the disease, and that such patients were almost equally likely to have become seizure-free even without a change in drug.
In patients who were having seizures but remained on the same AED, our data revealed no effect of changing doses. This corroborates pre-existing data showing that patients who become seizure-free overwhelmingly do so at low doses(Kwan and Brodie, 2001). Duration of pre-existing seizure-freedom also had no bearing on likelihood of remaining seizure-free after drug switch, nor did there seem to be better results in the small group of patients whose seizures had immediately been controlled on the first drug. Thus, the notion that quickly- or lengthily-controlled patients have an “easier” form of the disease which will respond equally well to alternative treatment does not receive support from our findings.
We also found no effect of AED mechanism; i.e. those switched from the older drugs to LTG, a drug with a similar effect on sodium channels, had outcomes no different than those switched to TPM or LEV, drugs which lack similar sodium channel effects. This suggests that presumptive mechanism of action may not be greatly relevant to therapeutic outcome, though the subgroups result in numbers too small to draw firm conclusions.
The inclusion of a matched control group in this study has added an important methodologic feature missing from previous investigations, but our study design has its own limitations. The greatest one is the discrepancy between AEDs in the groups: all switched patients were initially taking PHT or CBZ, whereas the matched controls were taking any of 10 different AEDs. A better design would have been to compare the switched patients to those taking CBZ or PHT who did not switch, but this was simply not possible, as restricting ourselves to CBZ- and PHT-treated patients would have precluded the performance of the study completely. A second theoretical limitation is that our results could have been tainted by the occurrence of withdrawal seizures due to taper of the original drug. This appears to have been a non-factor in our results, however, since we found only a single patient who had one or two seizures soon after the switch followed by seizure freedom, and no patient had a seizure during the drug crossover period.
The final major limitation is the retrospective nature of the control group and the matching process, thus yielding groups with substantive differences along with the potential for limited information or subtle biases. Our analyses attempted to account for these, but that there may be other undiscovered yet relevant differences between the cases and controls which were not accounted for. These caveats notwithstanding, it is doubtful that any study of this nature could be done with better group comparability, as there are simply too many parameters to control for. The only way to eliminate these limitations would be to prospectively randomize patients to either stay on their AED or switch to another; conducting such a study at present would be daunting, if not impossible.
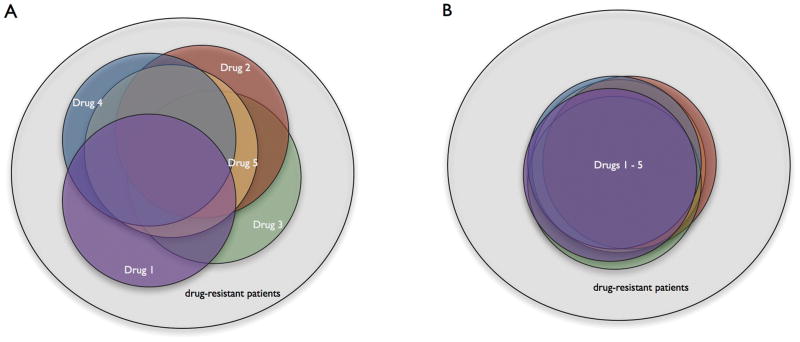
Our results point to an important unresolved issue in the field of epilepsy therapy. Head-to-head comparisons of AEDs have mostly shown equivalent efficacy among various compounds(Dam et al., 1989; Brodie et al., 1995; Privitera et al., 2003; Brodie et al., 2007; Marson et al., 2007). If one accepts this, then there are two models to conceptualize the relative efficacy of AEDs. The first possibility is that each of these drugs, though successful at about the same rate, is effective in slightly different segments of the epilepsy population (Fig 2A). This model predicts that successive AED trials will lead to more and more patients coming under control, but that such control may, in many patients, be dependent upon a specific drug. The second possibility is that all of these drugs are effective in the same subgroup of patients, and therefore ineffective in the same (complementary) subset of patients (Fig 2B). This model predicts that successive medication trials are very likely to be fruitless if the patient was not controlled on the first drug, but it also predicts that a patient who is controlled should likely remain controlled on any other AED as well. These competing models have heretofore been examined only in drug-resistant patients, but the present data on serial efficacy in seizure-free patients effectively serve to assess the same models, using a different approach. Our data might be construed as more supportive of the first model with regard to seizure-free patients, but more supportive of the second model with regard to non-seizure-free patients, a contradiction that does not admit of a ready resolution. Other investigations have also been contradictory in support of redundant or non-redundant AED efficacy(Kwan and Brodie, 2000; Luciano and Shorvon, 2007), and more research is clearly necessary to answer this fundamental question. It is possible that there are groups of patients for whom the first model is appropriate, and others for whom the second is more accurate.
Figure 2.
Two different models of relative AED efficacy.
In each diagram, the large circle represents the universe of epilepsy patients, while each colored circle represents the fraction of patients who may be rendered seizure-free by an individual drug. Patients resistant to all drugs are in the gray shaded area outside the colored circles. In the first model (A) each drug is similar in efficacy (i.e. the circles are of the same size), but most provide control over a portion of the population not controlled by the other agents. Thus, as more drugs are introduced, the proportion of drug-resistant patients diminishes, but the attainment of seizure-freedom may be dependent upon a particular drug. In the second model (B) each drug is not only similar in efficacy, but also almost wholly overlapping in its coverage. Thus, each successive drug introduction expands the pool of seizure-free patients only trivially. However, a patient who is amenable to treatment is likely to respond to any agent.
In conclusion, our findings indicate that while the large majority of seizure-free patients remain so when switched to a different agent, there is nonetheless a real risk of seizure recurrence, with no obvious predictors. For those who are not seizure-free after failing several drug trials, our data reinforce the idea that change in control of epilepsy is often unrelated to physician intervention. This implies that reacting to every seizure with a dose or drug change might not be warranted, as even those who subsequently enter remission are likely to have done so due to spontaneous disease fluctuation rather than alteration of the therapeutic regime. These findings require verification and expansion in future studies.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DISCLOSURES
Drs. Wang, Stuckert, and Zhan have nothing to disclose.
Dr. Mintzer has engaged as a speaker for Glaxo SmithKline and UCB Pharma and as a consultant for Sunovion, UCB Pharma, Eisai, and Supernus. Dr. Mintzer is also a member of the Epilepsy Study Consortium, through which he has engaged in consultation for Pfizer, Upsher-Smith, and Novartis. Dr. Mintzer is supported by NIH (K23NS058669).
Drs. Skidmore and Sperling have engaged as speakers for UCB Pharma and have received research support from UCB, Medtronic, Neuropace, Sunovion, Eisai, Marinus, Vertex, and Lundbeck. Dr. Skidmore has also engaged in consultation for UCB Pharma, Idrovo, and Upsher-Smith.
Dr. Nei has engaged as a speaker for Medtronic.
References
- Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, Bazil C, Pacia SV, Spencer SS. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–190. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Perucca E, Ryvlin P, Ben-Menachem E, Meencke HJ. Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68:402–408. doi: 10.1212/01.wnl.0000252941.50833.4a. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995;345:476–479. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- Dam M, Ekberg R, Loyning Y, Waltimo O, Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989;3:70–76. doi: 10.1016/0920-1211(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ, Kalviainen R, Yurkewicz L, Weaver J, Knapp LE. Efficacy and safety of pregabalin versus lamotrigine in patients with newly diagnosed partial seizures: a phase 3, double-blind, randomised, parallel-group trial. Lancet Neurol. 2011;10:881–890. doi: 10.1016/S1474-4422(11)70154-5. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42:1255–1260. doi: 10.1046/j.1528-1157.2001.04501.x. [DOI] [PubMed] [Google Scholar]
- Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62:375–381. doi: 10.1002/ana.21064. [DOI] [PubMed] [Google Scholar]
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJ, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PE, Smith CT, Vanoli A, Williamson PR. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean MJ, Bukhari AA, Wamil AW. Effects of topiramate on sodium-dependent action-potential firing by mouse spinal cord neurons in cell culture. Epilepsia. 2000;41(Suppl 1):S21–4. [PubMed] [Google Scholar]
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Medical Research Council Antiepileptic Drug Withdrawal Study Group. Lancet. 1991;337:1175–1180. [PubMed] [Google Scholar]
- Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, Sperling MR. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009;65:448–456. doi: 10.1002/ana.21615. [DOI] [PubMed] [Google Scholar]
- Mintzer S, Skidmore CT, Rankin SJ, Chervoneva I, Pequinot E, Capuzzi DM, Sperling MR. Conversion from enzyme-inducing antiepileptic drugs to topiramate: Effects on lipids and c-reactive protein. Epilepsy Res. 2012;98:88–93. doi: 10.1016/j.eplepsyres.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003;107:165–175. doi: 10.1034/j.1600-0404.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology. 2008;70:54–65. doi: 10.1212/01.wnl.0000286959.22040.6e. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. Springer; New York: 2002. [Google Scholar]
- Zona C, Niespodziany I, Marchetti C, Klitgaard H, Bernardi G, Margineanu DG. Levetiracetam does not modulate neuronal voltage-gated Na+ and T-type Ca2+ currents. Seizure. 2001;10:279–286. doi: 10.1053/seiz.2000.0504. [DOI] [PubMed] [Google Scholar]