Abstract
Targeting the tumor stroma in addition to the malignant cell compartment is of paramount importance to achieve complete tumor regression. In this work, we modified a previously designed tumor stroma-targeted conditionally replicative adenovirus (CRAd) based on the SPARC promoter by introducing a mutated E1A unable to bind pRB and pseudotyped with a chimeric Ad5/3 fiber (Ad F512v1), and assessed its replication/lytic capacity in ovary cancer in vitro and in vivo. AdF512v1 was able to replicate in fresh samples obtained from patients: (i) with primary human ovary cancer; (ii) that underwent neoadjuvant treatment; (iii) with metastatic disease. In addition, we show that four intraperitoneal (i.p.) injections of 5 × 1010 v.p. eliminated 50% of xenografted human ovary tumors disseminated in nude mice. Moreover, AdF512v1 replication in tumor models was enhanced 15–40-fold when the tumor contained a mix of malignant and SPARC-expressing stromal cells (fibroblasts and endothelial cells). Contrary to the wild-type virus, AdF512v1 was unable to replicate in normal human ovary samples while the wild-type virus can replicate. This study provides evidence on the lytic capacity of this CRAd and highlights the importance of targeting the stromal tissue in addition to the malignant cell compartment to achieve tumor regression.
Introduction
Ovarian cancer is one of the leading gynecologic malignancies globally; Scandinavia, Israel, and North America have the highest rates of incidence (10–15/100,000) while developing countries and Japan exhibit the lowest incidence (5/100,000).1,2,3 Although progress in conventional therapies (surgery, chemotherapy, and irradiation) has been achieved, the 5-year survival rate for patients with advanced stage ovarian cancer is still low.3
One of the potential approaches to tackle the advanced stages of the disease is the use of conditionally replicative adenoviruses (CRAds).4 Several oncolytic adenoviruses, including few CRAds, were assessed in ovary cancer models following grafting of human cells in immunodeficient mice.5,6,7 Different CRAds whose replication was driven by the promoters corresponding to VEGF,5 Cox-2,8 the leukoprotease inhibitor,9 CXCR4, Survivin, and Mesothelin6 also exhibited important therapeutic efficacy on disseminated ovarian cancer models and extended mice survival, but none of them was reported to be able to eliminate disseminated tumors.5,6,8,9 In all cases, the target of the viruses was the malignant epithelium with no specific consideration on the capacity of the viruses to target the stromal cell compartment. The nonreplicative adenovirus Ad5.SSTR/TK.RGD where gene expression is driven by an immediate-early CMV promoter,10 the E1B-55kd gene deleted oncolytic adenovirus ONYX-015 with no specificity for ovarian cancer,11 and the CRAd Ad5-δ24RGD,12 entered clinical trials. Despite the lack of partial or complete responses the trials highlighted the feasibility and safety of oncolytic adenoviruses use in the clinics, and reinforced the need to enhance viral replication and specificity.
Recent studies have shown that most aggressive ovarian cancer, especially those disseminated in the peritoneum and the omentum, exhibited a high content of genes expressed by stromal cells.13 Stringent follow up studies demonstrated that patients with tumors showing enhanced activity of stromal genes exhibited the poorest survival.13 Type I and III collagen produced by the tumor stroma were proposed as predictors of poor outcome in human ovarian cancer.14 Thus, novel approaches based on medicines that target the tumor stroma compartment can be highly effective in elusive tumors such as advanced ovarian cancer.
SPARC (secreted protein, acidic, rich in cysteine) is a secreted glycoprotein that has been associated with most aggressive human cancers.15,16 Elevated immunostaining was observed in 40–80% of human malignant ovarian carcinomas.17,18,19 Interestingly, SPARC was mainly expressed in the tumor stroma, including endothelial cells and fibroblasts, in close contact with the leading edge of the tumor; in addition, almost 15% of ovary carcinomas exhibited SPARC expression in epithelial cells;17 however, in situ hybridization showed no SPARC reactivity in malignant ovary epithelial cells suggesting that in most cases SPARC is secreted by stromal fibroblasts and internalized by epithelial cells at the tumor-stromal interface.20 It appears that SPARC expression is downregulated in several types of epithelial cancer cells due to promoter methylation.21
With the aim of targeting the stromal compartment of the tumor mass, we have previously designed a CRAd based on a specific fragment of the SPARC promoter (Ad-F512). Ad-F512 was also active on pancreatic cancer cells with silenced SPARC expression due to promoter methylation; however, Ad-F512 efficacy was greatly dependent on the presence of the accompanying stromal cells both in xenografted human melanoma and pancreatic cancer models.22 Here, we demonstrate a strong therapeutic effect of an improved version of Ad-F512 (named AdF512v1), where the F512-SPARC promoter drives the expression of E1A mutated in one of the pRb-binding sites, and the CRAd was pseudotyped with a chimeric fiber Ad5/3. We show that AdF512v1 replicated in fresh tissue explants obtained from ovarian cancer patients that received or not neoadjuvant chemotherapy and in disseminated tumors, but exhibited no replication in nonmalignant human ovary tissue explants; AdF512v1 was also therapeutically effective in a human ovarian cancer model disseminated in the peritoneum and cured 50% of the mice. Moreover, AdF512v1 showed enhanced replication in vitro in ovary cancer xenografts that contained human stromal cells holding promise regarding its potential utility in solid desmoplastic tumors.
Results
In vitro activity of different versions of Ad-F512 on ovary cancer cell lines
In previous studies, we observed that Ad-F512 was active both in human melanoma cells and certain pancreatic cancer cells lines regardless of SPARC mRNA levels.22 In order to assess whether the F512-SPARC promoter is active in epithelial ovary cancer cells we transduced three ovary cancer cell lines with nonreplicative adenoviral vectors pseudotyped or not with the chimeric fiber 5/3 and expressing luciferase under the control of F512-SPARC. These studies confirmed that F512-SPARC was active in ovary cancer cells regardless of SPARC mRNA levels (Figure 1a and Supplementary Table S1). Moreover, F512-SPARC was as active as the SV40 promoter and the viral vector carrying the chimeric fiber 5/3 showed 2 to almost 80-fold—enhanced activity compared to the viral vector carrying the native type 5 fiber (Figure 1a).
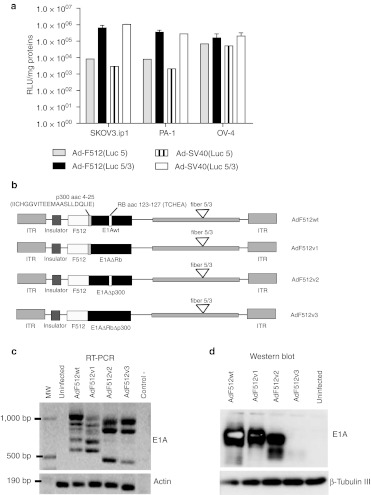
Figure 1.
F512-SPARC promoter activity in ovary cancer cells. (a) Luciferase activity of F512-SPARC and SV40 promoters in three ovary cancer cells lines. The cancer cell lines (7 × 10 4 cell/MW24) were infected with 4 E1-deleted viruses, Ad-SV40(Luc 5), Ad-SV40(Luc 5/3), Ad-F512(Luc 5), and Ad-F512(Luc 5/3), and 48 hours later luciferase activity was analyzed. Relative light units (RLU) data are shown relative to milligram of protein. Error bars represent mean ± SD. (b) Genomic organization of the different conditionally replicative adenoviruses (CRAds) used in this work. (c) Reverse transcription-PCR (RT-PCR) and (d) western blot analysis of E1A in following infection of SKOV3-luc cells with the different viruses (for more details see Supplementary Materials and Methods). β-Tubulin III was used as the loading control of western blots and β-actin as a control of the reverse transcription-PCR.
Therefore, we decided to construct four novel versions of Ad-F512 pseudotyped with the chimeric fiber 5/3 and carrying different variants of mutated E1A that can restrict viral replication in nonmalignant tissue. AdF512v1 includes a deletion that restricts E1A binding to pRb; AdF512v2 includes an E1A deletion that restricts its binding to p300 and AdF512v3 includes E1A mutated both in the pRB- and p300-binding sites; AdF512wt contains the E1A wild type (Figure 1b). By reverse transcription-PCR analysis we observed that AdF512v1 and AdF512wt exhibited quite similar patterns of E1A RNA expression with the presence of five bands of different molecular weights; part of these bands were absent in AdF512v2 and AdF512v3 (Figure 1c); in coincidence with the mRNA pattern, AdF512v1 and AdF512wt showed very similar E1A protein pattern, while AdF512v2 and the double mutant exhibited lower or faint levels respectively, of the highest molecular weight E1A band and the appearance of E1A bands of lower molecular weights (Figure 1d).
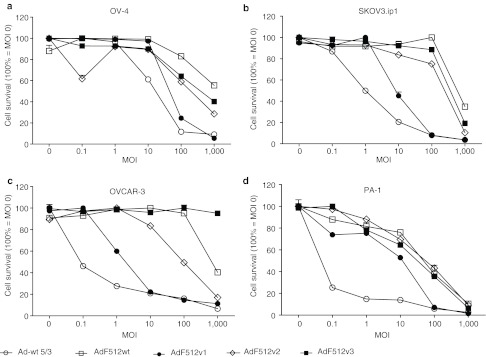
Based on the differences in E1A expression pattern at the mRNA and protein level between the different CRAds we decided to compare their lytic capacity in four different ovary cancer cell lines. AdF512v1 exhibited the best lytic effect in all the cell lines assayed (Figure 2a–d). The percentage of remaining viable cells after infection with AdF512v1 (at multiplicity of infection (MOI) 100) was 25, 8, 12, and 7% for OV-4, SKOV3.ip1, OVCAR-3, and PA-1 ovary cancer cells, respectively. At the lowest MOIs (0.1–10), Ad-wt 5/3 was slightly more effective than AdF512v1 OV-4, OVCAR-3, and SKOV3.ip1 and more effective in PA-1 cells although at MOI 100 both viruses exhibited a similar lytic effect. In order to confirm the lytic capacity of AdF512v1, we transduced three of the ovary cancer cell lines with AdF512v1 at MOI 100. We observed by flow cytometer analysis the absence of viable cells 4 days after infection confirming the lytic capacity of AdF512v1 on ovary cancer cells (Supplementary Figure S1). Ad-wt 5/3 was used as a control of cell lysis.
Figure 2.
Conditionally replicative adenovirus (CRAd) activity in ovary cancer cells. (a–d) Correspond to ovarian cancer cell lines OV-4, SKOV3.ip1, OVCAR-3, and PA-1. Oncolytic capacity of the CRAds. Six days postinfection cell viability was assessed quantitatively using the MTS assay. Data was normalized to uninfected cells. Error bars represent the mean ± SEM (n = 3).
Ex vivo replication of AdF512v1 in fresh human ovary cancer explants
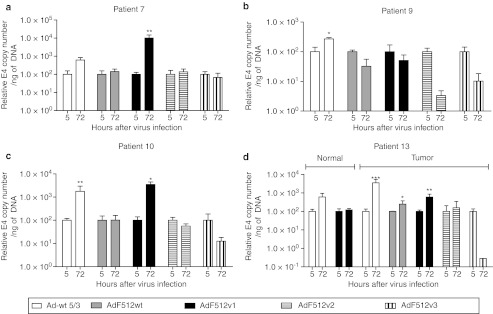
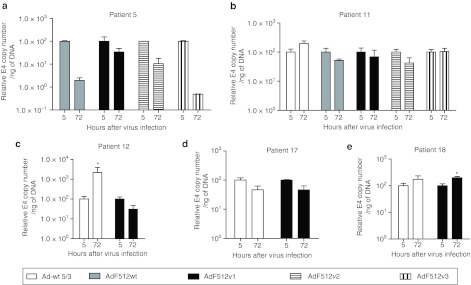
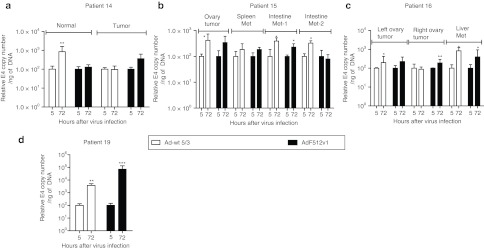
Instead of pursuing the comparison of the different CRAds only in terms of their in vitro lytic capacity on malignant cell lines, we decided to further explore their capacity to replicate ex vivo on freshly available tumor explants. The use of these explants is becoming a valuable tool to assess viral replication since they resemble the situation CRAds face in the clinics. The explants used in the present studies exhibited epithelial cells nests intermingled in abundant stroma containing mainly fibroblasts and some endothelial vessels that exhibited intense SPARC staining (Supplementary Figure S2a). Freshly available explants were obtained from patients undergoing surgery due to a primary or metastatic ovarian carcinoma; normal ovary samples were obtained from patients undergoing surgery for other pathologies and expressed faint levels of SPARC (Supplementary Figure S2b). Initial samples transduced with the nonreplicative Ad-F512(Luc 5/3) confirmed that F512-SPARC was at least 10-times more active in the ovary cancer explants than in normal ovaries (Supplementary Figure S2c). CRAds replication was evaluated in explants obtained from 13 patients. Samples were infected at ~500 v.p./cell of the different Ad-F512 versions, and compared to Ad-wt 5/3. In preliminary studies we determined that the best time for E4 assessment as a surrogate marker of viral replication is 72 hours after infection (data not shown). We analyzed samples from 4 primary tumors obtained from untreated patients, 5 primary tumors from 4 patients with previous chemotherapy, 4 metastatic samples obtained from 2 patients and 5 normal ovary samples. AdF512v1 was the only CRAd that replicated in ovary cancer samples 7, 10, and 13 (Figure 3a, c and d). Interestingly, AdF512v1, that we confirmed was unable to bind pRb (Supplementary Figure S3), did not replicate in normal ovary explants obtained from postmenopausal patients 5, 11, 12, 13, and 17 (Figures 3d and 4). Interestingly, none of the novel CRAds-containing mutated E1A was able to replicate in the malignant explants (Figure 3).
Figure 3.
Viral replication in freshly excised human ovary cancer explants. (a–d) Correspond to samples obtained from patients with primary ovary cancer. Human ovary cancer explants were infected with 500 v.p./cell of the different viruses and after 5 or 72 hours samples were collected and DNA was isolated. E4 levels were determined by real-time PCR. Levels of E4 at 72 hours were normalized with the data at 5 hours. Sample 13 also includes the contralateral normal ovary. Error bars represent the mean ± SEM, n = 3 for each sample. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 4.
Viral replication in explants of nonmalignant human ovary explants. (a–e) Correspond to samples obtained from patients with normal ovary. Nonmalignant ovary samples were analyzed as described in Figure 3. Error bars represent the mean ± SEM, n = 3 for each sample. *P < 0.05.
In further studies, we were able to evaluate AdF512v1 replication in samples from patients that ended paclitaxel and carboplatin neoadjuvant chemotherapy 1 month before surgery (samples 14, 15, 16, and 19, Figure 5); in two cases (samples 15 and 16) we also included disseminated tumor tissue obtained from different regions of the peritoneal cavity and in case 14 we also obtained a sample from a nonmalignant ovary (Figure 5). AdF512v1 significantly replicated in malignant samples obtained from intestine and liver metastasis indicating that remnant cells after neoadjuvant chemotherapy are sensitive to the CRAd lytic activity (Figure 5). Histological analyses confirmed that remnant malignant cells were viable with no evidence of necrotic cells (data not shown). With the exception of cancer sample 14 and normal sample 17, Ad-wt 5/3 could replicate in all samples regardless of whether they were cancer or noncancer ovary tissue (Figures 3–5). Interestingly, the replication rates of AdF512v1 were superior to Ad-wt 5/3 in cancer samples 7, 10, and 19; Ad-wt 5/3 replication increased sixfold at 72 hours in patient 7, whereas AdF512v1 replication increased 100-fold at the same time point. In patient 10, we observed sixfold increase with Ad-wt 5/3 and 46-fold increased replication with AdF512v1, while in patient 19 was 40 and 714, respectively (Figures 3a,c and 5d, and Supplementary Table S2). Mostly important, with the exception of a slight replication in sample 18, AdF512v1 was essentially unable to replicate in normal ovary tissue. Thus, the overall ex vivo data with human samples prompted us to select AdF512v1 to further in vivo studies in animal models of disseminated solid human ovary cancer.
Figure 5.
AdF512v1 replication in explants obtained from patients that underwent neoadjuvant chemotherapy. (a–d) Correspond to samples obtained from patients with normal, malignant or metastatic ovary cancer. Ovary cancer tissue explants and metastases were analyzed as described in Figure 3. Error bars represent the mean ± SEM, n = 3 for each sample. *P < 0.05 and **P < 0.01, and ***P < 0.001.
In vivo studies with AdF512v1
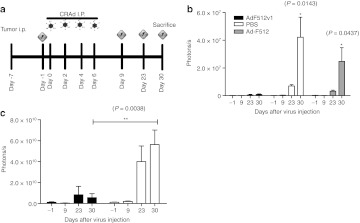
Therapeutic efficacy on a disseminated xenograft model. To further assess the in vivo therapeutic efficacy of AdF512v1 we injected 3 × 106 luciferase-expressing SKOV3-luc ovary cancer cells in female nude mice peritoneum. Carcinomatosis developed in 6 days when visible tumors were observed. Treatment was initiated the next day by administering 4 injections intraperitoneal (i.p.) of 5 × 1010 v.p./400 µl of virus or control vehicle every other day (Figure 6a). Tumor growth was evaluated in two independent experiments by bioluminescent imaging follow up at days 9, 23, and 30 after the initiation of AdF512v1 administration (Figure 6b and c). In one experiment, we also included the parental CRAd Ad-F512 as an additional control. By day 30 mice were sacrificed and the number and weight of metastases was determined. We observed a strong increase in the luminescence signal in control and Ad-F512-treated animals whereas in mice treated with AdF512v1 the signal intensity was strongly inhibited (Figure 6b and c). In fact, we were unable to detect luminescence signal in 1/5 (first experiment) and 3/6 (second experiment) AdF512v1-treated mice indicating tumor absence. Macroscopic and microscopic examination of the peritoneal cavity at autopsy revealed no evidence of viable tumor tissue in cured mice while control mice showed areas of luminescence emission confirmed as micrometastases in spleen, diaphragm and pancreas (Supplementary Figure S4a). Quantification of visible metastases revealed an average of 4 metastatic foci in control mice, 3 metastatic foci in Ad-F512-treated mice while mice treated with AdF512v1 showed none or a maximum of 1 metastatic nodule per mice in both experiments (Supplementary Figures S4b and c). Similar differences between AdF512v1-treated and control or Ad-F512-treated mice were observed when the weight of the metastatic mass was compared (Supplementary Figures S4d and e). Although, AdF512v1 was not designed for systemic use we established its efficacy on this model of disseminated cancer after intravenous administration through the tail vein. No therapeutic effect was observed with a single administration of 1010 v.p. of AdF512v1 administered 7 days after SKOV3-luc ovary cancer cells injection (Supplementary Figure S5a). In a second experiment mice were administered twice with the CRAds with 1 week difference, and despite the fact that we have not seen statistical differences (Supplementary Figure S5b), 1/5 mice treated with AdF512v1 exhibited a visible reduction in the tumor mass (Supplementary Figure S5c).
Figure 6.
AdF512v1 treatment of mice-harboring intraperiotoneally disseminated ovary cancer. (a) Protocol followed in the in vivo assays (b, c) evaluation of the antitumor effect of AdF512v1, Ad-F512 or vehicle [phosphate-buffered saline (PBS)] quantified as photons per experimental time point in two independent experiments. Error bars represent the mean ± SEM. Number of mice in (b): AdF512v1 (n = 5), Ad-F512 (n = 5), and PBS (n = 4); in (c): AdF512v1 (n = 6), and PBS (n = 5). P < 0.05, and **P < 0.01.
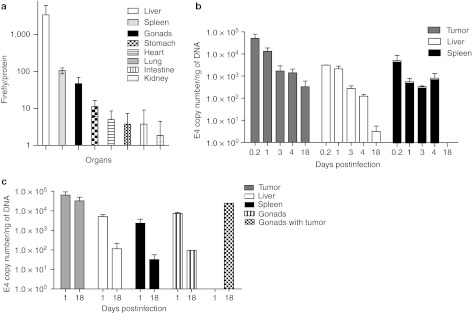
Viral retention in the tumor niche
Based on the previous in vivo data, our next aim was to establish whether differential CRAd retention at the tumor niche was associated with the in vivo therapeutic effect. Initially, we injected i.p. 5 × 1010 v.p. of Ad-F512(Luc-5/3) in healthy mice and 48 hours later we observed considerable luciferase activity restricted mainly to the liver and to a lesser extent to the spleen and gonads (Figure 7a). The other organs showed negligible luciferase expression. Next, we grafted mice with SKOV3-luc ovary cancer cells followed by AdF512v1 administration once i.p. at day 7 and removed tumor, liver, and spleen 5 hours, 1, 3, 4, and 18 days post-AdF512v1 injection to establish viral retention by the different organs; gonads were discarded in this initial experiment due to the presence of infiltrating malignant cells that were detected own to the routine luminescence analysis performed to avoid contamination by infiltrating malignant cells. We observed that the tumor mass was very efficiently infected since there were 10–16 more AdF512v1 particles in tumor samples at 5 hours and at day 1 compared to liver and spleen (Figure 7b). In addition, no viral particles were retained in liver and spleen by day 18 whereas the tumor showed the presence of almost 500 E4 copies per ng of DNA (Figure 7b). In a second experiment viral particles were evaluated at days 1 and 18 and once more we observed that AdF512v1 retention by the tumor was very high compared to the normal organs (Figure 7c). Interestingly, AdF512v1 levels in tumor-infiltrated gonads at 18 days was 250-fold higher than the levels in tumor-free gonads indicating that the virus targets and is retained preferentially by the tumor niche (Figure 7c).
Figure 7.
AdF512v1 retention at different organs. (a) Naive mice were intraperitoneal (i.p.) injected once with Ad-F512 (Luc 5/3) and 48 hours later the main organs were collected and luciferase activity was assayed. Results are presented as relative light units (RLU) normalized for total protein concentration. (b) Mice-harboring tumors disseminated in the peritoneum were i.p. injected with AdF512v1 and after 5 hours and 1, 3, 4, and 18 days, two to three mice were sacrificed and DNA was extracted to assess E4 levels. Data is expressed as E4 copy number per nanogram of DNA. (c) E4 levels were assessed at days 1 and 18 postinfection. Error bars represent the mean ± SEM, where n = 2 or 3.
Ex vivo and in vivo effect of AdF512v1 on tumors composed of malignant and stromal cells
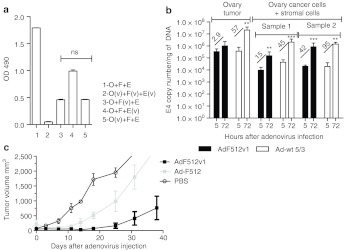
The previous data demonstrated that AdF512v1 is a more potent version of Ad-F512 that was designed to target both the malignant and the stromal compartment of the tumor mass. Human adenoviruses such as AdF512v1 do not replicate in murine stromal cells that are recruited to the growing human ovary tumor xenograft. Therefore, we performed a series of experiments to establish the relevance of the stromal cell component on AdF512v1 therapeutic efficacy. In initial experiments we observed that human fetal fibroblasts WI-38 that exhibit characteristics that resemble cancer-associated fibroblasts, and transformed-microendothelial cells (HMEC-1) could support replication of AdF512v1 that can lyse these type of stromal cells (Supplementary Figure S6). In a second series of experiments we plated (ratio 1:1:1) a mix of SKOV3-luc ovary cancer cells, WI-38 fibroblasts and HMEC-1 transformed-microendothelial cells previously infected or not with AdF512v1 and evaluated total cell viability with MTS and viable SKOV3-luc cells through luciferase expression. The strongest in vitro lytic effect (both by MTS and luciferase expression) was observed when the three cell types were previously infected with AdF512v1 (Figure 8a and Supplementary Table S3 and Supplementary Figure S7). Moreover, previous infection of stromal cells, once at a time, with AdF512v1, led to significant reduction in the amount of remaining viable cells and to almost the complete elimination of SKOV3-luc cells, suggesting that stromal cells supported viral replication and spreading that led to the elimination of coplated ovary cancer cells (Figure 8a and Supplementary Table S3).
Figure 8.
Ex vivo replication and in vivo antitumor effect of AdF512v1 on tumor xenografts composed of malignant and stromal cells. (a) MTS assays after 6 days cocultures of SKOV3-luc (O), WI-38 fibroblasts (F), and HMEC-1 endothelial cells (E) previously infected (v) or not with AdF512v1. ns, nonstatically significant. (b) Explants of xenografted SKOV3-luc tumors (with or without stromal cells) were infected at 500 v.p./cell with AdF512v1 or Ad-wt 5/3 and after 5 or 72 hours DNA samples were isolated and E4 levels were determined by real-time-PCR. Fold of increment between 5 and 72 hours are shown between bars. Error bars represent the mean ± SEM (n = 3 or 4). (c) In vivo growth of subcutaneous tumors made of SKOV3-luc/WI-38/HMEC-1-treated intratumorally with three administrations of AdF512v1, Ad-F512, or phosphate-buffered saline (PBS).
To further assess the involvement of the stromal compartment in viral efficacy, we established SKOV3-luc subcutaneous tumors in nude mice combined or not with a mix of HMEC-1 and WI-38 cells. When tumors reached 500 mm3 animals were sacrificed and tumor explants were ex vivo treated and infected as described for human tumor samples. We observed that mixed tumors containing ovary cancer and stromal cells were infected with less efficiency than tumors without stromal cells (see E4 copy number levels at 5 hours postinfection, Figure 8b). Ad-wt 5/3 replication at 72 hours was marginally affected by the presence of stromal cells (Figure 8b). Interestingly, AdF512v1 showed increased rates of replication in tumors containing stromal cells clearly suggesting that stromal cells can enhance viral replication leading to increased lytic activity (Figure 8b). Interestingly, similar experiments performed with another ovary cancer cell line PA-1 that express SPARC but was less sensitive to AdF512v1 lytic effect than SKOV3 cells, also showed an improvement in viral replication in the presence of stromal cells (Supplementary Figure S8). In fact both Ad-wt 5/3 and AdF512v1 were unable to replicate in tumors made of cancer cells alone (Supplementary Figure S8) indicating that stromal cells play a relevant role probably by supporting viral replication and secreting soluble factors that rendered malignant cells more sensitive to the virus.22
We finally established the therapeutic efficacy in vivo of AdF512v1 on tumors made of SKOV3-luc cells mixed with WI-38 and HMEC-1 cells. The cells mix was implanted subcutaneous for the clear limitation of injecting the mix directly into the peritoneum. Once tumors reached 100 mm3 we treated mice with three intratumor administrations of AdF512v1, Ad-F512 or phosphate-buffered saline (PBS) (at days 0, 3, and 7). At the end of the experiment one of three mice treated with AdF512v1 was completely free of tumor and the other two showed greatly reduced tumor volumes compared to the group of mice treated with Ad-F512 or PBS. The differences between the AdF512v1-treated group and the other two groups were statistically significant (Figure 8c and Supplementary Figure S9).
Discussion
According to Globocan data base more than 200,000 cases of ovarian cancer are diagnosed each year. It is estimated that more than 125,000 women with ovarian cancer die each year. The most important determination of survival seems to be disease stage at diagnosis. Only ~20% of women are diagnosed at an early stage, but in most of the cases the disease is detected at an advanced stage leading to a poor prognosis.2,23 Early disease stage has a 5-year survival rate of greater than 70%, but for those diagnosed with advanced disease stage, it is below 15%. Currently available methods prove quite unable to detect ovarian cancer at an early stage,24 therefore new therapeutic tools are urgently needed.
Here, we characterized a novel CRAd that has been designed to target the tumor-associated stromal cell compartment since its replication is driven by a promoter fragment of the stroma-associated gene SPARC. We showed here that this novel CRAd was therapeutically effective in a xenograft model of disseminated/metastatic human ovary cancer inducing a major growth inhibitory effect including the complete remission of the tumor in 50% of the cases. Mostly important, this novel CRAd was also capable of replicate ex vivo in explants of fresh samples of primary human ovary cancer, obtained from patients that underwent or not neoadjuvant chemotherapy, and in ovary cancer metastases; but contrary to the wild-type virus it was almost unable to replicate in noncancerous ovary samples. This oncolytic virus was able to replicate ex vivo and eliminate in vivo tumors made of malignant and stromal cells.
Initial uses of oncolytic viruses for therapeutically targeting ovarian cancers involved the use of ONYX-15 an oncolytic virus with broad cancer spectrum and nonselective replication capacity.11 The limited success of these initial trials was attributed at least in part to the reduced infective capacity of type 5 adenoviruses that entered cells through CAR receptor.11 In a second study, a replicative-competent Edmonston B measless vaccine strain that infects cells through CD46 was administered to patients with recurrent ovarian cancer.25 Fourteen of the 21 patients exhibited stable disease and CA-125 levels were reduced by >30% in 5 of the 21 patients.25 A more recent trial in recurrent malignant gynecologic diseases including ovarian cancer has been published.12 This trial was based on an infectivity enhanced-oncolytic adenovirus carrying E1A mutated in the Rb-binding site whose transcriptional regulation was under the control of the wild-type promoter and the viral fiber was pseudotyped with an RGD motive to enhance viral infectivity. The authors found no severe adverse effect, and although no partial or complete responses were observed after 1 month follow up, reduced CA-125 levels were observed in 7 of 21 patients.12
AdF512v1 is an improved version of Ad-F512 that was shown to inhibit melanoma and pancreatic cancer growth in preclinical models.22 AdF512v1 was improved by (i) the incorporation of an insulator sequence upstrem of the F512-SPARC promoter, (ii) the incorporation of a mutated form of E1A unable to bind pRb, and (iii) virus pseudotyping with a chimeric 5/3 that binds preferentially to type 3 adenoviral receptors. The decision to assess the therapeutic efficacy in ovarian cancer of AdF512v1 was due to the fact that SPARC is expressed mainly in stromal cells (fibroblasts and endothelial cells) in close contact with the forefront of the epithelial tumor mass.17,18 Interestingly, AdF512v1 was also able to replicate in ovarian cancer cells that express negligible SPARC levels. Silencing of SPARC expression in some epithelial ovarian cancer cells is mainly due to promoter methylation.17,20 As shown in previous studies, methylation of the internal promoter would not hinder the activity of the oncolytic virus provided the transcription factors that regulate SPARC promoter activity are still expressed in the target cells.22
In addition to the use of a promoter active both in the malignant and in the stromal cell compartment we decided to pseudotype the virus with a chimeric 5/3 fiber that retargets the virus to enter malignant cells through CD46, although recent evidence points to desmoglein as an additional receptor for type 3 adenoviral fiber.26 We observed that vectors expressing the 5/3 chimeric fiber exhibited at least 40% increased infectivity and lytic effect on ovary cancer cells compared to the virus expressing the type 5 fiber. This is consistent with previous evidence showing that the Ad5/3 chimera displays enhanced infectivity in ovary cancer cell lines and purified primary tumor cells (~10-fold).5,6,27,28 Recently, it was shown that a full serotype 3 CRAd was as useful as the 5/3 pseudotype virus.29
Reduced binding of E1A to pRb and p300/CPB emerged as a strategy to limit adenoviral replication in normal cells.30 Therefore, we decided to mutate E1A to eliminate its capacity to bind to either of the two proteins or to both of them. Indeed, we observed that with the sole exception of sample 18, AdF512v1 was completely unable to replicate in normal human ovary explants, while the wild-type virus replicated both in malignant and normal ovary explants. In addition to the almost complete abrogation of its replication capacity in fresh human normal ovary samples AdF512v1 was also unable to replicate in the permissive Syrian hamster organs (data not shown). Thus, it appears that mutation in the pRB-binding site restricted AdF512v1 capacity to replicate in normal ovaries and greatly attenuated its potential harmful effect on normal organs. On the other hand, we observed that AdF512v1 exhibited an enhanced in vitro activity even at the lowest MOI of 1 to 10 and ex vivo activity in human ovary cancer explants. This enhanced lytic/replication capacity was in close coincidence with previous studies showing that CRAds carrying an E1A mutation in the pRb-binding site might exhibit similar or even better lytic effect in vitro than CRAds carrying the E1Awt gene.30,31,32 It was hypothesized that in the absence of pRb-binding capacity, there is an augmented E1A transactivation function either because pRb sequestration no longer occurs due to the abrogation of E1A feedback inhibition, or a diminished E1A ubiquitination as a result of a decreased phosphorylation status.31
Fresh cancer explants are being increasingly used as a reliable tool to assess viral capacity to replicate and lyse ex vivo human samples that resemble the situation the virus might face in a clinical setting. This methodology has been already used in few works to assess the replication and lytic capacity of CRAds in breast and ovary explants.33,34,35,36 Besides, ex vivo liver tissue slices were used to assess CRAd-mediated liver toxicity.6,35,37 However, we show for the first time that a CRAd such as AdF512v1 was also able to replicate in ovary cancer samples and metastatic tissue obtained from patients that underwent mainstay neoadjuvant chemotherapy that included paclitaxel and carboplatin to reduce the tumor mass and allow subsequent surgery. Microscopic examination confirmed no necrotic tissue in selected samples and high levels of cells viability. Few studies have shown that paclitaxel can enhance the in vitro adenoviral activity either by increasing E1A levels or the expression of cell surface receptors for the virus.38,39 More recently it was shown that CRAd treatment followed by paclitaxel triggered apoptotic cell death.40 Here, we show for the first time that a CRAd was able to replicate and lyse remnant cells that were resistant to mainstay chemotherapy indicating that viral therapy can be effective on cell clones that can override frontline chemotherapy.
Studies in nude mice with the nonreplicative version of AdF512v1 expressing the luciferase gene demonstrated that the main target organs were liver, spleen and gonads. Interestingly, necropsies of mice treated with AdF512v1 revealed no toxicity in liver, spleens and gonads. These data demonstrate that adenoviruses based on the chimeric 5/3 fiber exhibit a narrow spectrum of target organs and are less toxic than adenovirus type 5, as this serotype can infect at high levels lung, kidney, and prostate and was extremely toxic to the liver in animal models.41 Besides, AdF512v1was retained at the tumor mass and infiltrated gonads with greater avidity compared to liver, spleen, and non-infiltrated gonads suggesting that the tumor niche might create a favorable environment for AdF512v1 replication. In this context it must be pointed out that E4 levels in the tumor mass are underestimated due (i) to the fact that the study was performed after only one single administration of the virus and (ii) since we were unable to quantify viral particles released by the tumor mass to the peritoneum.42
Increasing evidence indicates the importance of stromal cells (such as fibroblasts and endothelial cells) in early relapse and overall survival in ovary cancer.13 Ovary cancer patients with a high stromal molecular signature had the poorest survival;7,13 moreover, collagen levels have been linked to chemotherapy resistant in ovary cancer.14,43 Ad-F512 was originally designed to target mainly, but not only, the stromal fibroblasts and endothelial cells with the understanding that both the stromal and malignant epithelium compartments should be eliminated to achieve complete remission of tumors such as ovary cancer, with large desmoplastic reaction. Since AdF512v1 replicated in WI-38 fetal fibroblasts and HMEC-1 microendothelial cells, we also evaluated viral replication in vitro on coplated malignant and stromal cells, and ex vivo and in vivo on explants and tumors, respectively, composed of a mix of malignant and stromal cells. Interestingly, the virus replicated (as assessed by E4 copy levels) at a better extent in the combined malignant/stromal tumors than in tumors composed of malignant cells alone, and greatly reduced the in vivo tumor growth of tumors composed of malignant and stromal cells suggesting overall that stromal cells might enhance viral efficacy by supporting viral replication and spreading. Moreover, we have previously shown that stromal cells can enhance viral efficacy not only because they can act as a viral reservoir but also because they can release factors that promote cell entry in a cell cycle phase that is more permissive to viral lytic activity.22 Thus, assessing CRAds activity in mixed tumors composed of malignant and stromal cells and in human explants might be essential to predict the therapeutic potential of oncolytic adenoviruses.
Materials and Methods
Cell lines and cell culture. All the cells used in this work are described in the Supplementary Materials and Methods section.
Construction and production of adenoviruses. Adenoviruses were developed as described in the Supplementary Materials and Methods section by using homologous recombination in bacteria. Viral constructs were confirmed by restriction pattern and automatic DNA sequencing (ABI PRISM 377 DNA Sequencer; Applied Biosystems, Foster City, CA).
Luciferase assays. 7 × 104 cells /well (seeded in 24-well plates) were transduced with Ad-SV40(Luc 5), Ad-SV40(Luc 5/3), Ad-F512(Luc 5), or Ad-F512(Luc 5/3), at 25 × 106 v.p./ml in 200 µl of 2% DMEM/F12. Luciferase expression was assessed as described.22
Preparation and use of human tissue explants. Human primary ovarian tumor samples were obtained from the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL and from the Hospital Municipal de Oncología Marie Curie, Buenos Aires, Argentina. Institutional review board approval was obtained at the time of initial debulking from patients with histologically confirmed ovarian adenocarcinoma or nonmalignant ovary samples. The declaration of Helsinki protocols were followed and patients gave written informed consent. Samples were kept on ice in University of Wisconsin (UW) solution (ViaSpan; Barr Laboratories, Pomona, NY) until slicing or alternatively in RPMI medium (Invitrogen, Carlsbad, CA). Time from harvest to slicing was kept at an absolute minimum (<2 hours). The Krumdieck tissue slicing system (Alabama Research and Development, Birmingham, AL) was used in accordance with the manufacturer's instructions and previously published techniques;35 in few cases samples were sliced manually. Viral infections were performed with a MOI of 500 in 500 µl of 2% vol/vol FCS RPMI with 1% antibiotics, 1% L-glutamine into 24-well plates.35 Infections were allowed to proceed for 5 hours (E4 assay) or 24 hours (luciferase assay), and then the medium was removed and replaced with 10% vol/vol FCS RPMI.
Luciferase assays: Infected tissue slices were placed in cell culture lysis buffer (Promega, Madison, WI) with beads and homogenized with an ultra sonicator (Fisher Scientific Model 100, Pittsburgh, PA) at a setting of 15 watts for 10 seconds. The homogenate was centrifuged to pellet the debris, and luciferase activity was measured as described.22 Experiments were performed in triplicate. Protein concentration of the tissue homogenates was determined using a Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA) to allow normalization of luciferase expression.
Assessment of virus replication: DNA purification from infected tissue slices and qPCR for E4 was performed as described44 with slight differences since we selected 72 hours as the end of the experiment because we observed no decrease in explants viability up to 96 hours in culture (data not shown). Briefly, DNA was purified with the DNeasy Tissue kit (Qiagen, Santa Clarita, CA) or Genomic DNA extraction kit tissue (Real Genomics, RBC, Taiwan). The primers used for amplification of the E4 were forward E4Fuab (Supplementary Table S4) and reverse E4Ruab (Supplementary Table S4) and detected with a E4 probe (Supplementary Table S4).45 Negative controls without template were performed for each reaction series, and an internal control (human β-actin) or total DNA were used to normalize the copy number for the E4 gene. Comparison of replication rates of different treatment groups were performed with a Student's t-test.
In vitro cytotoxicity assay. For determination of virus-mediated cytotoxicity, 1 × 104 cells were seeded in 24-well tissue culture plates and infected with the CRAds at indicated titers.22 After 6 days, cell viability was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay; Promega).
Ex vivo replication of CRAds in tumors obtained from nude mice. Five to six-weeks-old female athymic N:NIH(S)-nu mice (obtained from Instituto Leloir Facility or from the animal facility of the Faculty of Veterinary, University of La Plata, Argentina) were subcutaneous injected in one flank either with 3 × 106 SKOV3-luc cells or 4 × 106 PA-1 cells to produce an homogeneous ovary tumor or a mix of 3 × 106 or 4 × 106 SKOV3-luc or PA-1 cells respectively, mixed with 1 × 106 HMEC-1 and 1 × 106 WI-38 fibroblasts in 200 µl of PBS to produce an heterogeneous tumors made of malignant and stromal cells. When the average tumor volume reached 500 mm3, mice were sacrificed and the tumors were sliced and infected in vitro as described previously for patient samples.
In vivo studies. The i.p. tumors were established by injecting 3 × 106 SKOV3-luc cells/200 µl into female nude mice (n = 4–5 /group). On day 7 when the tumor is already established (Supplementary Figure S10), 5 × 1010 v.p. of AdF512v1 or vehicle were injected i.p. in 400 µl of PBS. Imaging was performed before treatment on day -1 and then at days 9, 23, and 30 after adenovirus injection with anesthetized animals injected with 150 mg/kg of D-luciferin i.p. After 10 minutes, the bioluminescent images were collected with a CCD, using the IVIS Imaging System (Xenogen, Alameda, CA), with the field of view set at 25-cm height. The photographic images used a 0.2-second exposure, 8 f/stop, 2 binning (resolution), and open filter. The bioluminescent and gray-scale images were overlaid using LivingImage software (Xenogen). Regions of interest were drawn around the i. p tumors, and the total counts (photons) were summed in the entire tumor areas. None of the mice showed signs of wasting or other visible indications of toxicity and all animals under study received food and water ad-libitum. For studies on viral distribution, 5–6-weeks-old athymic N:NIH(S)-nu female mice were injected i.p. with 5 × 1010 v.p./400 µl of Ad-F512(Luc 5/3). After 48 hours mice were sacrificed and livers, spleens, gonads, kidneys, lungs, stomachs, hearts, and intestines were harvested. Organ sections were used to evaluate the luciferase activity as we described for tumor samples. To establish viral clearance 5–6-weeks-old athymic N:NIH(S)-nu female mice were injected i.p. with 3 × 106 SKOV3-luc cells /200 µl. Ten days later mice were injected once with AdF512v1 (5 × 1010 v.p./400 µl); groups of 2–3 mice were sacrificed at the indicated times for tissue isolation. E4 levels were assessed as describe above.
Five to six-weeks-old female nude mice were subcutaneous injected either with tumor cells alone or with a mix of tumor cells and stromal cells (SKOV3-luc (3 × 106); WI-38 (1 × 106); HMEC-1 (1 × 106)). The in vivo treatment started when the average tumor volume reached 100 mm3; mice were randomly separated in groups that received three intratumoral injections of 1 × 1010 viral particles/mouse of either Ad-F512 or AdF512v1 on days 0, 3, and 7. Tumor volumes were estimated weekly from caliper measurements (volume = 0.5 × (width)2 × length). Mice were sacrificed when tumors reached an average of 2,500 mm3. None of the mice showed signs of wasting or other visible indications of toxicity.
Ethics statement. All experiments were approved by the Institutional Animal Care and Use Committee of the Fundación Instituto Leloir (Protocol #30OP). The Fundación Instituto Leloir has an approved Animal Welfare Assurance as a foreign institution with the Office of Laboratory Animal Welfare, Number A5168-01.
SPARC mRNA quantification. Quantification was performed as previously described22.
SUPPLEMENTARY MATERIAL Figure S1. Flow cytometer analysis of ovary cancer cells after infection with Ad-wt or AdF512v1. Figure S2. SPARC immunostaining and luciferase assay in patient samples. Figure S3. Immunoprecipitation of E1A/host protein complexes. Figure S4. In vivo bioluminescence assays following i.p. treatment. Figure S5. In vivo bioluminescence assays following i.v. treatment. Figure S6. Viability of stromal cells after infection with different viruses. Figure S7. In vitro lysis of cells following previous infection with AdF512v1. Figure S8. Ex vivo replication of AdF512v1 in tumor xenografts composed by malignant (PA-1) and stromal human cells. Figure S9. Survival curve of mice-harboring subcutaneous tumors (malignant + stromal cells) treated with the different viruses or PBS. Figure S10. Intraperitoneal tumor at day 7. Table S1. Relative expression of SPARC mRNA levels in ovary cancer cell lines. Table S2. Analysis of CRAd performance in human tissue slices. Table S3. Luciferase expression of SKOV3-luc cells following coculture with stromal cells previously infected or not with AdF512v1. Table S4. Specific primers used in this work. Materials and Methods.
Acknowledgments
This work was supported by grants from the National Agency for Promotion of Science and Technology (Anpcyt) Argentina, Fundación Bunge y Born, Argentina, the Programa Bicentenario—BancoMundial, Conicyt, Chile CTE-06 and grant number 5P50CA101955-07. We are indebted to the continuous support of Amigos de la Fundacion Instituto Leloir para la Investigación en Cancer (AFULIC) Foundation, Argentina. We acknowledge the technical support of Florencia Straminsky and Cecilia Rotondaro from Fundación Instituto Leloir and Christine Dorvault from the Pathology Department at the University of Alabama at Birmingham, AL. This work was done in Ciudad Autónoma de Buenos Aires, Argentina and Birmingham, AL.
Supplementary Material
Flow cytometer analysis of ovary cancer cells after infection with Ad-wt or AdF512v1.
SPARC immunostaining and luciferase assay in patient samples.
Immunoprecipitation of E1A/host protein complexes.
In vivo bioluminescence assays following i.p. treatment.
In vivo bioluminescence assays following i.v. treatment.
Viability of stromal cells after infection with different viruses.
In vitro lysis of cells following previous infection with AdF512v1.
Ex vivo replication of AdF512v1 in tumor xenografts composed by malignant (PA-1) and stromal human cells.
Survival curve of mice-harboring subcutaneous tumors (malignant + stromal cells) treated with the different viruses or PBS.
Intraperitoneal tumor at day 7.
Relative expression of SPARC mRNA levels in ovary cancer cell lines.
Analysis of CRAd performance in human tissue slices.
Luciferase expression of SKOV3-luc cells following coculture with stromal cells previously infected or not with AdF512v1.
Specific primers used in this work.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T.et al. (2008Cancer statistics, 2008 CA Cancer J Clin 5871–96. [DOI] [PubMed] [Google Scholar]
- Matthews KS, Alvarez RD., and, Curiel DT. Advancements in adenoviral based virotherapy for ovarian cancer. Adv Drug Deliv Rev. 2009;61:836–841. doi: 10.1016/j.addr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Riman T, Nilsson S., and, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstet Gynecol Scand. 2004;83:783–795. doi: 10.1111/j.0001-6349.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Lam JT, Kanerva A, Bauerschmitz GJ, Takayama K, Suzuki K, Yamamoto M.et al. (2004Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy J Gene Med 61333–1342. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Lu B, Park M, Makhija SK, Numnum TM, Kendrick JE.et al. (2008Development of an optimized conditionally replicative adenoviral agent for ovarian cancer Int J Oncol 321179–1188. [DOI] [PubMed] [Google Scholar]
- Spentzos D, Levine DA, Ramoni MF, Joseph M, Gu X, Boyd J.et al. (2004Gene expression signature with independent prognostic significance in epithelial ovarian cancer J Clin Oncol 224700–4710. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Bauerschmitz GJ, Yamamoto M, Lam JT, Alvarez RD, Siegal GP.et al. (2004A cyclooxygenase-2 promoter-based conditionally replicating adenovirus with enhanced infectivity for treatment of ovarian adenocarcinoma Gene Ther 11552–559. [DOI] [PubMed] [Google Scholar]
- Rein DT, Breidenbach M, Kirby TO, Han T, Siegal GP, Bauerschmitz GJ.et al. (2005A fiber-modified, secretory leukoprotease inhibitor promoter-based conditionally replicating adenovirus for treatment of ovarian cancer Clin Cancer Res 111327–1335. [PubMed] [Google Scholar]
- Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z.et al. (2012A Phase I Clinical Trial of Ad5.SSTR/TK.RGD, a Novel Infectivity-Enhanced Bicistronic Adenovirus, in Patients with Recurrent Gynecologic Cancer Clin Cancer Res 183440–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S.et al. (2002Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer J Clin Oncol 201562–1569. [DOI] [PubMed] [Google Scholar]
- Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W.et al. (2010A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases Clin Cancer Res 165277–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Australian Ovarian Cancer Study Group et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- Santala M, Simojoki M, Risteli J, Risteli L., and, Kauppila A. Type I and III collagen metabolites as predictors of clinical outcome in epithelial ovarian cancer. Clin Cancer Res. 1999;5:4091–4096. [PubMed] [Google Scholar]
- Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E., and, Llera AS. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:691–705. doi: 10.1007/s10555-008-9146-7. [DOI] [PubMed] [Google Scholar]
- Chlenski A., and, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Paley PJ, Goff BA, Gown AM, Greer BE., and, Sage EH. Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol Oncol. 2000;78 3 Pt 1:336–341. doi: 10.1006/gyno.2000.5894. [DOI] [PubMed] [Google Scholar]
- Köbel M, Turbin D, Kalloger SE, Gao D, Huntsman DG., and, Gilks CB. Biomarker expression in pelvic high-grade serous carcinoma: comparison of ovarian and omental sites. Int J Gynecol Pathol. 2011;30:366–371. doi: 10.1097/PGP.0b013e31820d20ba. [DOI] [PubMed] [Google Scholar]
- Porter PL, Sage EH, Lane TF, Funk SE., and, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Shaw PA, Karp X, Huynh MH, Begley H., and, Ringuette MJ. Activation of SPARC expression in reactive stroma associated with human epithelial ovarian cancer. Gynecol Oncol. 1999;75:25–33. doi: 10.1006/gyno.1999.5552. [DOI] [PubMed] [Google Scholar]
- Socha MJ, Said N, Dai Y, Kwong J, Ramalingam P, Trieu V.et al. (2009Aberrant promoter methylation of SPARC in ovarian cancer Neoplasia 11126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MV, Viale DL, Cafferata EG, Bravo AI, Carbone C, Gould D.et al. (2009Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses PLoS ONE 4e5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J., and, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Brewer M, Zou C, Hernandez MA, Daley M, Ozols R.et al. (2007Prevention and early detection of ovarian cancer: mission impossible Recent Results Cancer Res 17491–100. [DOI] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA.et al. (2010Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer Cancer Res 70875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T.et al. (2011Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14 Nat Med 1796–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ.et al. (2002Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells Clin Cancer Res 8275–280. [PubMed] [Google Scholar]
- Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM.et al. (2002Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses Mol Ther 5695–704. [DOI] [PubMed] [Google Scholar]
- Hemminki O, Bauerschmitz G, Hemmi S, Lavilla-Alonso S, Diaconu I, Guse K.et al. (2011Oncolytic adenovirus based on serotype 3 Cancer Gene Ther 18288–296. [DOI] [PubMed] [Google Scholar]
- Ulasov IV, Tyler MA, Rivera AA, Nettlebeck DM, Douglas JT., and, Lesniak MS. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–1603. doi: 10.1002/jmv.00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A.et al. (2000An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy Nat Med 61134–1139. [DOI] [PubMed] [Google Scholar]
- Sauthoff H, Pipiya T, Heitner S, Chen S, Bleck B, Reibman J.et al. (2004Impact of E1a modifications on tumor-selective adenoviral replication and toxicity Mol Ther 10749–757. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Stoff A, Rivera AA, Mathis JM, Everts M, Wang M.et al. (2005Gene transfer to carcinoma of the breast with fiber-modified adenoviral vectors in a tissue slice model system Cancer Biol Ther 41203–1210. [DOI] [PubMed] [Google Scholar]
- Rocconi RP, Zhu ZB, Stoff-Khalili M, Rivera AA, Lu B, Wang M.et al. (2007Treatment of ovarian cancer with a novel dual targeted conditionally replicative adenovirus (CRAd) Gynecol Oncol 105113–121. [DOI] [PubMed] [Google Scholar]
- Kirby TO, Rivera A, Rein D, Wang M, Ulasov I, Breidenbach M.et al. (2004A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity Clin Cancer Res 108697–8703. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Stoff A, Michael Mathis J, Rocconi RP, Matthews QL.et al. (2007Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer Int J Cancer 120935–941. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Le LP, Stoff A, Everts M, Contreras JL.et al. (2006Employment of liver tissue slice analysis to assay hepatotoxicity linked to replicative and nonreplicative adenoviral agents Cancer Gene Ther 13606–618. [DOI] [PubMed] [Google Scholar]
- AbouEl Hassan MA, Braam SR., and, Kruyt FA. Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer Gene Ther. 2006;13:1105–1114. doi: 10.1038/sj.cgt.7700984. [DOI] [PubMed] [Google Scholar]
- Seidman MA, Hogan SM, Wendland RL, Worgall S, Crystal RG., and, Leopold PL. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol Ther. 2001;4:13–21. doi: 10.1006/mthe.2001.0414. [DOI] [PubMed] [Google Scholar]
- Ingemarsdotter CK, Baird SK, Connell CM, öberg D, Halldén G., and, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene. 2010;29:6051–6063. doi: 10.1038/onc.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Huyn S, Burton J, Sato M., and, Wu L. Differential biodistribution of adenoviral vector in vivo as monitored by bioluminescence imaging and quantitative polymerase chain reaction. Hum Gene Ther. 2006;17:1262–1269. doi: 10.1089/hum.2006.17.1262. [DOI] [PubMed] [Google Scholar]
- Merron A, Baril P, Martin-Duque P, de la Vieja A, Tran L, Briat A.et al. (2010Assessment of the Na/I symporter as a reporter gene to visualize oncolytic adenovirus propagation in peritoneal tumours Eur J Nucl Med Mol Imaging 371377–1385. [DOI] [PubMed] [Google Scholar]
- Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR.et al. (2003Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells Cancer Cell 3377–386. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG.et al. (2003Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus Mol Ther 8449–458. [DOI] [PubMed] [Google Scholar]
- Yang SW, Cody JJ, Rivera AA, Waehler R, Wang M, Kimball KJ.et al. (2011Conditionally replicating adenovirus expressing TIMP2 for ovarian cancer therapy Clin Cancer Res 17538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometer analysis of ovary cancer cells after infection with Ad-wt or AdF512v1.
SPARC immunostaining and luciferase assay in patient samples.
Immunoprecipitation of E1A/host protein complexes.
In vivo bioluminescence assays following i.p. treatment.
In vivo bioluminescence assays following i.v. treatment.
Viability of stromal cells after infection with different viruses.
In vitro lysis of cells following previous infection with AdF512v1.
Ex vivo replication of AdF512v1 in tumor xenografts composed by malignant (PA-1) and stromal human cells.
Survival curve of mice-harboring subcutaneous tumors (malignant + stromal cells) treated with the different viruses or PBS.
Intraperitoneal tumor at day 7.
Relative expression of SPARC mRNA levels in ovary cancer cell lines.
Analysis of CRAd performance in human tissue slices.
Luciferase expression of SKOV3-luc cells following coculture with stromal cells previously infected or not with AdF512v1.
Specific primers used in this work.