Abstract
Heat shock protein 27 (Hsp27) is highly overexpressed in castration-resistant prostate cancer (CRPC) and an antisense inhibitor (OGX-427) is currently in phase II clinical trials. In order to understand mechanisms of action of Hsp27 and find new therapeutic targets specific of CRPC, we screened for Hsp27 client proteins. Here, we report that translationally controlled tumor protein (TCTP) is a new Hsp27 client protein involved in Hsp27 cytoprotection. We found that TCTP expression is absent or weak in normal prostate cells, moderately expressed in 18.5% of treatment naive PC, and becomes uniformly and strongly expressed in 75% of CRPC. To define TCTP function, we developed and worldwide patented a TCTP antisense oligonucleotide (ASO). Interestingly, we found that CRPC progression correlates with TCTP overexpression and loss of P53. TCTP knockdown restored P53 expression and function, suggesting that castration-sensitivity is directly linked to P53 expression. Collectively, these findings provide a new Hsp27 cytoprotection mechanism in CRPC, and preclinical proof-of-concept that combining ASO-mediated TCTP knockdown with castration and/or docetaxel therapy could serve as a novel strategy to treat CRPC, with no or little toxicity for normal prostate cells.
Introduction
Prostate cancer (PC) is one of the most common cancers in industrialized countries. Patients with localized disease may be treated with surgery or radiation, whereas androgen withdrawal (castration) is used as first-line therapy in patients with metastatic disease. While most patients initially respond well to castration, they most ultimately become unresponsive and recur within 2 years as castration-resistant PC (CRPC).1 Recently, docetaxel-based regimens have demonstrated improved survival in men with CRPC in two different phase III studies.2,3 However, median overall survival was prolonged by only ~2–3 months. Additional therapeutic strategies targeting molecular mechanisms-mediating resistance are required. One strategy to improve therapies in advanced PC involves targeting genes that are activated by androgen withdrawal, either to delay or prevent the emergence of the CR phenotype. Recently, we identified Heat Shock Protein 27 (Hsp27) as a highly overexpressed gene in CRPC. Hsp27 is well referenced as a therapeutic target in cancer4 because its increased expression in several types of tumor cells correlates with aggressiveness, lack of response to therapies, and poor prognosis.5,6 As a molecular chaperone, Hsp27 is highly induced during stress responses and forms oligomers to interact with a wide variety of client proteins to prevent aggregation. We previously reported that Hsp27 knockdown using antisense oligonucleotides (ASOs) and small interference RNA (siRNA) increased apoptotic rates and enhanced castration therapy (CT) and chemotherapy in PC.7,8,9 We developed and worldwide patented a second generation ASO targeting Hsp27 that has been licensed (OGX-427) and phase II clinical trials are currently underway in prostate and bladder cancer.10,11 The functional role of stress-induced Hsp27 in castration or chemotherapy-induced apoptosis remains incompletely defined. The purpose of this study is to elucidate the pathways leading Hsp27 action in CRPC and find new specific therapeutic targets and treatment strategy for CRPC that would have less toxicity for normal tissues.
In order to understand Hsp27 mechanisms of action, we screened Hsp27 partner proteins using a two-hybrid SOS recruitment system. Expression profiles of these partners were then performed on normal (N, PNT2C2), castration sensitive (CS, LNCaP), castration resistant (CR, C4-2), and androgen independent (AI, PC-3) cell lines using western blot and on human normal, benign, CS, and CR tumor samples using tissue microarray (TMA). These experiments directed our focus on the translationally controlled tumor protein (TCTP), also called histamine-releasing factor (HRF), tumor protein translationally controlled 1 (Tpt1), p23 or fortilin. TCTP has been implicated in many cellular processes like cell cycle progression, cell growth, regulation of pluripotency or apoptosis, and silencing TCTP reverses the malignant phenotype.12 Molecular mechanisms involving TCTP in cell cycle progression, cell growth, and regulation of pluripotency have been proposed.13 Gachet et al., demonstrated that, in mitotic cells, TCTP interacts with microtubules during G1, S, G2, and early M phase of cell cycle and detaches from the spindle during the metaphase-anaphase transition.14 TCTP also interacts with the checkpoint with forkhead and ring finger domain (CHFR) E3 ubiquitin ligase that binds microtubules, a key enzyme involved in cell cycle progression. Upon depolymerization of the microtubules, CHFR and TCTP interaction is diminished.15 TCTP has also been identified as a substrate of polo-like kinase 1 (Plk1), which is involved in the formation and function of bipolar spindles. Abolishing Plk1 phosphorylation on two serine residues of TCTP induced mitotic defects.16 When microtubules are damaged, CHFR ubiquitinates Plk1, leading to Plk1 degradation and cell cycle blockage.17 TCTP has also been reported to be involved in cell growth via the activation of the target of rapamycin pathway. It has been reported that TCTP interacts with the GTPase Rheb (Ras homolog enriched in brain), a crucial enzyme for target of rapamycin pathway activation and protein synthesis. TCTP binds to Rheb and acts as guanine nucleotide exchange factor by stimulating GDP/GTP exchange.18 TCTP is also described to be a key regulatory factor of stem cells self-renewal and pluripotency via its interaction with the promoter or distal promoter of homeodomain transcription factors Oct4 and Nanog.19 However, little is known about the molecular mechanisms underlying its antiapoptotic activities. One hypothesis is that TCTP stabilizes the antiapoptotic protein myeloid cell leukemia sequence-1 (Mcl-1)20 and interferes with the homodimerization of BCL2-associated X protein (BAX).21 Recently, it has been reported a feedback loop between TCTP and the key regulator of apoptosis and tumor suppressor P53. Amson et al., demonstrated that P53 binds to a P53 responsive element that is present in the promoter of TCTP, leading to the transcriptional repression of TCTP. They also found that TCTP directly associates with the E3 ubiquitin ligase MDM2, increasing MDM2-mediated ubiquitination of P53 and promoting its degradation.22
In this study, we aimed to understand how TCTP mediates Hsp27 cytoprotection in CRPC and assess the effect of TCTP silencing on chemotherapy- and CT-induced apoptosis.
Results
TCTP is a new Hsp27 client protein overexpressed in CRPC
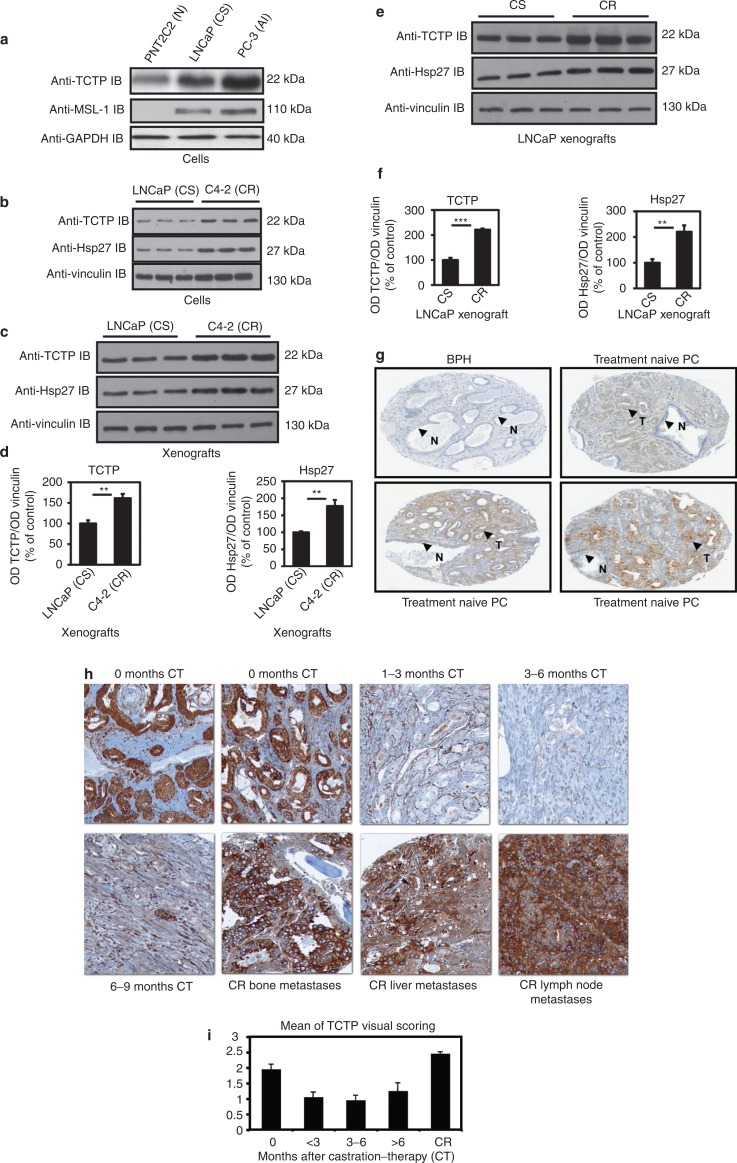
To define new mechanisms mediating the protective role of Hsp27 and identify new Hsp27 partners specific to CRPC, we used the CytoTrap approach as previously described by Thalappilly et al.23 After a PubMed analysis of more than 200 identified partner proteins, we focused specifically on partners linked to cancer progression: male-specific lethal-1 homolog (Msl-1), TCTP, heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2B1), and S100 calcium binding protein A4 (S100A4). The expression levels of these four partners were then determined on normal (PNT2C2), CS (LNCaP), CR and AI (C4-2, PC-3) cell lines using western blot and on human normal, benign, CS and CR tumor samples using a TMA. We found that neither hnRNPA2B1 nor S100A4 were overexpressed in the androgen receptor negative PC-3 cell line (data not shown). However, while TCTP and Msl-1 were only slightly expressed in normal immortalized PNT2C2 cells, their expression strongly increased in CS LNCaP cells and even more in AI PC-3 cells (Figure 1a). We then looked at their expression in human samples and found that Msl-1 was equally expressed in normal and PC samples (data not shown). Conversely, for TCTP protein levels we found a 1.7-fold increase in CR C4-2 cells and xenografts compared with CS LNCaP models (**P ≤ 0.01; Figure 1b–d). Furthermore, TCTP protein levels increased twofold in CR LNCaP xenograft tumors harvested 40 days after castration compared with CS LNCaP tumors harvested before castration (***P ≤ 0.001; Figure 1e,f). Figure 1d,f demonstrate that TCTP protein levels correlate with Hsp27 levels (1.7-fold, **P ≤ 0.01; Figure 1d and twofold, **P ≤ 0.01; Figure 1f). We then looked at the expression of TCTP in human samples using two different TMAs. TCTP was expressed in 18.5% of treatment naive PC, with no or weak expression in normal (N) and benign samples (benign prostate hyperplasia, BPH; prostate intraepithelial neoplasia, PIN). Even in normal (N) glands disseminated inside the tumor (T), no or weak expression of TCTP is detectable (Figure 1g). In order to study TCTP during CR progression, we defined TCTP expression in a second TMA of castration-treated patients. TCTP expression was found to be significantly downregulated after CT to become uniformly highly expressed in 75% of CR metastasis (Figure 1h). The mean intensity of positive cells in the non-treated versus castration-treated patients (<3 months, 3–6 months, >6 months or CR) was 1.8, 1.02, 0.8, 1.25, and 2.5, respectively (Figure 1i).
Figure 1.
TCTP protein level increases in castration-resistant (CR) prostate cancer cells and human tumors. (a,b) Proteins from normal (PNT2C2), castration sensitive (LNCaP), androgen independent (PC-3), and CR (C4-2) cells were extracted. TCTP, MSL-1, Hsp27, vinculin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels were analyzed by western blot. (c) Expression profile of TCTP, Hsp27, and vinculin were analyzed by western blot in CR C4-2 and CS LNCaP xenograft. (d) Average intensity of bands for TCTP and Hsp27 were normalized with vinculin and scoring with Image J software (**P ≤ 0.01). (e) CS (harvested before castration) and CR (harvested 40 days after castration) LNCaP tumors were used to analyze TCTP, Hsp27, and vinculin protein levels by western blot. (f) Average intensity of TCTP and Hsp27 bands was scored with Image J software after normalization with vinculin (**P ≤ 0.01 and ***P ≤ 0.001). (g) Representative microscopic fields of TCTP immunostaining in prostate benign hyperplasia (BPH) and treatment naive prostate cancer (PC) tissue microarray. Micrographs illustrate that 18.5% of treatment naive PC show TCTP immunoreactivity in tumor glands epithelium (T, arrows). In BPH, the epithelium that borders normal glands (N, arrows) is not immunoreactive for TCTP. We can notice that within prostate tumor, normal gland is not immunoreactive. (h) Representative microscopic fields of TCTP immunostaining in castration-treated PC tissue microarray. (i) Mean TCTP staining after castration therapy (CT). Specimens were graded from 0 to +3 intensity, representing the range from no staining to heavy staining by visual scoring and automated quantitative image analysis by proplus image software. Data from 112 samples were used to calculate (average) mean ±SE. All comparisons of stain intensities were made at ×200 magnification. AI, androgen independent; CS, castration sensitive; Hsp27, heat shock protein 27; MSL-1, male-specific lethal-1 homolog; N, normal; OD, optical density; TCTP, translationally controlled tumor protein.
Hsp27 interaction protects TCTP from the ubiquitin-proteasome degradation
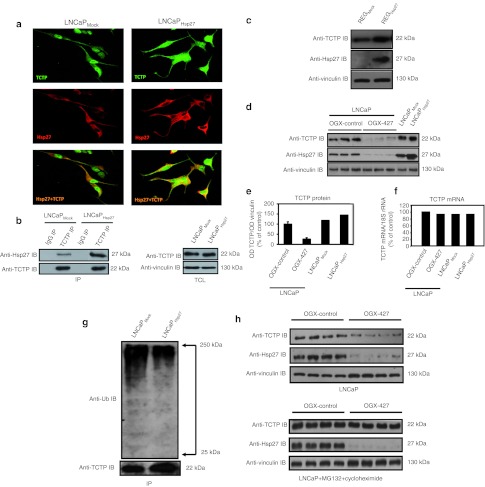
To further confirm and define the role of Hsp27 interaction with TCTP, we examined whether Hsp27 colocalizes and interacts with TCTP using immunofluorescence and co-immunoprecipitation. Confocal microscopy shows that TCTP (green) and Hsp27 (red) colocalize (yellow) in the cytoplasm of LNCaPMock and LNCaPHsp27 cells, and the intensity of TCTP staining and colocalization with Hsp27 was higher in LNCaPHsp27 relative to LNCaPMock cells (Figure 2a). We then confirmed the interaction between Hsp27 and TCTP by co-immunoprecipitation (Figure 2b). In absence of Hsp27, decreased levels of Hsp27 client proteins, such as androgen receptor, procaspase-3, signal transducer and activator of transcription 2 and 3 (STAT2 and 3), eukaryotic translation initiation factor 4E (eIF4E), and histone deACetylase 6 (HDAC6), have been reported.7,24,25,26,27 To determine whether Hsp27 also regulates TCTP, we used Hsp27 stably transfected REG (REGHsp27) and LNCaP (LNCaPHsp27) cells. We found that TCTP levels increased by 30% in REGHsp27 and LNCaPHsp27 cells compared with respective Mock stably transfected cells (Figure 2c–e). Conversely, Hsp27 knockdown in LNCaP cells using OGX-427 induced a 70% decrease in TCTP protein level compared to LNCaP treated with OGX-control (Figure 2d,e), without altering mRNA expression levels (Figure 2f), suggesting that Hsp27 regulates TCTP expression at a post-translational level. To elucidate how Hsp27 regulates TCTP protein levels, we tested Hsp27 effect on TCTP ubiquitination rates, and its subsequent proteasomal degradation using LNCaPMock and LNCaPHsp27 cells. Figure 2g shows that Hsp27 overexpression decreased ubiquitinated TCTP levels by up to 55%, as shown by the ladder of high molecular weight species, which is a characteristic of polyubiquitinated protein. To determine whether the ubiquitination of TCTP results in its proteasomal degradation, TCTP half-life was determined after 48 hours of OGX-427 treatment in the presence or absence of the proteasome inhibitor MG132, combined with protein synthesis inhibitor cycloheximide. Lysates from LNCaP ± MG132 cells (10 µmol/l) and cycloheximide (10 µg/ml), were analyzed using western blot with both anti-TCTP and -Hsp27 antibodies. As shown in Figure 2h, MG132 (+cycloheximide) treatment reverses the effect of OGX-427 and prolonged TCTP half-life, indicating that decreases in TCTP protein levels after OGX-427 treatment occurs via proteasomal degradation. Supplementary Figure S1 shows time-dependent decrease of TCTP protein level after cycloheximide treatment alone.
Figure 2.
Hsp27 interaction protects TCTP from the ubiquitin-proteasome degradation. (a) LNCaPMock and LNCaPHsp27 stained with rabbit polyclonal TCTP (green immunofluorescence, IF) and mouse monoclonal Hsp27 (red immunofluorescence, IF) antibodies. Yellow is for colocalized foci (merged) and green or red represent non-colocalization foci. (b) LNCaPMock and LNCaPHsp27 cell lysates were used to immunoprecipitate (IP) TCTP using rabbit anti-TCTP or rabbit anti-immunoglobulin (IgG) antibodies. The membrane was then immunoblotted with anti-Hsp27 antibody. Total cell lysate (TCL) represents proteins from LNCaPMock versus LNCaPHsp27 cells, extracted from cultured cells and blotted as control with anti-TCTP or anti-vinculin antibodies. (c) REG cells, stably transfected with Hsp27 (REGHsp27) or empty vector (REGMock), were harvested and proteins extracted. TCTP, Hsp27, and vinculin protein levels were analyzed by western blot. (d) Proteins were extracted from culture of LNCaPMock, LNCaPHsp27, and LNCaP treated with OGX-427 or OGX-control and analyzed by western blot with TCTP, Hsp27, and vinculin antibodies. (e) The graph represents TCTP protein levels, after normalization to vinculin protein levels, for each condition by scoring the band's intensity with Image J software. (f) Total RNAs was extracted from culture of LNCaPMock, LNCaPHsp27, and LNCaP treated with OGX-427 or OGX-control. TCTP and 18S levels were analyzed by quantitative reverse transcription-PCR (qRT-PCR). TCTP mRNA levels were analyzed after normalization to 18S ribosomal RNA (rRNA) levels. Results are expressed as percentage of LNCaP cells transfected with the OGX-control (100%). (g) Protein lysates from LNCaPMock and LNCaPHsp27 cells were used to immunoprecipitate (IP) TCTP, followed by western blotting with an anti-ubiquitin (Ub) antibody. (h) PC-3 cells were treated with OGX-427 or OGX-control for 2 days and then harvested for protein extraction or pretreated with cycloheximide (10 µg/ml) followed by MG-132 (10 µmol/l) for 48 hours and tested by western blot analysis using an anti-TCTP, anti-Hsp27, and anti-vinculin antibodies. Hsp27, heat shock protein 27; OD, optical density; TCTP, translationally controlled tumor protein.
TCTP is involved in cytoprotection induced by Hsp27 and TCTP knockdown inhibits PC cell growth and enhances chemotherapy in vitro
TCTP is overexpressed in several cancers including PC.28,29 Gene expression analysis showed that TCTP is a highly expressed gene in PC cells28 and functions to block apoptosis.30 Recently, TCTP knockdown has been shown to decrease the number of stem-like cancer cells in breast.22 In the present study, we have found that TCTP is involved in cytoprotection mediated by Hsp27, as TCTP knockdown using siRNA (Figure 3a) in CS LNCaP stably transfected with Hsp27 (LNCaPHsp27) or empty vector (LNCaPMock), reverses the cytoprotection to androgen withdrawal (serum-free media) and docetaxel treatments normally conferred by Hsp27 overexpression (Figure 3b; **P ≤ 0.01; lanes 3, 7 versus 4, 8). This result reveals that TCTP is an important effector of Hsp27 cytoprotective function. To determine whether TCTP inhibition affects PC progression in vitro, ASO- or siRNA-induced inhibition of TCTP was determined in AI PC-3 cells by western blot analysis. As shown in Figure 3c–e, significant inhibition of TCTP protein levels (Figure 3c,d) and mRNA levels (Figure 3e, **P ≤ 0.01; ***P ≤ 0.001) were observed after TCTP-siRNA or -ASO treatment. To assess the effect of TCTP downregulation on AI cell growth, PC-3 cells were treated with TCTP-siRNA or -ASO and incubated with docetaxel. Figure 3f,g (lanes 1, 2) show a 50% reduction in PC-3 cell growth after TCTP-siRNA or -ASO treatment compared with respective controls (***P ≤ 0.001). Furthermore, Figure 3g shows that TCTP downregulation using ASO treatment can enhance docetaxel sensitivity by up to 25% (**P ≤ 0.01; lanes 2, 5). TCTP downregulation (Figure 3h) also decreases CR C4-2 cell growth by up to 65% (***P ≤ 0.001; lanes 1, 2) and enhances docetaxel sensitivity by up to 20% (Figure 3i; ***P ≤ 0.001; lanes 2, 5). Conversely, TCTP overexpression using pDEST vector containing TCTP cDNA (pDEST-TCTP) compared with control (pDEST-Mock) in transiently transfected CS LNCaP and CR C4-2 cells (Figure 3j) leads to 35% (Figure 3k; **P ≤ 0.01; lanes 4, 5) and 60% (Figure 3i; ***P ≤ 0.001; lanes 4, 5) increase in cell viability, respectively, after docetaxel treatment with or without androgen withdrawal.
Figure 3.
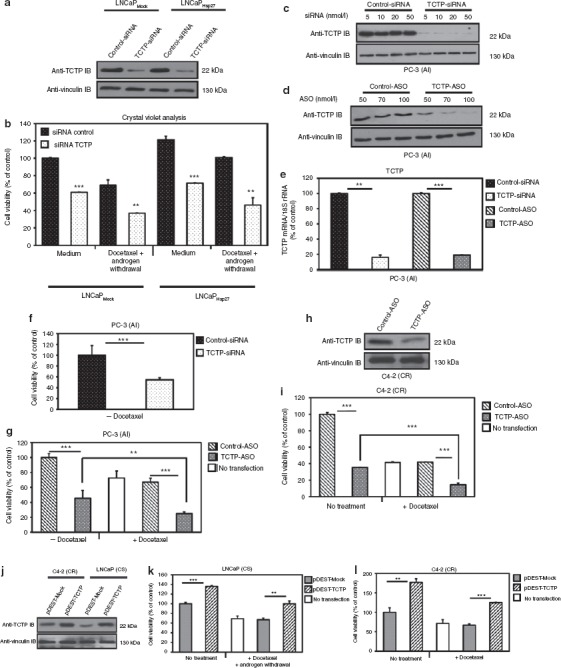
Cytoprotection induced by Hsp27 is in part mediated by TCTP and TCTP silencing inhibits prostate cancer cells growth and enhances chemotherapy in vitro. (a) LNCaPMock and LNCaPHsp27 cells were treated with TCTP- or control-siRNA (5 nmol/l), then proteins were extracted and analyzed by western blot. (b) LNCaPMock and LNCaPHsp27 cells were treated with 5 nmol/l TCTP- or control-siRNA. After 2 days, serum-free media (mimics androgen withdrawal in vitro) and docetaxel was added for 24 hours and cells were analyzed for growth rates using crystal violet dye. The experiment was repeated in triplicate and statistical analysis was done using Statview software (***P ≤ 0.001; **P ≤ 0.01). (c) PC-3 cells were treated with indicated concentrations of TCTP- or control-siRNA, and 2 days after proteins were extracted and analyzed by western blot. (d) Proteins were extracted from PC-3 cells treated with the indicated concentrations of TCTP- or control-ASO. TCTP and vinculin protein levels were analyzed by western blot. (e) PC-3 cells were treated for 1 day with 5 nmol/l of TCTP- or control-siRNA, and for 2 days with 100 nmol/l of TCTP- or control-ASO. Total RNA was extracted and TCTP levels were analyzed by qRT-PCR. After the normalization of TCTP mRNA with 18S rRNA levels, results were analyzed with the 2(-δδCT) Method. Each sample was analyzed in triplicate. **Differs from PC-3 transfected with control-siRNA (P ≤ 0.01) and ***with control-ASO (P ≤ 0.001) using Statview software. (f) PC-3 cells were treated with 5 nmol/l of TCTP- or control-siRNA. After 2 days, cell growth rates were analyzed using MTT test (***P ≤ 0.001). (g) Cell viability analysis of PC-3 treated with TCTP-ASO combined with docetaxel using MTT test. The experiment was repeated in triplicate. ***Differs from PC-3 transfected with control-ASO ± docetaxel (P ≤ 0.001) and **differs between PC-3 treated with TCTP-ASO monotherapy and TCTP-ASO plus 50 nmol/l docetaxel (P ≤ 0.01) treatments using Statview software. (h) TCTP-ASO efficiency on C4-2 was tested by western blot after 100 nmol/l of TCTP- or control-ASO treatment for 48 hours, the proteins was extracted. TCTP and vinculin levels were analyzed by western blot. (i) C4-2 cell viability was determined using crystal violet dye. TCTP inhibition decreases C4-2 cell survival after 24 hours treatment with docetaxel in serum-free media (mimics androgen withdrawal in vitro). Error bars represent the SE, ***P ≤ 0.001 by Statview software. (j) C4-2 and LNCaP cells were transiently transfected with pDEST-TCTP or pDEST-Mock vectors for 2 days and proteins were extracted. TCTP and vinculin levels were analyzed by western blot. (k,l) LNCaP and C4-2 cells overexpressing TCTP, increased cell survival after combined androgen withdrawal and docetaxel-chemotherapy treatments. After 48 hours, cell viability was determined using the crystal violet assay. Error bars represent the SE, **P ≤ 0.01 and ***P ≤ 0.001 by Statview software. AI, androgen independent; ASO, antisense oligonucleotide; CR, castration resistant; CS, castration sensitive; Hsp27, heat shock protein 27; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium; qRT-PCR, quantitative reverse transcription-PCR; rRNA, ribosomal RNA; siRNA, small interference RNA; TCTP, translationally controlled tumor protein.
TCTP-ASO and -siRNA silencing induces cell cycle arrest and apoptosis in CR cells in vitro
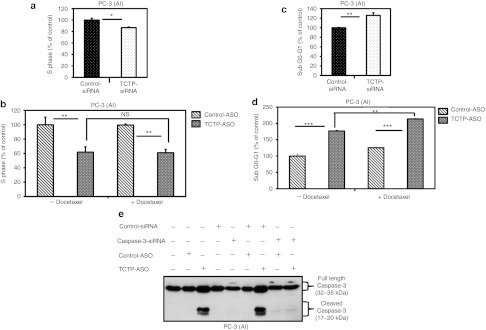
Induction of apoptosis and cell cycle blocking by TCTP-ASO or -siRNA was demonstrated by flow cytometry (Figure 4). In AI PC-3 cells, the fraction of cells in S phase was reduced by 15% (*P ≤ 0.05; Figure 4a) and 40% (**P ≤ 0.01; lanes 1, 2, Figure 4b) after TCTP-siRNA or -ASO treatment respectively, compared with the controls. Furthermore, the fraction of PC-3 cells undergoing apoptosis (sub G0-G1 fraction) was 25% (**P ≤ 0.01; Figure 4c) to 75% (***P ≤ 0.001; lanes 1, 2; Figure 4d and Supplementary Figure S2) higher with TCTP-siRNA or -ASO, respectively, compared with the controls. Figure 4d and Supplementary Figure S2 illustrate that combining TCTP-ASO plus docetaxel increased apoptosis by up to 40% compared with TCTP-ASO alone (**P ≤ 0.01; lanes 2, 4). As TCTP has been reported to inhibit caspase-3 activation,31 Figure 4e (lanes 4, 7) confirms the presence of active-cleaved caspase-3 fragments (17–20 kDa) in AI PC-3 cells treated with TCTP-ASO compared with control. As expected, this caspase-3 cleavage was not observed in cells when caspase-3 was knockdown by means of a specific siRNA (Figure 4e, lane 9). Collectively, these data suggest that TCTP knockdown induces cell cycle arrest and apoptosis in AI PC-3 cells by enhancing caspase-3 activation.
Figure 4.
TCTP silencing induces cell cycle arrest and apoptosis in prostate cancer cells in vitro. (a-d) Flow cytometry was used to quantify the percentage of PC-3 cells in each cell cycle phase, 2 days after siRNA or ASO treatment. The plot represents the mean of phase S and sub G0-G1 fractions from three independent flow samples. The results are expressed in percentages, PC-3 cells transfected with control-siRNA or -ASO representing 100%. (a,b) Rate of PC-3 cells in phase S was determined after (a) TCTP-siRNA and (b) -ASO treatments. * and **differs from PC-3 transfected with controls (P ≤ 0.05 and P ≤ 0.01, respectively). Nonsignificant (NS) difference was found between the phase S fractions of PC-3 treated with TCTP-ASO alone and TCTP-ASO plus chemotherapy. (c,d) Apoptotic determination of PC-3 cells treated with (c) TCTP-siRNA and (d) -ASO. ** and *** differs from PC-3 transfected with control-siRNA and -ASO alone (P ≤ 0.01 and P ≤ 0.001, respectively). A 40% increase (**P ≤ 0.01) of apoptotic PC-3 treated with TCTP-ASO plus docetaxel was found compared to PC-3 treated with TCTP-ASO monotherapy. (e) Effect of TCTP silencing on caspase-3 cleavage and activity. PC-3 cells, 24 hours after transfection with 80 nmol/l of control or caspase-3 siRNA, were treated with, TCTP- or control-ASO (100 nmol/l, 24 hours). Proteins were extracted for western blotting with a caspase-3 antibody that recognizes both full-length and cleaved caspase-3. AI, androgen independent; ASO, antisense oligonucleotide; siRNA, small interference RNA; TCTP, translationally controlled tumor protein.
TCTP-ASO treatment inhibits AI and CR tumor progression, enhances docetaxel chemotherapy, and delays CR progression after castration in vivo
In order to assess effects of TCTP silencing in AI growth in vivo, we evaluated the effects of TCTP-ASO treatment in PC-3 and LNCaP xenografts (Figure 5). Male nude mice bearing PC-3 tumors were randomly selected for TCTP- versus control-ASO treatment. Figure 5a shows that TCTP-ASO monotherapy significantly reduced AI PC-3 tumor volume from weeks 8–10 (**P ≤ 0.01). At killing, tumor volume was greater than twofold higher in controls (1,808 ± 471 mm3) compared with TCTP-ASO–treated group (881 ± 64 mm3; **P ≤ 0.01). Moreover, TCTP-ASO treatment significantly enhanced the apoptotic effects of docetaxel from week 4–13 (***P ≤ 0.001; **P ≤ 0.01; *P ≤ 0.05). At killing 13 weeks after treatment, tumor volume was greater than sixfold higher in controls (682 ± 186 mm3) compared with the TCTP-ASO–treated group (112 ± 44 mm3; *P ≤ 0.05; Figure 5b).
Figure 5.
TCTP-ASO treatment inhibits CR tumor progression, enhances docetaxel chemotherapy, and delays prostate cancer progression in vivo. (a,b) Mice bearing AI PC-3 tumors were randomly selected for treatment with (a) TCTP- or control-ASO alone, or (b) combined with docetaxel. When PC-3 tumors reached 50 mm3, 12.5 mg/kg/mouse of TCTP- or control-ASO were injected intraperitoneally (i.p.) daily for 10 weeks in animals receiving ASO monotherapy and for 13 weeks in animals receiving ASO combined with docetaxel. From days 7–14 docetaxel (see Material and Methods) was administered i.p. three times per week. Tumor volume was measured once weekly and calculated by the formula length × width × depth × 0.5236. Points, mean tumor volume in each experimental group containing 10 mice; bars, SE. *, **, and ***differ from control-ASO (P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively) by Statview software. (c) Effect of TCTP-ASO treatment on LNCaP tumor growth in vivo after castration. Twenty male mice bearing LNCaP tumors were randomly selected for treatment with TCTP- or control-ASO. Castration was performed when tumors reached a mean volume of 150 mm3. TCTP- or control-ASO were injected (12.5 mg/kg/day) daily from 1 to 9 weeks after castration. Tumor volume was measured weekly. Each point represents the mean tumor volume of 10 mice; bars, SE. *P ≤ 0.05; **P ≤ 0.01, differ from control by Statview software. (d) Photographs of PC-3– and LNCaP-harvested tumors from animals that received i.p. TCTP- or control-ASO. (e) Proteins were extracted from harvested PC-3 tumors treated with TCTP- or control-ASO alone. The effect of TCTP-ASO treatment on caspase-3 cleavage was analyzed by western blot. (f) Body weight of animals treated i.p. with ASOs over the duration of the experiment. t0 = body weight before the first injection. tf = body weight the day of killing. AI, androgen independent; ASO, antisense oligonucleotide; CR, castration resistant; CS, castration sensitive; TCTP, translationally controlled tumor protein.
In order to determine whether TCTP-ASO could delay the CR progression after castration, 20 male mice bearing CS LNCaP tumors were castrated 6–8 weeks after tumor implantation and were randomly selected for treatment with TCTP- versus control-ASO. As shown in Figure 5c, TCTP-ASO delays CR progression of LNCaP xenografts during the 9 weeks of analysis, compared with controls. At killing, tumor volume was greater than fourfold higher in control (1,650 ± 281 mm3) compared with the TCTP-ASO–treated group (397 ± 132 mm3; *P ≤ 0.05). Harvested tumors from animals treated with TCTP-ASO ± castration tended to be two (PC-3) to fourfold (LNCaP) smaller compared with respective controls (Figure 5d). TCTP-ASO increases apoptosis of PC-3 and LNCaP tumors, as demonstrated by the presence of cleaved caspase-3 fragments compared with controls (Figure 5e). Under the experimental conditions described above, no adverse effects were observed. Figure 5f shows that TCTP-ASO did not cause general toxicity to animals as indicated by the absence of change in animal behavior or body weight.
TCTP silencing inhibits CR tumor growth by inducing P53 expression and function
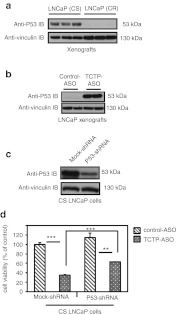
In mammary tumor cells, it has been recently reported that TCTP knockdown increased P53 expression, underlining the relevance of TCTP in cancer.22 In PC, the biological role of P53 is unclear due to the contradictory nature of several studies.32,33 However, CS LNCaP cells retain a wild-type functional P53 protein, whereas AI PC-3 cells lack P53.34 In order to assess interactions between P53 and TCTP in CRPC progression, we measured P53 expression in LNCaP tumors overexpressing TCTP and resistant to castration. Interestingly, we found that CR progression after castration correlated with the loss of P53 expression (Figure 6a). In order to assess a direct correlation between TCTP expression and P53, we looked at P53 content in LNCaP-xenografted tumors after TCTP- or control-ASO treatment previously described in Figure 5c. We observed that TCTP silencing using ASO was able to restore P53 expression (Figure 6b) and castration-sensitivity (Figure 5c), suggesting that castration-sensitivity of PC is linked to P53 expression. In order to assess the relevance of TCTP-induced P53 expression, we performed a rescue experiment by depleting P53 using short hairpin RNA (shRNA) (Figure 6c) in TCTP-ASO–treated LNCaP cells. As shown in Figure 6d (lanes 2, 4), the inhibition of P53 by P53-shRNA in LNCaP TCTP-ASO–treated cells restores cell viability (approximately twofold, ***P ≤ 0.001), suggesting that TCTP silencing leads to increased P53 expression and function.
Figure 6.
TCTP silencing inhibits CR tumor growth by inducing P53. (a) Proteins were extracted from CS (harvested before castration) and CR (harvested 40 days after castration) LNCaP tumors. P53 and vinculin protein levels were analyzed by western blot. (b) CR LNCaP tumors treated with TCTP- or control-ASO after castration, were used to analyze P53 and vinculin protein levels by western blot. (c) P53 protein levels were analyzed by western blot after P53- or mock-shRNA treatment. (d) LNCaP cell survival after combined P53- or mock-shRNA and TCTP- or control-ASO treatments was analyzed. After 2 weeks, cell viability was determined using a crystal violet assay. Error bars represent the SE, **P ≤ 0.01 and ***P ≤ 0.001 by Statview software. ASO, antisense oligonucleotide; CR, castration resistant; CS, castration sensitive; shRNA, short hairpin RNA; TCTP, translationally controlled tumor protein.
Discussion
Hsp27 expression is induced by various stressors, such as chemotherapy and androgen withdrawal, and can act at multiple control points across various apoptotic pathways, to ensure that stress-induced damage does not trigger PC cell death.35 Several mechanisms account for the cytoprotective effect of Hsp27, including chaperone inhibitor of misfolded proteins aggregation, inhibition of key effectors of the apoptotic machinery at the pre- and post-mitochondrial level,7,9,36,37 and proteasome-mediated degradation of proteins under stress conditions.35 Although many mechanisms by which Hsp27 inhibits apoptosis are defined, its role in CR growth is still the subject of much studies.7,38,39
Here, we found that Hsp27 colocalizes and binds to TCTP (Figure 2a,b), a 172 amino acid antiapoptotic polypeptide whose presence, both extra- and intracellular, has been implicated in many cellular functions related to cell growth and apoptosis regulation.16,29 A recent study demonstrated that overexpression of TCTP can prevent stress-induced mammalian cell apoptosis.40 TCTP has been recently described to regulate cell survival in many human cancers, including PC,28 and it is considered as an interesting target in cancer therapy.40,41,42 In spite of the importance of TCTP, little is known about its role in CRPC progression and the factors regulating its expression levels. In this study, we identify Hsp27 as an upstream regulator of TCTP (Figure 2c–e). Transcription of TCTP gene is regulated in response to a wide range of extracellular signals and cellular conditions.43 However, mechanisms regulating TCTP degradation remain incompletely defined. The ubiquitin-proteasome pathway is an important factor controlling the turnover and activity of regulatory proteins.44 Here, we found that Hsp27 regulates TCTP expression at post-translational level (Figure 2f) and in Hsp27 absence; TCTP is rapidly ubiquitinated and degraded by the proteasome (Figure 2g,h). Accordingly, the half-life of TCTP is markedly decreased in LNCaP cells treated with OGX-427 (Figure 2d). OGX-427 mediated Hsp27 knockdown, by inducing the proteasomal degradation of TCTP, reduces cell viability after androgen withdrawal and/or chemotherapy. The most plausible explanation of this effect is that Hsp27 associates to and stabilizes TCTP, thereby maintaining the protein in a properly folded state.
These results are consistent with our recent work showing that the interaction between Hsp27 and the eIF4E decreases eIF4E ubiquitination and proteasomal degradation.24 Furthermore, TCTP silencing using siRNA suppressed the cytoprotection afforded by high Hsp27 levels (Figure 3a,b), suggesting that TCTP is at least in part, responsible for Hsp27 antiapoptotic activity. In order to see whether TCTP is an Hsp27 client protein involved in CRPC, we evaluated its expression in human tumors before and after androgen withdrawal (Figure 1). We found using TMA of 112 PC patients that TCTP levels significantly decrease after castration to become uniformly and highly overexpressed in metastatic CR tumors (Figure 1h,i). The highly uniform expression of Hsp27 in 75% of metastatic CR lesions obtained from rapid autopsy specimens, further underscored the association of TCTP with the lethal phenotype of this disease. Moreover, using another TMA of 99 normal, benign, and Gleason pattern PCs, we found that only 18.5% of cancers expressed TCTP (Figure 1g). No or weak expression is detectable in normal or benign prostate samples (N, PIN, BPH) or in the normal glands (N) disseminated inside the tumors, making this target very interesting for PC therapy to avoid undesirable toxicity in normal prostate tissues. These results are consistent with a recent study showing high expression of TCTP in a fraction of breast cancers that correlates with poor prognosis.22 TCTP expression was also not detectable in normal breast parenchyma.
To assess the role of TCTP as therapeutic target in CRPC, we chose AI PC-3 and CR C4-2 cells to study the effect of TCTP downregulation. ASO and siRNA are specific strategies to silence gene expression. Pre-treatment of PC-3 or C4-2 cells with TCTP-ASO reduced cell viability, blocked cell cycle, and enhanced apoptosis via caspase-3 activation (Figures 3 and 4). These results support recent data showing that TCTP antagonizes apoptosis by enhancing the antiapoptotic actions of Mcl-1 and BCL-XL by anchoring into the mitochondrial membrane and inhibiting dimerization of proapoptotic protein BAX.21 Consistent with these in vitro data, systemic administration of TCTP-ASO monotherapy suppressed PC-3 and LNCaP tumor growth in vivo and also significantly enhanced docetaxel activity (Figure 5). Previously, a report also demonstrates that TCTP overexpression is associated with loss of sensitivity to artesunate in tumor cells.45 More interestingly, we found that CR progression after castration correlates with TCTP overexpression and loss of P53 (Figure 6a). We observed that TCTP silencing using ASO is able to restore P53 expression (Figure 6b) and function (Figure 6d), suggesting that castration-sensitivity is linked to P53 expression. A similar regulation pathway has recently been described in mammary tumor cells. Amson et al., reported a feedback loop between TCTP and tumor suppression P53. They found that TCTP directly associates with the E3 ubiquitin ligase MDM2, increasing MDM2-mediated ubiquitination of P53 and promoting its degradation. They also nicely demonstrated that P53 binds to a P53 responsive element that is present in the promoter of TCTP, leading to the transcriptional repression of TCTP.22 Although the biological role of P53 is less defined in PC,32,33 our study demonstrates for the first time a direct correlation between TCTP overexpression, lack of P53, and CRPC progression. Additional study is required in order to definitely assess the role of the tumor suppressor P53 in PC progression.
In summary, we highlight a novel mechanism mediating Hsp27 cytoprotection in CRPC after androgen withdrawal and chemotherapy. Hsp27 interacts and stabilizes TCTP by inhibiting its stress-induced ubiquitination and proteasomal degradation, leading to decreased P53 content and function. Furthermore, we provide preclinical proof-of-principle that combining ASO-mediated TCTP knockdown with castration and/or docetaxel therapy could serve as a novel strategy to treat CRPC, with no or little toxicity for normal prostate cells.
Materials and Methods
Cell lines and cell culture conditions. The AI prostate cancer cell line PC-3 was purchased from the American Type Culture Collection (Rockville, MD) and maintained in Dulbecco's Modified Eagle's Medium (Invitrogen, Cergy Pontoise, France), supplemented with 10% fetal calf serum (FCS). The human CS prostate cancer cell line LNCaP cells were kindly provided by the University of Virginia (Charlottesville, VA), the human CR prostatic cancer cell line C4-2 was made by Pr. M.G. (The Vancouver Prostate Centre, Vancouver, British Columbia, Canada) and the normal prostate cell line PNT2C2 was kindly provided by the York Cancer Research (University of York, York, UK). Those cells were maintained in RPMI 1640 (Invitrogen) supplemented with 10% FCS. Colorectal cancer stably transfected REGMock and REGHsp27 cells were kindly provided by Dr C.G. and maintained in F10 medium (Invitrogen) supplemented with 10% FCS.
Lentiviral infection of Hsp27 into LNCaP cells. The full-length cDNA for human Hsp27 was subcloned into the lentiviral vector pHR′-CMV-EGFP at the BamHI and XhoI sites and stably transfected to LNCaP as described before by Rocchi et al.7
Yeast two-hybrid SOS recruitment system. We used the yeast two-hybrid SOS recruitment system cytotrap from Stratagene (Agilent technologies, Massy, France) to identify new Hsp27 partners involved in CRPC progression following the same protocol recently published by Thalapilly et al.23
Xenografted tumors. 10 × 106 LNCaP and C4-2 cells were inoculated subcutaneously with 0.1 ml of Matrigel (BD Biosciences Discovery Labware, Le Pont de Claix, France) in male athymic nude mice (Charles River Laboratories, L'Arbresle, France). C4-2 tumors were harvested 2 months after inoculation and LNCaP tumors were harvested before (4 weeks after inoculation) or 40 days after castration (9 weeks after inoculation). Proteins from tumors were extracted and analyzed by western blot.
Western blot analysis. Western blot analysis was performed as described previously8 with 1:5,000 rabbit anti-Hsp27 polyclonal antibody (Assay Designs, Villeurbanne, France), 1:2,000 rabbit TCTP polyclonal antibody (Abcam, Cambridge, UK), 1:250 rabbit Msl-1 polyclonal antibody (Abcam), 1:500 mouse anti-ubiquitin monoclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany), 1:1,000 rabbit anti-caspase-3 polyclonal antibody (Cell Signaling Technology, Danvers, MA) or 1:500 mouse anti-P53 (DO-1) monoclonal antibody (Beckman Coulter, Krefeld, Deutschland). Loading levels were normalized using 1:5,000 rabbit anti-glyceraldehyde-3-phosphate dehydrogenase polyclonal (Abcam) or 1:2,500 mouse anti-vinculin monoclonal antibodies (Sigma Chemical, St Louis, MO).
TMA construction. TCTP expression was assessed in two different TMA from samples before and after castration. The first TMA was made by Pathology Department of Hôpital Nord (Marseille, France), with different non-treated prostate samples (n = 99) obtained from patients who underwent radical prostatectomy in the Department of Urology of the Hôpital Nord of Marseilles as described by S.G.46 Several cores were punched (i.e., 2, 4 or 6 cores per patient) with a total of 352 cores (see the map of the TMA in a Supplementary Figure S3a). This TMA includes 75 normal (N) prostate tissues, 6 PIN, 12 BPH, 168 PC Gleason grade 3 (G3), 67 PC Gleason grade 4 (G4), and 14 PC Gleason grade 5 (G5). The second TMA was made by Vancouver Prostate Centre with CT PC samples (n = 112) obtained from the Tissue Bank of Vancouver Prostate Centre.7 TMA was made with triplicate cores per patient with a total of 336 cores (see the map of the TMA in Supplementary Figure S3b). Specimens were chosen to represent various castration treatment duration before radical prostatectomy ranging from no treatment (n = 21) to 0–3 months (n = 21), 3–6 months (n = 28), and 6 months (n = 28) treated patients. CR tumors were also identified (n = 14). All the tumors were obtained from radical prostatectomy specimens while CR tissues were obtained from metastatic lesions from the warm autopsy specimens of 14 men who succumbed to PC. All radical prostatectomy specimens in the array were clinical stage T1 or T2. One metastatic prostate lesion was obtained per patient from 7 bones, 3 lymph nodes, 2 livers, 1 adrenal, and 1 lung lesions.
TMA and image analysis. The first TMA was analyzed as described previously47,48 with 1:100 anti-TCTP antibody (Abcam). And the second TMA analysis was performed as described previously8 with 1:50 anti-TCTP antibody (Abcam).
Immunofluorescence. Immunofluorescence analysis was performed as described previously24 on LNCaPMock and LNCaPHsp27 cells with primary antibodies mouse monoclonal Hsp27 (StressGen, Ann Arbor, MI) or rabbit polyclonal TCTP (Abcam) and secondary fluorescent antibodies goat anti-mouse Alexa Fluor 546 (Invitrogen) or goat anti-rabbit Alexa Fluor 488 (Invitrogen). Images were captured using a Zeiss 510 META fluorescence confocal microscope plan 63X/1.4 (Zeiss, Le Pecq, France) followed by analysis of focal colocalization performed with Image Proplus 6 software (MediaCybernetics, Wokingham, UK) with an assignment of yellow for colocalized foci and green or red as non-colocalization.
Immunoprecipitation. Cleared lysates (250 µg) with adjusted protein concentration (Protein assay; Bio-Rad, Marnes-la-Coquette, France) were used for immunoprecipitation with 1:50 rabbit anti-TCTP antibody (Abcam) O/N at 4 °C. Immune complexes were precipitated after 1 hour incubation with 30 µl of TrueBlot anti-rabbit Immunoglobulin beads (eBiosciences, Paris, France). The complexes were resuspended in protein sample buffer (Bio-Rad) and boiled for 5 minutes before western blot as described before. We used the rabbit True Blot anti-rabbit IgG secondary antibody (eBiosciences) to reveal the western blot.
Analysis of TCTP mRNA expression. The quantitative reverse transcription PCR analysis was done on a LightCycler detection system (Roche Applied Science, Meylan, France). Expression levels of 18S subunit of the ribosome gene were used as an internal control. TCTP and 18S were detected using the SYBR Premix Ex Taq (Takara Bio, St Germain en Laye, France) following the manufacturer's instructions with primers indicated in Supplementary Table S1. Each sample was analyzed in triplicate and the experiment was repeated three times. Results were analyzed using RealQuant data analysis software (Roche, Neuilly-sur-seine, France) and the 2(-δδCT) method.49
ASOs and siRNA sequences. 2′-O-(2-methoxyethyl) ASO, OGX-427, was obtained from OncoGenex (Vancouver, British Columbia, Canada). The sequence of OGX-427 corresponds to the human Hsp27 translation initiation site (5′-GGGACGCGG-CGCTCGGTCAT-3′). A mismatch ASO (ASO; 5′-CAGCAGCAGAGTATTTATCAT-3′) was used as a control. TCTP phosphorothioate ASO targeting the human TCTP (5′-ACCAATGAGCGAGTCATCAA-3′) and control oligonucleotide (5′-CGTGTAGGTACGGCAGATC-3′) were purchased from Operon (Eurofin MWG, Courtaboeuf, France). TCTP siRNA (5′-AACCCGUCCGCGAUCUCCCdGdG-3′) was validated and purchased from Qiagen (Courtaboeuf, France). The control siRNA was also from Qiagen. Caspase-3 siRNA (5′-UGGAUUAUCCUGAGAUGGGTT-3′) was purchased from Cell Signaling (Caspase-3 siRNA I; Ozyme, St Quentin Yvelines, France). The control siRNA was also from Cell Signaling.
Treatment of cells with ASO and siRNA. Cells were plated at a density of 25,000 (PC-3), 50,000 (C4-2) and 75,000 (LNCaP) by 1.9 cm2 and treated the day after with indicated siRNA or ASO for 1 or 2 days respectively. Oligofectamine, a cationic lipid (Invitrogen), was used to increase siRNA or ASO uptake into the cells. Cells were treated with indicated siRNA or ASO concentrations after a preincubation for 20 minutes with 3 mg/ml of oligofectamine in serum-free OPTI-MEM (Invitrogen). Four hours later, 1 ml of FCS was added in the medium. To study the effects on rates of TCTP proteasome degradation, cycloheximide (10 µg/ml) and MG132 (10 µmol/l) were added at the end of the second transfection with ASO in replaced medium for 24, 48, and 72 hours.
Transient transfection into C4-2 and LNCaP cells. LNCaP and C4-2 cells were transfected with pcDNA 3.2/V5-DEST vector (Invitrogen) containing wild-type TCTP (pDEST-TCTP) or empty vector control (pDEST-Mock) using FuGENE HD transfection reagent (Promega, Charbonnieres, France). The transfection complex was added to the cells following the manufacturer's instructions for 7:2 ratio. LNCaP cells were transfected with P53-shRNA subcloned into a lentiviral vector pLentilux RSV-CMV-EGFP kindly provided by the university of Michigan (Ann Arbor, MI). The transfection complex, containing 1:10 ratio of the lentiviral vector and 10 µg/ml Polybrene (1,5-Dimethyl-1,5-diazaundecamethylene polymethobromide; Millipore, Molsheim, France) in RPMI 1640 supplemented with 10% FCS, was added to the cells during 48 hours.
In vitro mitogenic assay. Crystal violet assay (LNCaP and C4-2) or bromure de 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay (PC-3) were used as previously described.8,50 In brief, cells were seeded in each well of 12 wells microtiter plates (30,000–50,000 cells per well). PC-3 cells were transiently transfected the day after seeding with 5 nmol/l of TCTP-siRNA for 48 hours or 100 nmol/l of TCTP-ASO for 72 hours. Cells were then treated with 50 nmol/l (half maximal inhibitory concentration (IC50)) of docetaxel and MTT assays were performed after 24 hours. LNCaPMock and LNCaPHsp27 cells were transiently transfected the day after seeding with 5 nmol/l of TCTP siRNA for 48 hours. LNCaP and C4-2 were transiently transfected with 0.02 µg/µl of pDEST-TCTP or -Mock for 48 hours. Cells were then treated with 1 nmol/l (IC50) of docetaxel in serum-free media (mimics androgen withdrawal in vitro) and crystal violet assays were performed the day after. Each assay was performed in triplicate. Docetaxel concentrations were previously determined for each cell line based on the IC50.
Flow cytometric analysis. Flow cytometry of propidium iodide-stained nuclei was performed as described previously.8 In brief, PC-3 cells were plated at the density of 106 cells into 10 cm dishes. Cells were treated the day after seeding with 5 nmol/l of TCTP or control-siRNA and 100 nmol/l of TCTP- or control-ASO. Cells were then treated with 50 nmol/l of docetaxel and analyzed after 24 hours for relative DNA content on a dual laser flow cytometer (FACSCalibur; Becton Dickinson Biosciences, Le Pont de Claix, France). Each assay was performed in triplicate.
Assessment of in vivo tumor growth. Approximately 3 × 106 PC-3 cells and 10 × 106 LNCaP cells were inoculated subcutaneously with 0.1 ml of Dulbecco's Modified Eagle's Medium (Invitrogen) supplemented with 10% FCS for PC-3 or with 0.1 ml of Matrigel (BD Biosciences) for LNCaP in the flank region of 4-week-old male athymic nude mice (Charles River Laboratories) via a 23-gauge needle. Mean tumor volume was similar in all groups before therapy. When PC-3 tumors reached 50 mm3, usually 3–4 weeks after injection, mice were randomly selected for treatment with TCTP-ASO alone, control-ASO alone, TCTP-ASO plus docetaxel or control-ASO plus docetaxel. Mice bearing LNCaP tumors between 100 and 200 mm3 in volume were castrated via scrotal approach and randomly assigned to a treatment arm. Mice were treated 1 week after castration with TCTP- or control-ASO alone. For both experiments, each experimental group consisted of 10 mice and the tumor volume measurements performed once weekly and calculated by the formula length × width × depth × 0.5236.50 After randomization, 12.5 mg/kg TCTP- or control-ASO were injected intraperitoneally once daily for 9 or 10 weeks for ASO monotherapy groups and 13 weeks for the ASO plus docetaxel groups. A total of 35 mg/kg docetaxel was administrated intraperitoneally three times per week from days 7–14. Data points were expressed as average tumor volume levels ± SE. All animal procedures were performed in accordance with protocols approved by French laws and the European directives and with appropriate institutional certification.
P53 rescue experiments. LNCaP cells were transiently transfected with P53-shRNA and plated at the density of 75,000 cells by 1.9 cm2. The day after, cells were treated with TCTP- or control-ASO for 2 days. The crystal violet assay was performed 2 weeks after. Each assay was performed in triplicate.
Chemotherapeutic and chemical agents. Docetaxel was obtained from Sanofi-aventis (Paris, France). Cycloheximide and MG132 were purchased from Calbiochem (Merck Chemicals, Nottingham, UK).
Statistical analysis. All the results were expressed as mean ± SE. Statistical analysis was performed using one-way analysis of variance followed by Fisher's protected least significant difference test (Statview 512; Brain Power, Calabasas, CA). *P ≤ 0.05 was considered significant, with **P ≤ 0.01 and ***P ≤ 0.001.
SUPPLEMENTARY MATERIAL Figure S1. Estimation of TCTP half-life by a cycloheximide treatment in LNCaP cells. Figure S2. TCTP inhibition increases apoptosis in CR PC-3 cells and enhanced docetaxel chemotherapy. Figure S3. Prostate cancer tissue microarray MAPs and scoring. Table S1. Sequences of primers used in this study.
Acknowledgments
We thank Marie-Noëlle Lavaut (CRCM) for her excellent technical assistance in immunohistochemistry experimentation. We thank Nelson Dusetti and Odile Gayet (CRCM) for her excellent help in two-hybrid experiments. This work was supported by grants from the French Cancer Institute (InCa, PAIR prostate program), l'Institut National de la Santé et de la Recherche Médicale (Inserm), l'Association pour la Recherche sur le Cancer (ARC), l'Association pour la Recherche sur les Tumeurs de la Prostate (ARTP), the French Research Ministry (FRM), the National Research Agency (ANR), the Mediterranean University, and the competitivity pole Eurobiomed. C.G. team has the « label de La Ligue National contre le Cancer». The University of British Columbia has submitted patent applications, listing Dr Gleave and Dr Rocchi as inventors, on the Hsp27 antisense sequence (OGX-427) described in this paper. This patent has been licensed to OncoGenex Technologies, a Vancouver-based biotechnology company that Dr Gleave has founding shares in. The authors declared no conflict of interest.
Supplementary Material
Estimation of TCTP half-life by a cycloheximide treatment in LNCaP cells.
TCTP inhibition increases apoptosis in CR PC-3 cells and enhanced docetaxel chemotherapy.
Prostate cancer tissue microarray MAPs and scoring.
Sequences of primers used in this study.
REFERENCES
- Fusi A, Procopio G, Della Torre S, Ricotta R, Bianchini G, Salvioni R.et al. (2004Treatment options in hormone-refractory metastatic prostate carcinoma Tumori 90535–546. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME.et al. (2004Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer N Engl J Med 3511513–1520. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN.et al. (2004Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer N Engl J Med 3511502–1512. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A.et al. (2007Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets FEBS Lett 5813665–3674. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB., and, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ciocca DR., and, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C.et al. (2005Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis Cancer Res 6511083–11093. [DOI] [PubMed] [Google Scholar]
- Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L.et al. (2004Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer Cancer Res 646595–6602. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Jugpal P, So A, Sinneman S, Ettinger S, Fazli L.et al. (2006Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro BJU Int 981082–1089. [DOI] [PubMed] [Google Scholar]
- Hirte HW, McGuire III WP, Edwards RP, Husain A, Hoskins P, Michels JE.et al. (2010Clinical Science Symposium, Novel Therapies and Approaches for Management of Gynecologic Cancers J Clin Oncol 2815s [Google Scholar]
- Hotte SJ, Yu EY, Hirte HW, Higano CS, Gleave M., and, Chi KN. OGX-427, a 20 methoxyethyl antisense oligonucleotide (ASO), against Hsp27: Results of a first-in-human trial. J Clin Oncol. 2009;27:15s. [Google Scholar]
- Tuynder M, Fiucci G, Prieur S, Lespagnol A, Géant A, Beaucourt S.et al. (2004Translationally controlled tumor protein is a target of tumor reversion Proc Natl Acad Sci USA 10115364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TH, Chen L., and, Guan XY. Role of translationally controlled tumor protein in cancer progression. Biochem Res Int. 2012;2012:369384. doi: 10.1155/2012/369384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T., and, Bommer UA. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci. 1999;112 (Pt 8):1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- Burgess A, Labbé JC, Vigneron S, Bonneaud N, Strub JM, Van Dorsselaer A.et al. (2008Chfr interacts and colocalizes with TCTP to the mitotic spindle Oncogene 275554–5566. [DOI] [PubMed] [Google Scholar]
- Yarm FR. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol Cell Biol. 2002;22:6209–6221. doi: 10.1128/MCB.22.17.6209-6221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Chen J, Wong J., and, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol. 2002;156:249–259. doi: 10.1083/jcb.200108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Chern JJ, Cai Y, Liu M., and, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- Koziol MJ, Garrett N., and, Gurdon JB. Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr Biol. 2007;17:801–807. doi: 10.1016/j.cub.2007.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Peng HW, Cheng YS, Yuan HS., and, Yang-Yen HF. Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol Cell Biol. 2005;25:3117–3126. doi: 10.1128/MCB.25.8.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susini L, Besse S, Duflaut D, Lespagnol A, Beekman C, Fiucci G.et al. (2008TCTP protects from apoptotic cell death by antagonizing bax function Cell Death Differ 151211–1220. [DOI] [PubMed] [Google Scholar]
- Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D.et al. (2011Reciprocal repression between P53 and TCTP Nat Med 1891–99. [DOI] [PubMed] [Google Scholar]
- Thalappilly S, Suliman M, Gayet O, Soubeyran P, Hermant A, Lecine P.et al. (2008Identification of multi-SH3 domain-containing protein interactome in pancreatic cancer: a yeast two-hybrid approach Proteomics 83071–3081. [DOI] [PubMed] [Google Scholar]
- Andrieu C, Taieb D, Baylot V, Ettinger S, Soubeyran P, De-Thonel A.et al. (2010Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E Oncogene 291883–1896. [DOI] [PubMed] [Google Scholar]
- Baylot V, Andrieu C, Katsogiannou M, Taieb D, Garcia S, Giusiano S.et al. (2011OGX-427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer Cell Death Dis 2e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert B, Eckel B, Fasquelle L, Moulin M, Bouhallier F, Gonin V.et al. (2012Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins PLoS ONE 7e29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P.et al. (2007Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity Cancer Res 6710455–10465. [DOI] [PubMed] [Google Scholar]
- Arcuri F, Papa S, Carducci A, Romagnoli R, Liberatori S, Riparbelli MG.et al. (2004Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activity Prostate 60130–140. [DOI] [PubMed] [Google Scholar]
- Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R.et al. (2002Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1 Proc Natl Acad Sci USA 9914976–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ., and, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun. 1997;239:357–366. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Thirugnanam S, Zheng G, Chen A., and, Ramaswamy K. Gene silencing of translationally controlled tumor protein (TCTP) by siRNA inhibits cell growth and induces apoptosis of human prostate cancer cells. Int J Oncol. 2009;34:1241–1246. [PubMed] [Google Scholar]
- McDonnell TJ, Navone NM, Troncoso P, Pisters LL, Conti C, von Eschenbach AC.et al. (1997Expression of bcl-2 oncoprotein and p53 protein accumulation in bone marrow metastases of androgen independent prostate cancer J Urol 157569–574. [PubMed] [Google Scholar]
- Mehta R, Kyshtoobayeva A, Kurosaki T, Small EJ, Kim H, Stroup R.et al. (2001Independent association of angiogenesis index with outcome in prostate cancer Clin Cancer Res 781–88. [PubMed] [Google Scholar]
- Gan L, Wang J, Xu H., and, Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate. 2011;71:1158–1166. doi: 10.1002/pros.21331. [DOI] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E., and, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Garrido C, Schmitt E, Candé C, Vahsen N, Parcellier A., and, Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–584. [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY.et al. (2001Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death Nature 410549–554. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y.et al. (1999Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays J Natl Cancer Inst 911758–1764. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Muracciole X, Fina F, Mulholland DJ, Karsenty G, Palmari J.et al. (2004Molecular analysis integrating different pathways associated with androgen-independent progression in LuCaP 23.1 xenograft Oncogene 239111–9119. [DOI] [PubMed] [Google Scholar]
- Rho SB, Lee JH, Park MS, Byun HJ, Kang S, Seo SS.et al. (2011Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53 FEBS Lett 58529–35. [DOI] [PubMed] [Google Scholar]
- Chung S, Kim M, Choi W, Chung J., and, Lee K. Expression of translationally controlled tumor protein mRNA in human colon cancer. Cancer Lett. 2000;156:185–190. doi: 10.1016/s0304-3835(00)00460-2. [DOI] [PubMed] [Google Scholar]
- Deng SS, Xing TY, Zhou HY, Xiong RH, Lu YG, Wen B.et al. (2006Comparative proteome analysis of breast cancer and adjacent normal breast tissues in human Genomics Proteomics Bioinformatics 4165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diraison F, Hayward K, Sanders KL, Brozzi F, Lajus S, Hancock J.et al. (2011Translationally controlled tumour protein (TCTP) is a novel glucose-regulated protein that is important for survival of pancreatic beta cells Diabetologia 54368–379. [DOI] [PubMed] [Google Scholar]
- Mitchell BS. The proteasome–an emerging therapeutic target in cancer. N Engl J Med. 2003;348:2597–2598. doi: 10.1056/NEJMp030092. [DOI] [PubMed] [Google Scholar]
- Efferth T. Mechanistic perspectives for 1,2,4-trioxanes in anti-cancer therapy. Drug Resist Updat. 2005;8:85–97. doi: 10.1016/j.drup.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Giusiano S, Baylot V, Andrieu C, Fazli L, Gleave M, Iovanna JL.et al. (2012TP53INP1 as new therapeutic target in castration-resistant prostate cancer Prostate 721286–1294. [DOI] [PubMed] [Google Scholar]
- Charpin C, Giusiano S, Secq V, Carpentier S, Andrac L, Lavaut MN.et al. (2009Quantitative immunocytochemical profile to predict early outcome of disease in triple-negative breast carcinomas Int J Oncol 34983–993. [DOI] [PubMed] [Google Scholar]
- Charpin C, Secq V, Giusiano S, Carpentier S, Andrac L, Lavaut MN.et al. (2009A signature predictive of disease outcome in breast carcinomas, identified by quantitative immunocytochemical assays Int J Cancer 1242124–2134. [DOI] [PubMed] [Google Scholar]
- Livak KJ., and, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Gleave M, Tolcher A, Miyake H, Nelson C, Brown B, Beraldi E.et al. (1999Progression to androgen independence is delayed by adjuvant treatment with antisense Bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate tumor model Clin Cancer Res 52891–2898. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimation of TCTP half-life by a cycloheximide treatment in LNCaP cells.
TCTP inhibition increases apoptosis in CR PC-3 cells and enhanced docetaxel chemotherapy.
Prostate cancer tissue microarray MAPs and scoring.
Sequences of primers used in this study.