Abstract
Lentiviral vector (LV)-mediated gene transfer is a promising method of gene therapy. We previously reported that systemic injection of HIV-based LV triggers a transient inflammatory response. Here, we carried out studies to better characterize this response, and to develop a strategy to overcome the adverse effects of interferon (IFN) on LV-mediated gene transfer. We profiled gene expression in the liver after LV administration using deep-sequencing (RNA-seq), and identified several innate response pathways. We examined the response to LV in MyD88-TRIF knockout mice, which are incapable of toll-like receptor (TLR) signaling. Unexpectedly, the IFN response to LV was not reduced in the liver indicating that a non-TLR pathway can recognize LV in this organ. Indeed, blocking reverse transcription with azidothymidine (AZT) reduced the IFN response only in the liver, suggesting that proviral DNA can be a trigger. To block the inflammatory response, we pretreated mice with a short course of dexamethasone (Dex). At 4 hours post-treatment, all the IFN-induced genes were normalized. By blocking the inflammatory response, hepatocyte transduction was dramatically increased, which in turn doubled the level of human factor IX (FIX) produced by a hepatocyte-specific LV. Our studies uncover new insights into LV-induced immune responses in the liver, and provide a means to increase the safety and efficiency of LV-mediated gene transfer.
Introduction
Lentiviral vectors (LVs) are a promising tool for therapeutic gene transfer. At least three clinical trials have utilized LVs in humans to treat diseases ranging from cancer to HIV infection, and clinical benefits without adverse events from vector integration have been reported.1,2,3 We and others have begun to utilize LV to transfer genes to the liver for gene replacement therapy, and have shown that phenotypic correction can be achieved in animal models of hemophilia, Crigler-Najjar syndrome, and mucopolysaccharidosis type IIIA.4,5,6,7,8
Initial attempts to use LV to deliver a therapeutic transgene in vivo were limited by an immune response against the transgene product, and clearance of transduced cells.4,9 However, engineering the vector to avoid expression in professional antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages, provides a means to overcome the transgene-specific immune response.4,5,6,8 Interestingly, de-targeting transgene expression from hepatocytes, in addition to APCs, restores the adaptive immune response against the transgene.10 This highlights the potential of hepatocytes to promote immunological tolerance, and provides for the possibility of using the liver as a target for vaccines aimed at inducing tolerance instead of immunity. Indeed, a recent study utilized an integrase-defective LV (IDLV) to transiently express an antigen in the liver, and induce antigen-specific regulatory T cells.11 This type of strategy might one day be used for preventing autoimmune diseases.
Although the adaptive immune response can be avoided by targeting vector expression to hepatocytes, and the risk of insertional mutagenesis can be eliminated by using IDLV, one concern with in vivo administration of LV is the induction of an inflammatory response. In a previous study, we reported that LV can trigger a transient type I interferon (IFN) response in the liver of mice following intravenous injection.12 In general, viral induction of IFN occurs as a result of germline-encoded pattern recognition receptors (PRRs) being triggered by specific structures associated with pathogens, such as toll-like receptor (TLR)3, which recognizes double-stranded RNA.13 In vitro studies have suggested innate recognition of LV, as well as its parent virus HIV, a single-stranded RNA (ssRNA) virus, occurs as a result of triggering TLR7, a PRR for ssRNA. Further in vitro analysis indicated that plasmacytoid DCs (pDC), which constitutively express TLR7, but not conventional DCs, are activated by HIV and LV, implying that pDCs are the primary source of IFN in response to LV.12,14,15 However, the relevance of this mechanism in vivo has not been examined.
Here, we set out to better characterize the innate reaction to LV, and to evaluate a means for preventing this response. Our results indicate that TLR3 is also involved in recognizing LV in vivo, and that an additional sensor is capable of recognizing reverse-transcribed proviral DNA in the liver. We also show that glucocorticoids (GCs) can ameliorate the innate response to LV, and specifically improve hepatocyte transduction. These findings provide a crucial advancement for moving LV and IDLV toward the clinic for liver-directed therapeutic gene transfer and vaccination.
Results
Transcriptome analysis details the innate response to LV in the liver
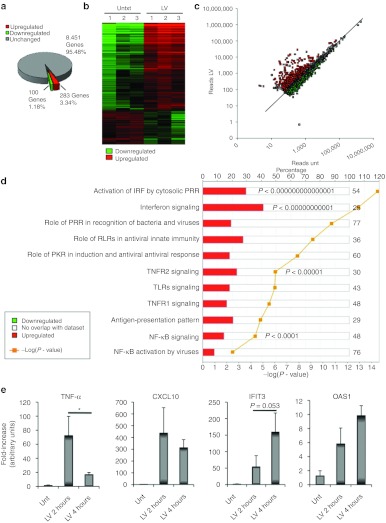
To determine the liver's response to LV, we injected mice intravenously with 3 × 108 transducing units (TU) of vector, or with saline, and performed RNA deep-sequencing (RNA-seq) on the liver after 4 hours. We obtained 12,000,000 100-nucleotide reads per sample, which were mapped to the mouse genome, and used to quantitate mRNA expression. We detected expression of more than 8,000 genes, of which only 383 genes were differentially expressed between LV and saline-injected mice. In LV-treated mice, 283 genes were significantly upregulated, and 100 genes were downregulated (Figure 1a,b and Supplementary Figure S1a). The downregulated genes were mostly associated with lipid metabolism, such as Ppargc1b, Lpin1, Fbpa1, and none of them were decreased by more than fourfold (Supplementary Figure S1b).
Figure 1.
RNA-seq reveals the effects of LV on the liver. Mice (n = 3) were intravenously injected with 3 × 108 TU of LV.PGK.GFP or saline, and RNA was isolated from the liver after 4 hours. (a) Percent of hepatic genes affected by LV injection. Average of three biological replicates is shown. (b) Cluster analysis of each treated mouse based on differential gene expression. A heat-map representation of those genes that were significantly changed by LV administration. Each column is for an individual mouse. The intensity of the color corresponds to the level of difference. (c) Comparison of hepatic gene expression between untreated and LV-treated mice. The mean number of reads for each treatment group (n = 3) was used. Black line shows an equal number of reads in both groups. Green dots represent statistically downregulated genes and red dots represent statistically upregulated genes. Gene expression was considered different when P < 0.05. (d) Analysis of molecular pathways affected by LV in the liver. Analysis was performed by Ingenuity by using Fisher's exact test, Benjamini-Hochberg correction for multiple testing. Red color represents percentage of upregulated genes within each pathway. Yellow line is the −log(P value). (e) The expression of Tnfα, Cxcl10, Ifit3, and Oas1 was determined in the liver 2 and 4 hours after the LV injection. Results are mean ± SD (n = 3–6 mice per group), all untreated mice (2 and 4 hours) are shown in the same group. *P < 0.05, LV-treated at time point 2 versus 4 hours after injection. IFN, interferon; IRF, interferon regulatory factor; LV, lentiviral vector; NF-κB, nuclear factor-κB; PRR, pattern recognition receptor; RLR, (RIG)-I–like receptor; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α TNFR, tumor necrosis factor receptor; TU, transducing unit; unt, untreated.
In contrast, 64% of the upregulated genes were increased by fourfold or more, and some were upregulated >100-fold (Figure 1a–c and Supplementary Figure S1c). Network analysis indicated PRR-mediated IFN regulatory factor and IFN-signaling pathways were the most significantly upregulated pathways (P < 10−14 and P < 10−11, respectively, Figure 1d). Among the upregulated IFN-induced genes were Mx1 and Mx2, and Oas and Ifit family genes (Supplementary Figure S1c). Many of the IFN-induced GBP GTPases (Gbp 2, 3, 4, 5, 6) and the IFN-induced p47 GTPAses (Iigp1 and Igtp) were also upregulated. In addition, other IFN-responsive genes were increased, such as some of the p200 family members (Ifi204 and Ifi203). Furthermore, the expression of a variety of key transcription factors involved in the signaling pathway of the IFN response was enhanced, including Stat1, Stat2, and Stat3 and the IFN regulatory factors Irf1, Irf7, and Irf9. Surprisingly, only three chemokines were increased after the LV injection. These were Ccl2 (MCP1), Cxcl10 (IP-10), and Cxcl9, whereas the commonly IFN-induced chemokine Ccl5 (Rantes) was not altered. Some other IFN-responsive genes were not increased, such as Ifitm1, Ifitm2 and Ifitm3, Ifi202 and Gip2 and Gip3.
The expression of genes that function in the recognition of pathogens were also increased, namely, Tlr2 and Tlr3, all three members of the cytoplasmic viral RNA sensor retinoic acid-inducible gene (RIG)-I–like receptors, RIG-I (Ddx58), Lgp2, and Mda5 and its homolog Ddx60, and the cytoplasmic sensor of the NOD-like receptor family (NLR) Nod1. Although the expression of Tlr7, Tlr8, and Tlr9 was not upregulated, expression of their downstream signaling molecule Myd88 was increased almost fourfold. The expression of Trif and Irf3, two molecules that act downstream of TLR3 signaling, was unchanged. Regarding the cytoplasmic sensors, the NOD1 effector molecule Ripk2 was also upregulated. This molecule is a kinase that activates both nuclear factor κB (NFκB) and MAPK (mitogen-activated protein kinase) pathways.16 Double-stranded DNA viruses such as adenoviruses trigger a sensor NLRP3-inflammasome–dependent response.17 However, here none of these inflammasome molecules including Asc (Pycard), Aim2, caspase1, or Nlrp3 were increased. However, Trex1, an exonuclease that has been shown to have a role in innate recognition of cytoplasmic DNA,18,19 was upregulated by sevenfold in response to vector injection.
Pathway analysis also indicated the NFκB pathway was upregulated (P < 0.0001). The expression of both Nfkb1 and Nfkb2 genes was increased after LV injection as well as the expression of the inhibitor of NFκB kinase subunit epsilon (Ikbke), which is a noncanonical I-κB kinase that activates NFκB.20 The overexpression of these receptors and signaling molecules may result from recruitment of inflammatory cells to the liver; however, hematoxylin and eosin staining did not reveal inflammatory infiltrates (Supplementary Figure S1d), suggesting that enhanced expression of the NFκB responsive genes was occurring in organ resident cells.
Despite increased expression of NFκB, there was no detectable increase in the expression of many NFκB-induced inflammatory cytokines, such as Mip-1α (Ccl3), Mip-1β (Ccl4), Il-8 (Cxcl8), Il-1b, Il-6, and Tnfα. Although Tnfα was not detected, three members of the tumor necrosis factor (TNF) superfamily were upregulated, Tnfrsf14, Tnfsf10 (TRAIL), and Tnfaip3 (also known as A20). Tnfrsf14 is a receptor that activates immune response pathways21 and Tnfsf10 is a proapoptotic TNF-ligand family member.22 Interestingly, Tnfaip3 is an anti-inflammatory cytoplasmic protein whose expression is induced by TNF-α.23 Accordingly, TNFR1 and TNFR2 signaling pathways were upregulated (P < 10−5, Figure 1d), suggesting TNF-α was present earlier than 4 hours after LV injection. To address whether A20 overexpression could be due to increased TNF-α production at an earlier time point, mice were treated with either saline or LV and liver was collected 2 hours later. Indeed, TNF-α expression was significantly higher at 2 hours compared with 4 hours (Figure 1e). Several IFN-induced genes were also measured, and no differences were observed between either time point.
Finally, we also found that a number of factors known to be involved in restricting HIV infection24 were also upregulated, including members of the tripartite motif containing (Trim) family, such as Trim 21, 34, 12 and 30.25 SamHD1, which was recently identified as the gene responsible for blocking HIV infection of DCs,26 was also induced in the liver upon LV administration. However, the significance of these genes to LV/HIV infection in mice is unknown.
LV can activate the innate response in the liver through a TLR-independent mechanism
A number of in vitro studies have shown that LV activates pDCs by triggering TLR7 (ssRNA sensor).14,27,28 With the exception of TLR3, all TLRs require MyD88 for intracellular signaling. Thus, to analyze whether the response that we found to LV in vivo was TLR-mediated, we injected LV intravenously into Myd88−/− mice, and measured the expression of several IFN-inducible genes compared with untreated wild-type (WT) mice, and WT mice that received the same dose of LV (Figure 2a). In the spleen, there was a decrease in the expression of Oas1 and Cxcl10 in Myd88−/− mice compared with the injected WT mice. Surprisingly, induction of the IFN-responsive genes was not altered in the liver of Myd88−/− (Figure 2a). This indicated that while MyD88-dependent TLRs play an important role in sensing LV in the spleen, other innate sensors are being activated by LV in the liver. The double-stranded RNA sensor TLR3 signals through TRIF and does not require MyD88. To determine whether TLR3 was responsible for sensing LV in the liver, we treated Myd88−/−Trif−/− mice with LV (Figure 2b). The expression of all the tested cytokines was decreased in the spleens of these mice when compared with WT-injected mice, indicating that the TRIF pathway can play a complementary role to the MyD88 pathway in lymphoid tissue. Interestingly, no difference was observed between Myd88−/−Trif−/− and WT mice in the response within the liver (Figure 2b), which further indicated that a non-TLR–mediated pathway was involved in sensing LV in the liver.
Figure 2.
LV-induced IFN response in the liver is triggered by a TLR-independent pathway. The expression of OAS1, CXCL10, and ISG15 was determined by q-PCR in both the spleen and liver 4 hours after intravenous injection of LV.PGK.GFP (3 × 108 TU) in: (a) MyD88−/− mice (n = 3). (b) MyD88-TRIF−/− mice (n = 3). (c) RIG-I−/− mice (n = 3). All samples are presented as the fold-increase compared with the corresponding untreated controls. Results are the mean ± SD. *P < 0.05 versus LV-treated control mice. (d) AZT-treated mice. The retrotranscription inhibitor AZT was intraperitoneally injected (1 mg/kg) 20 minutes before an intravenous injection of LV (3 × 108 TU). Results are presented as the mean ± SD (n = 4). WT mice refers to littermate controls and were thus age, gender, and weight matched. Mice were not age or weight matched between the experiments done with different knockouts (i.e., between a, b, c, and d). In the case of the RIG-I knockout mice, WT mice are on a CD1/B6/129 background, whereas the MyD88 and MyD88TRIF knockout mice are on the C57Bl/6 background. *P < 0.05 versus LV-treated mice. AZT, azidothymidine; IFN, interferon; LV, lentiviral vector; q-PCR, quantitative-PCR; TLR, toll-like receptor; TU, transducing unit; unt, untreated; WT, wild-type.
The LV proviral DNA is a trigger for the innate response in vivo
The existence of a non-TLR–mediated recognition pathway in the liver prompted us to investigate whether an intracellular sensor may be recognizing LV. RIG-I is a cytoplasmic RNA helicase that induces IFN in response to specific types of viral RNAs. To assess whether RIG-I is triggered by LV, we injected RIG-I–deficient mice intravenously with the vector, and monitored the IFN response at 4 hours. Similar to WT mice, there was a similar upregulation of OAS1, CXCL10, and ISG15, in RIG-I–deficient mice treated with LV both in liver and spleen compared to control littermates; indicating that RIG-I is not necessary for triggering the innate response to LV (Figure 2c).
Recently, the existence of a cytoplasmic sensor of viral DNA was suggested.18 Although LV is an RNA virus, its infection is dependent on reverse transcription into proviral DNA in the cytoplasm before genomic integration. To test whether the vector provirus can trigger an IFN response, we treated mice with 1 mg/kg of azidothymidine (AZT), a nucleoside analog that blocks reverse transcription, immediately before injection with LV. Although the inflammatory response in the spleen was not altered by this treatment, AZT was able to significantly decrease the induction of proinflammatory cytokine expression in response to LV in the liver (Figure 2d). These results suggest that the vector's proviral DNA is one of the triggers of the innate response in vivo.
We further characterized the response to LV in the liver by measuring the total number of leukocytes (CD45+ cells), macrophages (CD11b+ MHCII+), and neutrophils (Gr1+ CD11b+) 1 day after LV injection (Supplementary Figure S2a). We observed an increase in neutrophil numbers and a slight increase in macrophages, which was not significant (P = 0.07). No major differences were found in total leukocyte numbers. AZT treatment did not prevent the neutrophil infiltration. This was not unexpected since there appears to be multiple, distinct mechanisms of sensing LV (i.e., by TLR and non-TLR mechanisms), and thus blocking one recognition pathway is not sufficient to completely prevent the response to LV.
Macrophages and DCs detect LV through different pathways
To date, the majority of studies have focused on the response of DCs to HIV and LV infection. However, macrophages express many innate PRRs, and are a natural target of HIV infection.29 Moreover, macrophages are very abundant in the liver where they are efficiently infected by LV after systemic injection.30,31
To understand how macrophages respond to LV infection, we generated macrophages from WT and Myd88−/− mice. As a comparison, we also generated DCs from these mice. The cells were treated with equivalent concentrations of vector (multiplicity of infection 100), and after 12 hours we measured the expression of IFN-inducible genes. WT DCs and macrophages were both capable of responding to the vector. However, Myd88−/− DCs had an impaired response compared with WT cells (Figure 3a). Interestingly, the response of Myd88−/− and WT macrophages was similar suggesting that TLR7 and TLR9 signaling are not essential for recognition of LV by macrophages (Figure 3b). Instead, when we treated macrophages from Trif−/− or Myd88−/−Trif−/− mice with LV, the IFN response was drastically reduced. These results indicate that unlike DCs, macrophages use TLR3 to sense HIV-based LV.
Figure 3.
Macrophages and dendritic cells (DCs) sense LV through different TLR-dependent pathways. (a) Evaluation of LVs impact on mouse DCs. DCs were derived from the bone marrow of wild-type (WT) and MyD88−/− mice. On day 7, LV was added to the culture (MOI = 100) and 12 hours later, the expression of IFIT3, CXCL10, and ISG15 was determined by q-PCR. Results are presented as the mean ± SD of the fold-increase compared with untreated mice (n = 4). (b) Evaluation of LVs impact on mouse macrophages. Macrophages were generated from the bone marrow of MyD88−/−, TRIF−/−, MyD88-TRIF−/−, and WT mice. Macrophages were treated with LV (MOI = 100) for 12 hours and OAS1, CXCL10, and ISG15 were measured by q-PCR. Results are shown as mean ± SD of fold-increase compared with untreated (n = 4). *P < 0.05 versus LV-treated WT cells. (c) Flow cytometry analysis of macrophages transduced with LV in the presence of AZT. Macrophages were generated from WT mice and were treated with LV (MOI = 100) or LV + AZT (10 ng/ml) for 12 hours. Flow cytometry analysis was performed 2 days later to assess transduction. Representative flow cytometry analysis plots showing CD11b-positive and GFP-positive cells in the indicated treatments. (d) Assessment of OAS1 and CXCL10 expression in AZT-treated macrophages transduced with LV. (e) Flow cytometry analysis of BM-derived DCs transduced with LV or LV + AZT (10 ng/ml) for 12 hours. Representative flow cytometry plots showing CD11c-positive and GFP-positive cells in the indicated treatments 2 days later. (f) Assessment of OAS1, CXCL10, and TREX1 expression in AZT-treated DCs transduced with LV. RNA was isolated from BM-macrophages and BM-DCs 12 hours after being treated with LV in conjunction with AZT. The expression of the indicated cytokines was measured by q-PCR. Results are shown as mean ± SD of the fold-increase compared with untreated WT cells (n = 4). *P < 0.05 versus LV (no AZT)-treated cells. AZT, azidothymidine; BM, bone marrow; GFP, green fluorescent protein; LV, lentiviral vector; MOI, multiplicity of infection; q-PCR, quantitative-PCR; TLR, toll-like receptor; Unt, untreated.
Our studies in vivo with AZT indicated that proviral DNA was partly responsible for inducing a type I IFN response in the liver, but not the spleen. To specifically determine whether macrophages and DCs can be activated by the vector's reverse-transcribed DNA, we treated WT macrophages and DCs with AZT at the time of transduction. As expected, AZT was able to greatly decrease transduction efficiency (Figure 3c,e), indicating that reverse transcription was prevented. However, the IFN-induced cytokine expression levels did not change by the addition of AZT (Figure 3d,f). Thus, it appears that TLR3 and TLR7/9 are the major innate sensors of LV in macrophages and DCs, respectively. Of note, the expression of Trex1 was upregulated in both macrophages and DCs by transduction with LV and in the presence or absence of AZT. Since Trex1 rapidly degrades proviral DNA18 and prevents the IFN response to LV, this may explain why this pathway is less relevant in macrophages and DCs.
GCs block the innate response to LV and increase hepatocyte transduction
The transient inflammatory response to LV is a challenge for the use of these vectors for in vivo gene therapy. Thus, we sought to find a clinically relevant means to prevent the response. Since there appears to be multiple, distinct recognition pathways for LV, we looked for a target involved in the effector phase of the response to the vector. We re-analyzed the gene expression signatures of LV-treated mice, and found that GCs, a class of anti-inflammatory steroids, can inhibit several of the pathways upregulated by LV (Supplementary Figure S3a).
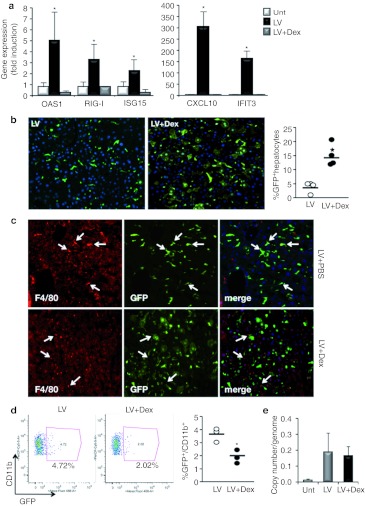
To test whether GCs could indeed avert the innate response to LV, we treated mice with the GC dexamethasone (Dex) at the time of vector administration. Although IFN-inducible genes were highly upregulated in LV-treated mice, mice that received LV + Dex showed a complete normalization of these genes in the liver (Figure 4a). Dex also reduced the inflammatory response in the spleen, although the reduction was not as complete as in the liver (Supplementary Figure S3b). Moreover, Dex treatment decreased the total number of neutrophils and macrophages in the liver compared with LV treatment alone (Supplementary Figure S2a). Although LV did not alter aspartate aminotransferase levels, it increased the serum levels of alanine aminotransferase and alkaline phosphatase (Supplementary Figure S2b–e). GC treatment was capable of partially normalizing serum levels of alkaline phosphatase and completely maintaining platelet numbers in the blood after LV injection (Supplementary Figure S2d,e).
Figure 4.
Glucocorticoids (GCs) block the innate response to LV and increase hepatocyte transduction. To evaluate the use of GCs on LV-mediated gene transfer, mice were pretreated with Dex (5 mg/kg) at 12 and 2 hours before LV injection and 4 hours later. (a) Assessment of the innate response to LV in the presence of GCs. At 4 hours postinjection, RNA was extracted from the liver and expression of OAS1, RIG-I, ISG15, IFIT3, and CXCL10 was measured. Results represent fold-increase compared with untreated mice and are the mean ± SD (n = 3 mice per group). *P < 0.05 t-test. (b) Analysis of LV transduction pattern in the liver. GFP immunostaining was used to determine the number of transduced hepatocytes 1 week after LV injection. The percent of transduced hepatocytes was enumerated by morphometrical analysis and shown on the right. *P <0.05. (c) Anti-F4/80 (red) was used to stain for macrophages, and anti-GFP was used to identify GFP-positive cells. White arrows indicate F4/80+ cells. (d) Flow cytometry analysis of liver non-parenchymal cells. The percentage of transduced CD11b-positive cells in the liver was determined by flow cytometry analysis. Representative dot plots are shown along with the percentages for each individual mouse. *P < 0.05. (e) Quantification of viral copies per mouse cell genome in the liver 1 week after LV injection. Samples were normalized by murine actin. Dex, dexamethasone; GFP, green fluorescent protein; LV, lentiviral vector; PBS, phosphate-buffered saline; unt, untreated.
We then assessed how blocking the inflammatory response with Dex affected LV transduction by analyzing green fluorescent protein (GFP) expression in the liver 1 week after vector injection. There was a striking difference in vector biodistribution between untreated and Dex-treated mice. In mice treated with LV alone, 4% of hepatocytes were transduced, whereas in mice treated with Dex, an average of 14% of hepatocytes were GFP-positive (Figure 4b). We confirmed these results with another LV that expressed GFP from a hepatocyte-specific promoter (Supplementary Figure S3c).
While Dex treatment improved hepatocyte transduction, Dex-treated mice actually had fewer transduced macrophages in the liver, as determined by immunostaining (Figure 4c) and flow cytometry analysis (Figure 4d). Quantitation of vector copy number in the liver showed that there was no difference between mice that were treated with LV in the presence or absence of Dex (Figure 4e). Since there was no net gain in vector content within the liver, but the cellular distribution of transduced cells changed, this indicates that Dex does not merely increase hepatocyte transduction, but actually increases hepatocyte transduction at the expense of macrophage transduction. The transduction of macrophages and other hematopoietic cells were unchanged in the spleen (Supplementary Figure S4a–c).
Improvement of factor IX and IDLV gene transfer by short-course steroids
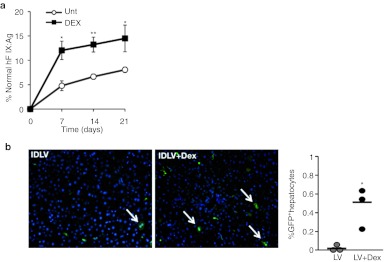
To test the therapeutic relevance of the increase in hepatocyte transduction, we evaluated whether Dex would improve factor IX (FIX) gene transfer. We used our previously described vector LV.ET.hFIX.142T, which is an integrating LV that expresses human FIX (hFIX) exclusively in hepatocytes due to a hepatocyte-specific promoter (ET) and target sites for miR-142-3p in the 3′UTR of the transgene.4 Mice pretreated with Dex or saline were injected with 2 × 108 TU of LV.ET.hFIX.142T and plasma levels of hFIX were monitored over time. In mice that received the vector alone, the hFIX expression levels reached ~6% of normal over the 3 weeks of analysis (Figure 5a). In contrast, in mice that were pretreated with Dex, hFIX levels reached as high as 15% of normal levels. Thus, by simply treating with a short course of steroid we were able to increase hFIX levels by 2.5-fold using a similar concentration of vector.
Figure 5.
Short-term GC treatment improves factor IX (FIX) gene transfer and increases transduction of integrative-defective LV (IDLV). (a) Quantitation of human FIX levels in mice treated with LV. Mice were injected with an LV-expressing human FIX from a hepatocyte-specific expression construct (LV.hFIX.142). A subset of mice received Dex at the time of LV administration. Human FIX levels in plasma were measured at the indicated time points by ELISA. Results are the mean ± SD (n = 4 mice per group). *P < 0.05, **P < 0.01. (b) Analysis of IDLV transduction of the liver in untreated and Dex-treated mice. GFP immunostaining was used to determine the frequency of transduced hepatocytes. A representative image is shown from mice 1 week after IDLV (2 × 108 TU/mouse) injection. The percentage of GFP+ hepatocytes was determined by morphometric analysis and the results for each mouse are shown on the right. *P < 0.05. Dex, dexamethasone; ELISA, enzyme-linked immunosorbent assay; GC, glucocorticoid; GFP, green fluorescent protein; LV, lentiviral vector; unt, untreated.
As recently demonstrated, IDLV is a promising method for tolerogenic vaccination.11 However, these vectors are poorly expressed, and thus require a high dose. We pretreated mice with Dex or saline, and injected them intravenously with 2 × 108 TU of an IDLV-encoding GFP. In mice that received saline plus IDLV, we could detect few GFP-positive hepatocytes, whereas in mice that were pretreated with Dex, an average of 0.6% of hepatocytes were GFP positive (Figure 5b). Thus, Dex similarly benefits IDLV-based vaccination by improving hepatocyte transduction. This is an important advancement for the use of IDLV because it will enable the effective dose of the vector to be lowered for vaccination approaches aimed at inducing immune tolerance via the liver.
Discussion
The studies described here provide the first evidence that LV triggers an innate host response in vivo through multiple distinct TLR and non-TLR pathways, and that the effect of this response can be blunted by coadministration of a clinical dose of GCs. Dex and other GCs have also been used to block the innate response to adenoviral vectors in mice32 and dogs,33 but they were not reported to affect vector transduction. We report that the use of Dex with LV not only blocks the innate response, but also specifically increases hepatocyte transduction. This enables the use of a lower vector dose to achieve similar efficiency of gene transfer. These results have important implications for moving LV and IDLV to the clinic for gene-based therapies.
Previous studies by ourselves and others indicated that LV, as well as its parent virus HIV, can induce IFN production, and IFN-inducible genes.12,14,15,27,34,35 The RNA-seq we have performed here provides the most comprehensive analysis so far of the effects of LV on an individual organ. This unbiased profiling approach revealed that there are actually relatively few genes whose expression is changed in response to LV. As noted, the majority of the genes that were upregulated are known to be IFN inducible, such as Mx1, Ifit1, and Oas1. Though surprisingly, the specific function of many of the upregulated genes in the innate immune response is still not known.36
In vitro studies with HIV suggested that induction of the IFN response was due to activation of TLR7 on pDCs, which implied that the viral ssRNA genome is the trigger.14 More recently, Breckport et al. elegantly demonstrated that myeloid DCs can also sense LV through TLR7, as well as TLR3.37 Our results suggest that macrophages primarily use TLR3 and DCs use TLR7 to sense LV implicating RNA from the vector as the trigger. The diminished IFN response we observed in the spleen of Myd88−/−Trif−/− mice indicated involvement of the TLR pathway in vector recognition in vivo by hematopoietic cells. However, the lack of change in the IFN response in the liver of Myd88−/−Trif−/− mice revealed that LV could be recognized in parallel in the liver by a non-TLR–mediated mechanism. Indeed, the existence of a non-TLR, DNA-dependent viral recognition pathway in the liver is suggested by our studies showing that treatment of mice with AZT at the time of LV injection reduces the level of the IFN response in the liver, but not the spleen. The fact that a partial decrease in IFN-induced genes' expression is only observed in the liver suggests that non-TLR–expressing cells are responding to the proviral DNA.
In support of this alternative recognition pathway, the Lieberman lab recently reported that during reverse transcription HIV produces DNA intermediates that can trigger an unknown intracellular DNA sensor.18 This pathway is not triggered when cells express the DNA exonuclease Trex1, which rapidly degrades cytoplasmic DNA. Our liver profiling found that expression of Trex1 is low in steady state (25.6 ± 2 reads/million). Although Trex1 levels are rapidly upregulated by the vector, the kinetics may not be sufficient to prevent vector-derived DNA from triggering a DNA sensor. As intracellular DNA sensors are a relatively new class of PRR,18,19,38 more work is needed to elucidate the nature and function of the genes involved in recognizing LV in the liver. Since human HIV can infect liver stromal cells, and actually promote an inflammatory response in the liver,39 uncovering the innate sensors of HIV may also be relevant for understanding liver pathogenesis associated with HIV infection.
Because LV activates the innate immune response through several non-overlapping pathways, blocking this initial response would be challenging. For this reason, we used a GC to block the upregulation of IFN-inducible genes. The effect of Dex on hepatocyte transduction is striking, and provides clear evidence that at least one or more of the genes upregulated by LV acts as a restriction factor for preventing infection of hepatocytes from being completed. A number of factors have been identified in humans and monkeys, which restrict HIV infection, such as APOBEC3G,40,41 TRIM5α,25 and very recently, SamHD1.26,42 The mouse homologous genes of TRIM5-α (TRIM12, 14, 21, and 30) were upregulated after LV injection. Similarly, SamHD1 was also upregulated, and so it is tempting to speculate that one of them may be involved in limiting hepatocyte transduction. However, it is not yet known whether these homologs would have similar effects on LV. Thus, it is possible, and even likely, that additional IFN-inducible genes, or more correctly, genes suppressed by Dex, are involved in restricting LV infection of hepatocytes.
The ability of Dex to suppress the inflammatory response and to increase hepatocyte transduction addresses two important issues with LV and IDLV gene transfer. The significance of using Dex as an adjuvant to vector administration is highlighted by the fact that we achieved more than double the level of circulating hFIX without increasing the vector dose. Importantly, Dex would not need to be given for more than 1–2 days to prevent the transient response to vector delivery, and would not need to be given at immunosuppressive doses since targeting vector expression to hepatocytes can avoid the immune response to the transgene product.4,5,6,8 Thus, we believe that Dex or other GCs will be an invaluable adjuvant for improving the success of liver-directed LV-based therapies.
Materials and Methods
Mice. C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), Myd88−/−, Trif−/−, Myd88−/−Trif−/− knockout mice were originally provided by Shizuo Akira and RIG-I–deficient mice were a gift from Adolfo García-Sastre. Vector was injected through the tail vein and Dex was delivered intraperitoneally. Mice received 5 mg/kg of body weight of dexamethasone (APP Pharmaceuticals, Schaumburg, IL) per injection in three consecutive injections: at 12 hours and 2 hours prior LV injection and 4 hours after. Mice that were sacrificed 4 hours after LV injection did not receive the last injection of Dex. AZT (APP Pharmaceuticals) was injected into the intraperitoneal cavity (1 mg/kg) 20 minutes before the intravenous injection of LV. All animal procedures were performed according to protocols approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee.
Immunohistochemistry. Tissues were fixed in 4% paraformaldehyde, equilibrated in 20% sucrose and embedded in optimal cutting temperature before sectioning as previously described;30 5-µm cryostate sections were stained with rat anti-mouse F4/80 (Serotec, Raleigh, NC) and rabbit anti-GFP (Invitrogen, Carlsbad, CA). Images were captured with a Coolsnap HQ camera and Eclipse E800 (Nikon, Melville, NY) microscope. Morphometric analysis was used to determine the frequency of GFP+ hepatocytes. Three random liver fields were measured per mouse. The number of DAPI (4′,6-diamidino-2-phenylindole)-positive nuclei, which were taken to belong to hepatocytes, was counted to determine the total number of hepatocytes per field. A minimum of 1,000 hepatocytes was counted per mouse. For hematoxylin and eosin staining, tissue was fixed in 10% formalin and embedded in paraffin.
Vector production. Third-generation vesicular stomatitis virus-pseudotyped vectors were produced by Ca3(PO4)2 transfection into 293T cells, as previously described.30 Supernatants were collected, passed through a 0.22-µm filter, and vector was concentrated by ultracentrifugation. Titer was estimated as TU/ml on 293T cells. IDLV was similarly produced. Titering was performed by pretreating 293T cells with mitomycin C to avoid vector dilution.
Gene expression analysis. Total RNA was extracted from tissue or cells using Tripure Isolation Reagent (Roche Molecular Biochemicals, Indianapolis, IN) and Glycogen-blue (Ambion, Carlsbad, CA) according to the manufacturers' instructions. For RNA-seq, 2 µg of total RNA per sample was fragmented by Covaris (Carlsbad, CA) and retrotranscribed and prepared with mRNA-seq Sample Prep. Kit (Illumina, San Diego, CA) following the manufacturer's instructions. Libraries were obtained from end-repaired cDNA by PCR with Phusion High-fidelity Taq polymerase (Finnzymes, Vantaa, Finland). Each library was prepared with different barcoded primers to permit sample multiplexing. The samples were sequenced on an Illumina Hi-Seq 2000. For quantitative-PCR, 0.5–2 µg total RNA was reverse-transcribed for 1 hour at 37 °C using RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). Quantitative-PCR was performed using the SYBR green qPCR master mix 2x (Fermentas, Glen Burnie, MD) and the following primers: actin forward: ctaaggccaaccgtgaaaag, actin reverse: accagaggcatacagggaca; CXCL10 forward: gctgccgtcattttctgc, CXCL10 reverse: tctcactggcccgtcatc; IFIT3 forward: tgaactgctcagcccaca, IFIT3 reverse: tcccggttgacctcactc; ISG15 forward: agtcgacccagtctctgactct, ISG15 reverse: ccccagcatcttcaccttta; OAS1 forward: gctgccagcctttgatgt, OAS1 reverse: tggcatagattctgggatca; RIG-I forward: gaagattctggaccccaccta, RIG-I reverse: gaatgtactgcacctcctca; TREX1 forward: ccttgctgcctgcttctc, TREX1 reverse: gaagatgagggtctgcatgtg.
Vector copy number. The vector copy number per genome was determined by quantitative-PCR as previously described.30 Briefly, 100 ng of DNA extracted from liver was used. Samples were normalized by murine actin. We used serial dilutions of viral backbone plasmid DNA for standard curves.
Cell culture. Bone marrow cells were obtained by flushing tibiae and femurs with media, lysing red bloods, and plating the remaining cells in RPMI 1640 media. To derive DCs, RPMI was supplemented with 50 ng/ml of GM-CSF and 50 ng/ml of IL-4 (Preprotech, Rocky Hill, NJ). To derive macrophages, RPMI was supplemented with 50 ng/ml M-CSF (Preprotech).
Flow cytometry analysis. Spleen and liver were digested in HBSS (Hank's buffer salt solution) containing 10% fetal bovine serum and 0.2 mg/ml collagenase IV (Sigma-Aldrich, St Louis, MO) for 30 minutes. After filtration through a 70-µm cell strainer (BD Biosciences, Franklin Lakes, NJ), red blood cells were lysed with RBC Lysis buffer (eBioscience) for 5 min at room temperature. Samples were stained with: CD19 (eio1D3)-PE, F4/80 (BM-8)-PercPCy5.5, CD11b (M1/70)-PercP-Cy5.5, CD11c (N418)-APC-Alexa780, CD45 (30F11)-APC, MHCII IA/IE (M5/114.15.2)-Alexa 450, Ly-6G (RB6-8C5)-PE from eBioscience. DAPI (Vector Laboratories, Burlingame, CA) was used to stain dead cells. LSR-Fortessa (BD Biosciences) was used to acquired the samples and FlowJo (Ashland, OR) was used to analyze the data.
Blood parameter determinations. Aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels were determined in serum as units/l by the Clinical Laboratory at the Mount Sinai Hospital. The platelet number was determined by collecting blood (0.02 ml) into EDTA-2 mmol/l 1% PBS buffer. After red blood cells lysis, all blood samples were examined microscopically and underwent manual differential count.
hFIX determination. Plasma was obtained from tail vein in tubes with 10% volume of sodium citrate (3.8%), and used to measure the levels of hFIX by the Human Factor IX ELISA (Affinity Biologicals, Hamilton, Ontario, Canada).
Statistical analysis. All values are expressed as the means ± SEM. Differences between groups were compared by Student's t-test. A P value <0.05 was considered statistically significant. RNA-seq pathway analysis was performed by Ingenuity (Redwood City, CA) by using Fisher's exact test, Benjamini-Hochberg correction for multiple testing.
SUPPLEMENTARY MATERIAL Figure S1. RNA-seq analysis reveals the liver's response to LV. Figure S2. GC decreases liver infiltration and partially improves blood parameters after LV injection. Figure S3. Glucocorticoids can block the type I IFN response to LV and increase hepatocyte transduction. Figure S4. GC does not alter LV transduction of the spleen.
Acknowledgments
We would like to thank Milind Mahajan and the DNA core in the Genetics and Genomic Sciences Department of Mount Sinai School of Medicine for the RNA-seq. We would also like to thank César Muñoz-Fontela and Scott L Friedman for scientific discussion and Adolfo García-Sastre (Mount Sinai School of Medicine) for scientific discussion and for mice. This work was supported by an NIH Pathfinder Award (DP2DK083052-01) and funding from the Juvenile Diabetes Research Foundation (JDRF 17-2010-770) to B.D.B. J.M.B. was supported by NIAID 1R01AI073899-01. J.A. was recipient of a Fulbright-Generalitat de Catalunya postdoctoral fellowship and a Beatriu de Pinós postdoctoral fellowship (Spain). The authors declared no conflict of interest.
Supplementary Material
RNA-seq analysis reveals the liver's response to LV.
GC decreases liver infiltration and partially improves blood parameters after LV injection.
Glucocorticoids can block the type I IFN response to LV and increase hepatocyte transduction.
GC does not alter LV transduction of the spleen.
REFERENCES
- Porter DL, Levine BL, Kalos M, Bagg A., and, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X.et al. (2006Gene transfer in humans using a conditionally replicating lentiviral vector Proc Natl Acad Sci USA 10317372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P.et al. (2007A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice Blood 1104144–4152. [DOI] [PubMed] [Google Scholar]
- Matsui H, Hegadorn C, Ozelo M, Burnett E, Tuttle A, Labelle A.et al. (2011A microRNA-regulated and GP64-pseudotyped lentiviral vector mediates stable expression of FVIII in a murine model of Hemophilia A Mol Ther 19723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F, Remy S, Dariel A, Flageul M, Pichard V, Boni S.et al. (2010Lentiviral vectors that express UGT1A1 in liver and contain miR-142 target sequences normalize hyperbilirubinemia in Gunn rats Gastroenterology 139999–1007, 1007.e1. [DOI] [PubMed] [Google Scholar]
- McIntyre C, Byers S., and, Anson DS. Correction of mucopolysaccharidosis type IIIA somatic and central nervous system pathology by lentiviral-mediated gene transfer. J Gene Med. 2010;12:717–728. doi: 10.1002/jgm.1489. [DOI] [PubMed] [Google Scholar]
- Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL.et al. (2005Persistent expression of factor VIII in vivo following nonprimate lentiviral gene transfer Blood 1061552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Sabatino G, Lombardo A, Boccaccio C., and, Naldini L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243–260. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L., and, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátrai J, Cantore A, Bartholomae CC, Annoni A, Wang W, Acosta-Sanchez A.et al. (2011Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk Hepatology 531696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A.et al. (2007In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance Blood 1092797–2805. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S., and, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG.et al. (2005Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions J Clin Invest 1153265–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A.et al. (2004Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells J Virol 785223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JV, Ni J., and, Dixit VM. RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem. 1998;273:16968–16975. doi: 10.1074/jbc.273.27.16968. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ.et al. (2008The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response Nature 452103–107. [DOI] [PubMed] [Google Scholar]
- Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA., and, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T., and, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RT, Liao SM., and, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M., and, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK.et al. (1995Identification and characterization of a new member of the TNF family that induces apoptosis Immunity 3673–682. [DOI] [PubMed] [Google Scholar]
- Opipari AW, Jr, Boguski MS., and, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- Luban J. Cyclophilin A, TRIM5, and resistance to human immunodeficiency virus type 1 infection. J Virol. 2007;81:1054–1061. doi: 10.1128/JVI.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P., and, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E.et al. (2011SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx Nature 474654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti M, Gregori S, Hauben E, Brown BD, Sergi LS, Naldini L.et al. (2011HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells Hum Gene Ther 22177–188. [DOI] [PubMed] [Google Scholar]
- Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A.et al. (2007MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands J Virol 818180–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CA., and, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–443. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L., and, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- van Til NP, Markusic DM, van der Rijt R, Kunne C, Hiralall JK, Vreeling H.et al. (2005Kupffer cells and not liver sinusoidal endothelial cells prevent lentiviral transduction of hepatocytes Mol Ther 1126–34. [DOI] [PubMed] [Google Scholar]
- Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T.et al. (2009Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy Mol Ther 17685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL., and, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- Tan PH, Beutelspacher SC, Xue SA, Wang YH, Mitchell P, McAlister JC.et al. (2005Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy Blood 1053824–3832. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Diebold SS, Gschmeissner S, Takeuchi Y, Ikeda Y, Collins MK.et al. (2007Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9 J Virol 81539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P.et al. (2011A diverse range of gene products are effectors of the type I interferon antiviral response Nature 472481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S.et al. (2010HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7 J Virol 845627–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SE, Baran M., and, Bowie AG. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011;32:574–581. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A.et al. (2010Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis Hepatology 52612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD., and, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L., and, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S.et al. (2011Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein Nature 474658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA-seq analysis reveals the liver's response to LV.
GC decreases liver infiltration and partially improves blood parameters after LV injection.
Glucocorticoids can block the type I IFN response to LV and increase hepatocyte transduction.
GC does not alter LV transduction of the spleen.