Abstract
Axl is a tyrosine kinase receptor that was first identified as a transforming gene in human myeloid leukemia. Recent converging evidence suggests its implication in cancer progression and invasion for several solid tumors, including lung, breast, brain, thyroid, and pancreas. In the last decade, Axl has thus become an attractive target for therapeutic development of more aggressive cancers. An emerging class of therapeutic inhibitors is now represented by short nucleic acid aptamers. These molecules act as high affinity ligands with several advantages over conventional antibodies for their use in vivo, including their small size and negligible immunogenicity. Furthermore, these molecules can easily form conjugates able to drive the specific delivery of interfering RNAs, nanoparticles, or chemotherapeutics. We have thus generated and characterized a selective RNA-based aptamer, GL21.T that binds the extracellular domain of Axl at high affinity (12 nmol/l) and inhibits its catalytic activity. GL21.T blocked Axl-dependent transducing events in vitro, including Erk and Akt phosphorylation, cell migration and invasion, as well as in vivo lung tumor formation in mice xenografts. In this respect, the GL21.T aptamer represents a promising therapeutic molecule for Axl-dependent cancers whose importance is highlighted by the paucity of available Axl-specific inhibitory molecules.
Introduction
Axl belongs to the TAM family of tyrosine kinase receptors (RTKs) that also includes Sky (Tyro3, Dtk) and Mer. They are characterized by an extracellular domain consisting of two immunoglobulin-like domains followed by two fibronectin type 3-like domains. Axl-family members are activated by Growth-arrest-specific gene 6 (Gas6), a member of the vitamin K-dependent protein family that resembles blood coagulation factors rather than typical growth factors.1,2,3,4 In addition to Gas6, Protein S can also activate Sky and Mer on different cells types under physiologic and/or pathologic conditions.5,6,7
Axl overexpression has been reported in many human cancers and is associated with invasiveness and/or metastasis in lung,8 prostate,9 breast,10 gastric11 and pancreatic12 cancers, renal cell carcinoma13 as well as glioblastoma.14 Furthermore, by a phosphoproteomic approach based on the profiling of phosphotyrosine signaling, activated Axl protein was detected in ~5% primary tumors of non–small-cell lung cancer.15 More recently, activation of Axl has been found in thyroid papillary and anaplastic carcinomas,16 cutaneous melanomas17 as well as in B-cell chronic lymphocytic leukemia.18
Furthermore, it has been reported that expression of Axl is induced by targeted and chemotherapy drugs and upregulation of Axl by chemotherapy confers drug resistance in acute myeloid leukemia19 and its overexpression has been shown to be one of the mechanisms that can promote resistance to epidermal growth factor receptor-family directed therapies in breast.20
Despite the importance of Axl in several tumors has been well established, its biological functions have only recently begun to be understood. Axl has been characterized as an oncogenic kinase by its promotion of cancer cell survival, proliferation, invasion and migration, and more recently, it has been shown that Axl is able to mediate the oncogenic roles of the FOS-related component Fra-1 on tumor cell motility21 and to drive YAP-dependent oncogenic functions leading to proliferation and invasion of cancer cells.22
These data indicate that Axl signaling represents a novel target class for tumor therapeutic development.23,24 To date, only few inhibitors of Axl have been reported that are completely unrelated to the anti-Axl aptamer both from the structural and mode of action point of view: (i) small-molecule inhibitors, such as R428, that block the catalytic activities of Axl;25 (ii) anti-Axl monoclonal antibody that blocks the ligand Gas6 binding to the receptor;26 (iii) proteins derived from the extracellular domain of Axl that inhibit its action by competition on ligand binding (International Patent application WO2008098139).
An emerging new class of therapeutic molecules against RTKs is composed of nucleic acid aptamers.27 Aptamers are short structured single-stranded RNA or DNA that bind with high affinity to their target molecules. Aptamers possess many advantages over proteins as therapeutic reagents, including low cost, convenient synthesis and modification with high batch fidelity, no immunogenicity, rapid tissue penetration, and long-term stability.28 Furthermore, in the last years, aptamers targeting cell surface proteins are being explored as promising delivery agents to specifically drive nanoparticles, small interfering RNAs, chemotherapeutic cargos and molecular imaging probes toward a distinct disease or tissue.29,30
Herein, we have developed and characterized a nuclease resistant 2′-fluoro pyrimidines RNA aptamer, named GL21.T, capable of binding and inhibiting Axl RTK. When applied to Axl-expressing cancer cells the aptamer strongly inhibits cell migration and invasion and interferes with spheroid formation by cancer cells. Furthermore, it strongly inhibits tumor growth in a mouse xenograft model of human non–small-cell lung cancer.
Taken together, the results show that GL21.T aptamer is a promising RNA-based molecule that can be developed as a more effective alternative to existing Axl inhibitors.
Results
Axl receptor is target of GL21
By using differential whole cell SELEX on human glioma cell lines we recently identified a 2′-F Py-containing RNA aptamer, named GL21, that binds the highly malignant U87MG cells with an apparent Kd of 221 nmol/l.31 Using a rational approach based on its predicted secondary structure31,32 we designed a 34mer truncated version of the 92mer original molecule, named GL21.T, that contains the active site of GL21 and preserves high binding affinity to the U87MG cells (Supplementary Figure S1). As a first attempt to identify the functional targets of GL21.T we performed a phospho-receptor tyrosine kinase (RTK) array analysis that provided us with convincing evidence that the target(s) of GL21.T may belong to the TAM receptor family (Supplementary Figure S2).
Therefore, to definitely determine the target of GL21.T we first performed a filter binding analysis with the soluble extracellular domain of human Axl, Dtk (Tyro3) and Mer as targets (here indicated as EC-Axl, EC-Dtk, and EC-Mer, respectively), that revealed a stronger affinity of GL21.T for EC-Axl (Kd of 13 nmol/l) than for EC-Dtk (Kd of 43 nmol/l) (Figure 1a) whereas no saturable binding was detectable for EC-Mer (data not shown). Since the EC-Axl and EC-Dtk used in the binding assay are disulfide-linked homodimers we determined as well the binding of GL21.T upon their reduction to monomers and showed that the aptamer binds in vitro the ectodomain of Axl and Dtk irrespective of whether proteins are present as dimers or monomers (Figure 1b).
Figure 1.
GL21.T aptamer specifically interacts with Axl. (a) Binding isotherm for GL21.T: EC-Axl (left) and GL21.T:EC-Dtk (right) complexes. (b) EC-Axl or EC-Dtk (40 nmol/l, with and without 5 mmol/l DTT treatment), were incubated with 1 nmol/l GL21.T, protein-bound RNA was collected by nitrocellulose filters and radioactivity quantified. (c) Left, binding of 50 nmol/l radiolabeled GL21.T on the indicated cell lines. Right, lysates from the indicated cell lines were immunoblotted with anti-Axl antibodies. (d), Left, binding of 50 nmol/l radiolabeled GL21 on U87MG, SkBr3, or SkBr3 cells following 72 hours-transfection with Axl TruClone (Axl). Right, lysates from SKBr3 or SKBr3 transfected with Axl were immunoblotted with anti-Axl antibodies. (e), Left, binding of 50 nmol/l radiolabeled GL21.T on U87MG cells following 72 hours-transfection with a specific Axl short hairpin RNA (shRNA) (shRNAAxl) or a nonrelated shRNA (shRNActrl). Right, lysates from U87MG cells following 72 hours-transfection with shRNAAxl or shRNActrl were immunoblotted with anti-Axl antibodies. Values below the blot indicate signal levels relative to shRNActrl-transfected cells, arbitrarily set to 1 (labeled with asterisk). Intensity of bands has been calculated using the NIH Image Program on at least two different expositions to assure the linearity of each acquisition. In (c–e), blots shown are representative of at least three independent experiments and anti-α–tubulin antibodies were used as an internal control. (f) Binding of 50 nmol/l radiolabeled GL21.T, prior incubated with 150 nmol/l EC-Axl for 15 minutes at 37 °C, on U87MG cells. In (b–f), the results are expressed relative to the background binding detected with the unrelated aptamer used as a negative control. (g) Internalization rate of GL21.T and unrelated uptamer. Results are expressed as percentage of internalized RNA relative to total bound aptamer. In (a–g), error bars depict means ± SD (n = 3).
Consistently with its ability to specifically bind to the membrane-bound Axl as well as to the soluble ectodomain of the receptor, binding of GL21.T in stable tumor-derived cell lines was solely detected for the Axl receptor-positive cells (Figure 1c). Accordingly, binding of the GL21.T aptamer to the human breast cancer cells, SkBr3, that do not express Axl, may be rescued by forced expression of exogenous Axl in the cell derivative, SkBr3/Axl (Figure 1d) and, conversely, binding to the U87MG target cells was abrogated by depletion of endogenous Axl with a specific short hairpin RNA (shRNA) (Figure 1e). Furthermore, we show that binding of GL21.T to the U87MG cells was strongly competed by the recombinant EC-Axl (Figure 1f), thus confirming that recognition of target cells is mediated by aptamer binding to the extracellular domain of Axl on the cell surface. Moreover, differently from other aptamers that we have generated as high-affinity ligands for respective targets (Kd value around 10 nmol/l) but that are not endocytosed into target cells (data not shown), GL21.T is readily internalized into U87MG cells, getting ~30% of cell internalization following 15-minute incubation and reached ~60% following 2 hours of aptamer treatment (Figure 1g).
Taken together, these results indicate bona fide that the GL21.T aptamer specifically recognizes Axl and, at a lower affinity, Dtk receptors either if expressed on the cell surface in their physiological context as well as the purified soluble extracellular domain of the receptor both in monomeric and dimeric form. Furthermore, because of its ability to rapidly internalize within Axl-positive target cells it is a highly promising candidate as cargo for tissue specific internalization.
The GL21.T aptamer inhibits the Axl signaling but does not hamper cell growth
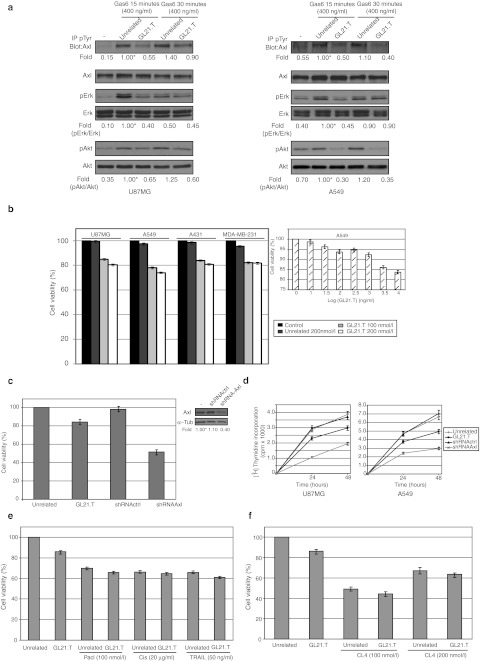
Gas6, the principal natural ligand of Axl,2 induces tyrosine phosphorylation of the receptor and the resulting activation of downstream signaling pathways that can lead to cell proliferation, migration, or to prevention of apoptosis.33 We first determined whether GL21.T could affect Axl activation following Gas6 stimulation. As shown in Figure 2a, treating either U87MG (left panel) or A549 (right panel) cells with GL21.T (200 nmol/l) drastically reduced the amount of tyrosine-phosphorylated Axl reaching around 50% inhibition at 15 minutes of Gas6 stimulation, whereas no effect was observed in the presence of an unrelated sequence used as a negative control. Consistently, treatment with the GL21.T aptamer reduces the extent of activation of two critical intracellular effectors of Axl, the extracellular-signal regulated kinase 1 and 2 (Erk1/2) and the PKB/Akt kinase,34 thus confirming that GL21.T acts as a competitive inhibitor of Axl.
Figure 2.
GL21.T inhibits Axl activation. (a) Serum-starved U87MG and A549 cells were either left untreated or treated for 3 hours with 200 nmol/l GL21.T or the unrelated aptamer and then stimulated for the indicated times with Gas6 in the presence of each aptamer. Cell lysates were either immunoprecipitated with anti-(phospho)-tyrosine (pTyr) antibodies and immunoblotted with anti-Axl antibodies or immunoblotted with anti-Axl, anti-(phospho)-Erk1/2 (pErk), anti-(phospho)-Akt (pAkt) antibodies, as indicated. Filters were stripped and reprobed with anti-Erk and anti-Akt antibodies, as indicated. Values below the blots indicate signal levels relative to 15 minutes-Gas6 stimulated unrelated aptamer control, arbitrarily set to 1 (labeled with asterisk). Quantitations were done as in Figure 1. Blots shown are representative of at least four independent experiments. (b) Indicated cell lines were left untreated or treated for 24 hours with increasing concentrations of GL21.T or the unrelated aptamer (as indicated), cell viability was analyzed as reported in Materials and Methods and expressed as percent of viable treated cells with respect to control, untreated cells. (c) Left, A549 cells were treated for 72 hours with GL21.T or the unrelated aptamer (200 nmol/l-final concentration) or A549 were transfected with shRNAAxl or shRNActrl and cell viability was analyzed as in b. Right, lysates from A549 cells following 72 hours-transfection with shRNAAxl or shRNActrl were immunoblotted with anti-Axl antibodies. Values below the blot indicate signal levels relative to mock-transfected cells, arbitrarily set to 1 (labeled with asterisk). Quantitation was done as in Figure 1. (d) U87MG and A549 cells were treated for 24 or 48 hours with GL21.T or the unrelated aptamer (200 nmol/l -final concentration) and proliferation was determined by [3H]-thymidine incorporation. (e) A549 cells were treated for 24 hours with GL21.T or the unrelated aptamer (200 nmol/l-final concentration) as single agents or in combination with TRAIL, cisplatin (Cis) and paclytaxel (Pacl) at the indicated concentrations. Cell viability was analyzed as in b. (f) A549 cells were treated for 24 hours with GL21.T or the unrelated aptamer (200 nmol/l-final concentration) as single agents or in combination with CL4 aptamer at the indicated concentrations. Cell viability was analyzed as in b. In (b–f) error bars depict means ± SD (n = 4). shRNA, short hairpin RNA.
Erk1/2 and the PKB/Akt are intracellular signaling effectors that promote cell survival and proliferation.33 Therefore, because of GL21.T inhibitory potential on the activation of both these pathways we determined whether GL21.T may reduces cell viability and proliferation. To this end, we analyzed the effects of GL21.T treatment on cell viability in four distinct cell lines. As assessed by the MTT assay, interfering with Axl function reduced the percent of viable cells of ~20% in all cell lines analyzed (Figure 2b, left) that remained stable up to 890 nmol/l-aptamer treatment (Figure 2b, insert), thus displaying a poor inhibitory potential. On the other hand, by using a specific shRNA to knock down Axl, we compared the effects on cell viability of the depletion of Axl to those of competitive inhibition by GL21.T. As shown in Figure 2c, interfering with Axl expression has a much stronger effect that aptamer treatment since it reduced the percent of viable cells to around 50%. Consistently with the poor effects of GL21.T on cell viability, inhibiting Axl with the aptamer had no relevant effects on cell proliferation (Figure 2d) and cell cycle (data not shown) in both A549 and U87MG cells. In A549 cells long-term serum withdrawal induces cell death reducing in 72 hours the percent of viable cells to ~30%. Since cell death was, however, not further increased by inhibiting Axl with GL21.T (data not shown), we thus investigated whether or not GL21.T may instead sensitize cells to external insults as conventional chemotherapeutics. To this end we treated A549 cells with three different chemotherapeutics that acts through different molecular mechanisms, cisplatin, paclitaxel, and TRAIL. As shown in Figure 2e, irrespective of the molecule used, all treatments reduce cell viability to ~60% and no measurable synergy was observed when combined with GL21.T. On the other hand, since the use of the multikinase inhibitor of Axl, Met, and vascular endothelial growth factor receptor GSK1363089, has been shown to enhance the effects of anti-HER1 and HER2 inhibitors,20 we used GL21.T together with the CL4 anti-HER1 aptamer, that cause selective apoptotic cell death,35 and analyzed their combined effects on cell viability. As expected, treating A549 cells with CL4 reduces the percent of viable cells to ~50%, however, the effect is not enhanced by the combination with GL21.T (Figure 2f). This indicates that hampering Axl activity and downstream signaling with the aptamer has poor effects on in vitro cell proliferation.
GL21.T interferes with cell migration and invasion
Even though the modest inhibitory effects of GL21.T on cell growth is in apparent discrepancy with its ability to interfere with Axl-dependent Erk and Akt activation, intracellular signaling initiated by Axl has been reported to be mostly involved in cancer cell migration and invasion rather than in cell proliferation.25,36,37,38 Indeed, besides promoting cell proliferation, one of the most relevant effects caused by the activation of Erk1/2 pathway is the regulation of cellular migration and invasion.39 Therefore, by using the Boyden chamber assay, we addressed the possibility that GL21.T might interfere with cell migration and invasion. As shown in Figure 3a treating cells with GL21.T aptamer (at 200 nmol/l) reduces U87MG and A549 cell migration either stimulated by 10% fetal bovine serum (FBS) (upper panels) or by the Axl physiological ligand, Gas6 (lower panels) of several folds (between 60 and 80% as compared to the unrelated aptamer, see upper right panel), the effect of the aptamer being dose dependent (see lower right panel). Next, we analyzed the interference of GL21.T on the invading capability of the U87MG cells by a chemoinvasion assay in which cells were plated on Matrigel coated filters and allowed to migrate. As shown in Figure 3b, U87MG cells possess a moderate but significant ability to migrate through Matrigel in the presence of 10% FBS, that is almost completely prevented by treatment with GL21.T.
Figure 3.
GL21.T aptamer inhibits cell migration and invasion. (a) Motility of U87MG and A549 cells was analyzed by Transwell Migration Assay in the presence of GL21.T or the unrelated aptamer for 24 hours toward 10% fetal bovine serum (FBS) or Gas6 as inducers of migration. (b) U87MG invasion through matrigel toward 10% FBS was carried out in the presence of GL21.T or the unrelated aptamer for 24 hours. In (a,b) the migrated or invaded cells, respectively, were stained with crystal violet and photographed. Representative photographs of at least three different experiments were shown. The results are expressed as percent of migrated or invaded cells in the presence of GL21.T with respect to cells treated with the unrelated aptamer. Vertical bars indicate the standard deviation values. (c) Serum-starved U87MG and A549 cells were either left untreated or treated for 3 hours with 200 nmol/l GL21.T or the unrelated aptamer and then stimulated with Gas6 in the presence of each aptamer. Rac1-GTP pull down assay was performed as described in Materials and Methods. The amount of total Rac1 was estimated by immunoblotting with anti Rac1 antibodies, as indicated. Values below the blots indicate signal levels relative to unrelated aptamer, arbitrarily set to 1 (labeled with asterisk). Quantitation was done as in Figure 1.
Because of the inhibitory effects of GL21.T on cell invasion and migration, we also examined the activation of Rac1. This is a member of the Rho family of GTPases that are involved in integrins-mediated cell adhesion, spreading, and migration through modulation of the actin cytoskeleton.40 As shown, a marked reduction in the active GTP-bound Rac 1 protein is observed following GL21.T treatment, that is consistent with inactivation of such signaling pathway (Figure 3c). Thus, in good agreement with previous reports that make use of a specific shRNA or the small drug R428,25,41 Axl inhibition by GL21.T treatment results in cell migration and invasiveness impairment.
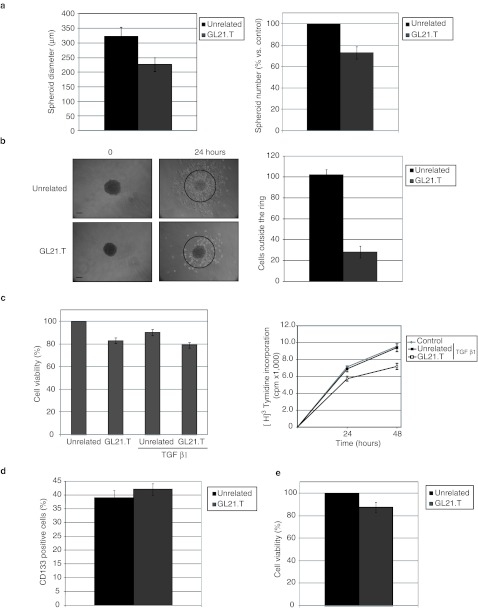
To further confirm the inhibitory effect of GL21.T, we thus determined the effect of the aptamer on spheroid formation and cellular motility. When grown in ultra-low adherent culture dish the U87MG cells forms spheroids that increase in size up to 10 days. As shown in Figure 4a, both the mean size and the number of spheroids was clearly decreased by GL21.T treatment (of ~30%). Furthermore, inhibition on glioma cell motility was then analyzed by using a spheroid radial migration assay. At 10 days tumor spheroids were plated on cell culture dishes and the number of cells spreading out from the spheroid of more than twice the mean radius of spheroids at 24 hours was determined. As shown in Figure 4b, upon treatment with the GL21.T aptamer cell motility is drastically impaired and cells remain clustered as spheroids or closely around them.
Figure 4.
GL21.T aptamer inhibits spheroid formation. (a) U87MG spheroid diameter (left) and number (right) have been calculated following 10 days of treatment in the presence of GL21.T or the unrelated aptamer. (b) Spheroids average ~200 µm in diameter were seeded onto 24-well plates and allowed to adhere and migrate for 24 hours. Left, representative photographs of the spheroids before and after migration. Right, quantitation of U87MG cells migrated from the initial spheroids, error bars depict means ± SD (n = 10). Bar: 100 µm. (c) A549 cells were left untreated or treated for 48 hours with transforming growth factors β1 (TGFβ1) either in the absence or in the presence of GL21.T or the unrelated aptamer as reported in Materials and Methods. Left, cell viability was analyzed as in Figure 2. Right, proliferation was determined by [3H]-thymidine incorporation. (d,e) U87MG spheroids of ~200 µm in diameter were treated with GL21.T or the unrelated aptamer for 72 hours. (d) Spheroids were stained with anti-CD133 antibodies. (e) Cell viability was analyzed as in Figure 2. In (a–e), error bars depict means ± SD (n = 4).
Increased cell motility and ability to invade the extracellular matrix characterize the epithelial-mesenchymal transition (EMT) in cancer cells.42 Since Axl has been recently shown to be involved in EMT transition and cell invasiveness41 we thus asked whether the aptamer GL21.T might specifically interfere with the acquisition of the mesenchymal phenotype. Several pleiotropic transcription factors, including Snail, Twist, and ZEB1/2 are known to mediate EMT that can be induced by several agents, including transforming growth factors β (TGFβ). In A549 cells, the GL21.T aptamer is unable to interfere with TGFβ1-induced entry of cells in the mesenchymal state as determined by its inability to interfere with increased expression of Snail and of the mesenchymal N-cadherin and with the repression of the epithelial marker E-cadherin levels (data not shown). Consistently GL21.T had no major effects on cell viability of TGFβ1-treated A549 cells (Figure 4c), thus suggesting that inhibiting Axl neither impairs the acquisition of a mesenchymal-like phenotype nor selectively interferes with proliferation of TGFβ-induced cells.
The efficiency of sphere formation is considered a measure of tumor aggressiveness being considered a typical feature of tumor-initiating cells.43,44 Since U87MG-derived spheroids are constituted in large part of CD133(+) cells, a subpopulation of cells that in human gliomas has been shown to bear stemness and tumor-initiating capabilities,44 we thus determined whether the GL21.T aptamer impairs sphere formation by selectively interfering with viability of CD133(+) U87MG cells. As shown in Figure 4d, the U87MG spheroids, if left growing for 7 days in medium supplemented with basic fibroblast growth factor and epidermal growth factor, are constituted of CD133(+) cells for around the 40%, as determined by fluorescence-activated cell sorting analysis. However, cell viability of such enriched CD133(+) population appears to be insensitive to GL21.T treatment (Figure 4e) thus indicating that Axl activity is required in vitro for anchorage independent cell growth rather than for cell survival.
GL21.T inhibits cell transformation
Because of its inhibitory potential on Axl activation and of the resulting impairment of Erk1/2 and Akt activation, we further determined whether GL21.T may interfere with the transforming potential of A549 and U87MG cells. To this end, we first assessed the effects of GL21.T on long-term cellular colony formation efficiency in semisolid media. Cells were treated with either the unrelated control or GL21.T aptamers and then plated in soft agar for 3 weeks. As shown in Figure 5a, as compared to the unrelated aptamer control, treatment with GL21.T led in both cell lines to a significant decrease in the efficiency of colony formation.
Figure 5.
GL21.T inhibits tumor growth. (a) Colony formation assay showing U87MG and A549 cells grown for 2 weeks in the presence of GL21.T or the unrelated aptamer. Representative photographs of at least three different experiments were shown. Bar: 100 µm. Colonies number of 15–20 random fields were counted and expressed as percent with respect to the unrelated aptamer-treated control. Vertical bars indicate the standard deviation values. (b) A549-luc xenografts were left growing for 30 days following implantation before aptamer injection. Mouse xenograft model bearing A549-luc cells tumors were injected intratumorally (left) or intravenously (right) with GL21.T or unrelated aptamer. Growth inhibition of tumors was measured as bioluminescence intensity (photon/sec) as indicated. Data shown are means ± SEM (n = 3 tumors). (c) Growth inhibition of tumors in a mouse xenograft model bearing A549 cells upon GL21.T treatment. Day 0 marks the first day of injection. Data are shown as means ± SEM (n = 8 tumors) (see Materials and Methods section for details). (d–g) Representative sections of tumors from unrelated aptamer or GL21.T-treated mouse were stained with (d,e) hematoxylin and eosin (H&E) and (f,g) Ki-67 antibody, as indicated. Magnification, ×200. (h) Three tumors per group selected randomly were excised, lysed, and the pooled or lysates were prepared from A549 cells treated for 24 hours with GL21.T or unrelated aptamer. Lysates were immunoblotted with anti-caspase-3, anti-PARP, and anti-α–tubulin antibodies, as indicated. Molecular weights of indicated proteins are reported.
Furthermore, by using xenografts of A549-luc or A549 cells into (nu/nu) immunodeficient mice we evaluated the ability of GL21.T to inhibit in vivo tumor growth. To this end we determined the bioluminescence intensity (total flux of photons) of A549-luc tumors as a measure of tumor cell mass. As shown in Figure 5b (left panel), at 7 days after intratumoral injection the bioluminescence increased of approximately six times in unrelated aptamer control tumors while only of approximately two times times in GL21.T-treated tumors. Similarly, 10 days of systemic administration of G21.T inhibited tumor growth as compared to the unrelated aptamer (Figure 5b, right panel). To confirm the in vivo growth inhibitory action of GL21.T we then evaluated the increases in tumor volume in A549-mouse xenografts. As shown in Figure 5c, in A549-mouse xenografts a pronounced reduction in tumor volume was observed in the presence of GL21.T treatment. Treatments were initiated at two weeks after A549 cell injection, when tumor mean volume was ~25 mm3, and followed for further three weeks until tumors treated with the unrelated control reached a volume of ~220 mm3 whereas those treated with GL21.T remained ~of 70 mm3.
At day 22, mice were sacrificed, tumor excised, embedded in paraffin, and six tumors per group randomly selected analyzed. As shown in the Figure 5d, the tumor sections showed, in every aspects, features of poorly differentiated carcinoma. Strikingly tumors from the treated group, but not controls, revealed marked degenerative features as extensive tumor necrosis and focal crystalline deposits (Figure 5e). To address further this issue we assessed the immunohistochemical staining for Ki-67. Notably the treated tumors showed a Ki-67 cutoff value <10% (Figure 5g), whereas in control tumors the value of Ki-67 was higher >75% (Figure 5f). Whether GL21.T inhibits tumor growth by allowing the apoptotic process to take place was thus addressed by immunoblot analysis of cleavage products of caspase3 and PARP. As shown in Figure 5h, at difference of what observed in vitro (left panel), in treated tumors GL21.T dramatically induces the activation of the apoptotic process (right panel).
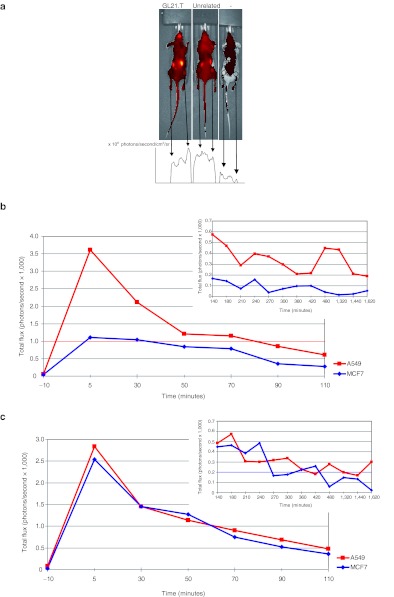
We thus verified whether the binding specificity of GL21.T for Axl-expressing cells is still preserved in vivo following intravenous administration of the aptamer. To this end, we determined the capability of GL21.T to spread into the body and to specifically accumulate in the Axl-expressing A549-derived tumor xenografts with respect to control MCF7 that do not express Axl. A549-luc cells were xenografted subcutaneously on the right flank and MCF7-luc cells were xenografted subcutaneously on the left flank. Thus we treated the mice so described with a single intravenous injection of 1,600 pmol of Alexa-labeled GL21.T and Alexa-labeled unrelated aptamer. Mice inoculated with fluorescent GL21.T showed an increased concentration of the aptamer corresponding to the A549 tumor region with respect to the MCF7 and to the whole body at 180 minutes. Conversely in mice treated with Alexa-labeled unrelated aptamer there are no significant differences in the concentration of aptamer in both the tumor masses (Figure 6a).
Figure 6.
GL21.T intravenous injection. (nu/nu) Mice-bearing MCF7-luc cells (left-hand side) and A549-luc (right-hand side) xenografts were injected intravenously either with 1,600 pmol of Alexa-labeled GL21.T or of unrelated aptamer. The aptamer amount in vivo was thus monitored by evaluating the intensity of fluorescence signal normalized for the tumor mass as determined by cell bioluminescence and measured at different times as indicated. (a) Typical image of one set of mice by IVIS camera at 180 minutes after injection. Arrows indicate the tumor regions (left-hand side MCF7-luc, right-hand side A549-luc). The corresponding fluorescent signal is reported for GL21.T (left mouse), unrelated aptamer (middle mouse), and sham control (right mouse). (b) The graph depicts the photon rate normalized by the bioluminescence signal (as measure of tumor volume) up to 110 minutes following GL21.T aptamer injection. The insert represents the signal measured from 140 to 1,620 minutes. (c) The same as in b for the mice injected with an unrelated aptamer sequence.
As shown in Figure 6b, over a period of 27 hours the Alexa-labeled GL21.T aptamer specifically accumulates in the A549 tumor region with respect to the MCF7, whereas the unrelated aptamer is retained at the same extents on both tumor types (Figure 6c). A major peak of accumulation was observed between 5 and 30 minutes. Taken together these results indicate that GL21.T may hamper Axl-dependent tumor formation.
Discussion
Here, we show that a short RNA-based aptamer, GL21.T, acts as a neutralizing ligand for the transmembrane RTK Axl on tumor cells. The aptamer, isolated with the use of a combinatorial selection-based approach, binds Axl at high affinity (Kd of 12 nmol/l) and high specificity. Indeed, we demonstrate that GL21.T may bind to living cells in culture provided that the human Axl is expressed on the cell surface and that such binding can be competed with an excess of the recombinant EC-Axl protein.
Axl has been recently implicated in several human cancers as being prognostic of a less favorable histiotype.8,9,10,11,12,13,14 The functional implication of Axl in tumors has been recently proposed in experimental models either by depletion with a specific shRNA or by functional inhibition with the TK inhibitor, R428,25 and by the mAb YW327.6S2.26 Furthermore, converging evidence indicate that Axl is likely involved in determining the EMT in cancer cells.41
We demonstrate that the binding of the GL21.T aptamer to Axl strongly reduces the receptor TK activity and the consequent activation of the two main downstream effectors Erk and Akt. According with the involvement of Axl in motility and invasion, we show that GL21.T strongly inhibits in vitro cell migration, extracellular matrix invasion, sphere formation and cell spreading. Given the potential consequences of these effects on metastatic potential it is possible that this anti-Axl aptamer could have therapeutic potential in metastatic spread. However, even impairing both Erk and Akt activation, as compared to Axl depletion, treating cells with GL21.T had poor effects on cell viability and proliferation that are both decreased by the treatment with the aptamer but only of ~20%. Based on previous reports of the need of Axl for EMT, we thus insight the mechanism that specifies the extent of cell death and asked whether the presence of an intrinsic heterogeneity in the cell culture may lead to a small population of Axl-dependent cells with a mesenchyme-like phenotype more sensitive to GL21.T. To address this possibility, we determined cell viability and proliferation either after TGFβ-dependent induction of the mesenchymal transition or in a partitioned CD133(+) cell population. However, it seems unlikely to be the case since in both cases we observed no increase of the inhibitory effect of GL21.T on cell growth. On the other hand, as recently shown, Axl interacts with other RTKs by multiple intracellular mechanisms that in concert contribute to the final cell phenotype45 thus likely determining responsiveness to GL21.T inhibition on cell viability.
We also show that, despite the poor effects on in vitro cell growth, in vivo the GL21.T aptamer efficiently inhibited tumor growth and induced apoptosis. This apparent discrepancy likely relies on the mechanism of action of GL21.T that by binding to Axl interferes with Gas6-induced receptor activation. Indeed, the expression of Axl and its ligand, Gas6, have been shown to be implicated in several tumors and metastasis. Activation of Axl in tumor cells implicates the production of high levels of Gas6 ligand by tumor infiltrating cells, including macrophages and leukocytes that thus promote cell growth (for a review, see ref. 46). Therefore, even if the molecular mechanisms remain to be investigated, the need of surrounding microenvironment for proper Axl function may provide a plausible explanation for the more drastic effects observed in vivo by GL21.T treatment.
Despite the still growing interest for the Axl-Gas6 axis as therapeutic targets for cancer,24 only few Axl inhibitors of in vivo tumor growth have been described to date, including the mAB YW327.6S2, the shRNA and the small TKI R428. The aptamer GL21.T binds Axl at high affinity and inhibits its TK activity likely interfering with Gas6-induced dimerization. Further GL21.T preserves in vivo its binding specificity, thus distinguishing Axl-expressing from nonexpressing tumor cells in the same animal. Therefore, it has a major advantage over other therapeutics since it couples high binding specificity and affinity to a low molecular weight, of ~10 kDa. Together with the monoclonal antibody,26 thus far the aptamer GL21.T is the only biomolecule that may act as inhibitory ligand for Axl thus revealing as a lead molecule not only as an inhibitory agent but also as a receptor specific ligand able to drive conventional therapeutics, nanoparticles and imaging agents for selective recognition of cancer cell surface.30,47 Most importantly, based on the recent development of aptamer-siRNA bioconjugates48,49,50 GL21.T appears as a prime candidate tool for the cell-specific receptor-mediated intracellular delivery of therapeutic RNAs.
Materials and Methods
Aptamers. GL21.T and the unrelated 2′-fluoropyrimidine aptamer used as a negative control were purchased from Sigma (Sigma-Aldrich, St Louis, MO).
GL21.T: 5′ AUGAUCAAUCGCCUCAAUUCGACAGGAGGC UCAC 3′.
Uunrelated aptamer: 5′UUCGUACCGGGUAGGUUGGCUUGCACAUAGAACGUGUCA3′
Before each treatment, the aptamers were subjected to a short denaturation–renaturation step (85 °C for 5 minutes, snap-cooled on ice for 2 minutes, and allowed to warm up to 37 °C). For cell treatments longer than 6 hours, RNA concentrations were determined to ensure the continuous presence of at least 200 nmol/l-concentration taking into account the 6 hours-half-life of the aptamer in 10% serum. For imaging assays aptamers have been internal-labeled with Alexa Fluor 647 fluorescent probe following the provider indications (Invitrogen, Carlsbad, CA).
Cell lines and transfection. Human glioma U87MG, human breast SKBr3, MCF7, MDA-MB-231 cells and epidermoid carcinoma A431 (American Type Culture Collection, Manassas, VA), were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 2 mmol/l L-glutamine (Invitrogen). Non–small-cell lung cancer A549 cells (American Type Culture Collection) were grown in RPMI (Invitrogen) supplemented with 10% FBS and 2 mmol/l L-glutamine. A549-luc-C8 and MCF7-luc-F5 (Caliper Life Sciences, Hopkinton, MA) were grown in RPMI supplemented with 10% FBS, 2 mmol/l L-glutamine and 150 µg/ml G418 (Sigma-Aldrich).
For Axl gene silencing, U87MG were transfected with shRNAAxl or shRNActrl (Open Biosystems, Rockford, IL). Axl expression in human breast SKBr3 cells was obtained by transfection of Axl TruClone (Origene, Rockville, MD). Cells (3.5 × 105 cells per 6-cm plate) were grown and overlaid with the transfection mixtures containing the shRNAAxl, shRNActrl, or Axl TruClone (6 µg) and Lipofectamine 2000 (Invitrogen) in Opti-MEM I reduced serum medium (Invitrogen). After 5-hour incubation, complete culture medium was added to the cells and incubation was prolonged up to 72 hours. Binding or [3H]-Thymidine incorporation assays with transfected cells were performed after 24 hours from transfection.
Binding assays. Binding to cells of GL21.T or unrelated aptamer as a negative control (50 nmol/l final concentration) was performed as described.35 Briefly, filter binding analysis with the soluble extracellular domain of human Axl, Dtk, and Mer as targets (R&D Systems, Minneapolis, MN), was performed by incubating 1 nmol/l of radiolabeled aptamers with 1, 3.2, 10, 32, 100, 320, and 1,000 nmol/l of EC-Axl, EC-Dtk, or EC-Mer as described.
In all binding assays the background values obtained with the unrelated aptamer were subtracted from the values obtained with the GL21.T.
To check the endocytosis rate, 100 nmol/l radiolabeled GL21.T or unrelated uptamer have been incubated on U87MG cells for increasing incubation times (from 15 minutes up to 2 hours) and at desired times, cells have been treated with 0.5 µg/µl proteinase K (Roche Diagnostics, Indianapolis, IN) at 37 °C. Following 30-minute treatment, the amount of RNA internalized has been recovered and counted.
Immunoblot analyses. To assess the effects of GL21.T aptamer on Axl activity, U87MG or A549 cells (1.5 × 105 cells per 3.5-cm plate) were serum-starved overnight, pre-treated with 200 nmol/l GL21.T aptamer or unrelated negative control aptamer for 3 hours and then stimulated with 400 ng/ml Gas6 (R&D Systems) either alone or in presence of each aptamer. Cell extracts, immunoprecipitation, and immunoblotting were performed as described.31 The primary antibodies used were: anti-phospho-ERK1/2 (E10), anti-phospho-AKT (Ser473), anti-phospho-AKT (Thr308), anti-AKT, anti-caspase-3, and anti-PARP (Cell Signaling Technology, Danvers, MA); anti-ERK1 (C-16) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho-tyrosine (4G10; Upstate Biotechnology Incorporated, Lake Placid, NY); anti-Axl (R&D Systems); anti-αtubulin (DM 1A) (Sigma-Aldrich). RTK antibody arrays (R&D Systems) were performed as recommended. Endogenous active GTP-bound Rac1 levels were detected using PAK-binding domain pull down assay (Cytoskeleton) according to the supplier's instructions.
Cell viability and [3H]-thymidine incorporation assays. Cell viability was assessed with CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to the supplier's instructions (4 × 103 cells/well in 96-well plates). To asses cell viability in the presence of TGFβ1, cells were maintained in DMEM 0.1% FBS for 24 hours and then treated with 50 ng/ml TGFβ1 (R&D Systems) alone or in presence of GL21.T or of the unrelated aptamer (200 nmol/l final concentration) for additional 48 hours.
For cell proliferation assay, A549 or U87MG cells (2 × 104 cells/well in 24-well plates) were treated for 24 hours or 48 hours with GL21.T or unrelated aptamer. During the final 6 hours, cells were pulsed with 1 µCi/ml [3H]-thymidine (45 Ci/mmol) (Amersham Bioscience, Piscataway, NJ) added in complete growth medium and incubated at 37 °C. At the end of each pulse, cells were harvested and [3H]-thymidine incorporation was analyzed by a Beckman LS 1701 Liquid Scintillation Counter.
Transwell migration/invasion and soft-agar colony formation assays. A549 or U87MG cells were pretreated for 3 hours either with 200 nmol/l GL21.T or with unrelated aptamer and then trypsinized, re-suspended in DMEM serum free, and counted. Cells (1 × 105 in 100 µl serum-free medium per well) were then plated into the upper chamber of a 24-well transwell (Corning Incorporate, Corning, NY) in the presence of increasing concentrations of either GL21.T or unrelated aptamer and exposed to Gas6 (400 ng/ml) or 10% FBS as inducers of migration (0.6 ml, lower chamber). For invasion assays the upper chamber of a 24-well transwell was coated with 20% Matrigel matrix (BD Biosciences, San Jose, CA) before plating of the cells. After incubation at 37 °C in humidified 5% CO2 for 24 hours, cells were visualized by staining with 0.1% crystal violet in 25% methanol. Percentage of migrated cells was evaluated by eluting crystal violet with 1% sodium dodecyl sulfate and reading the absorbance at 570 nm wavelength.
For soft-agar colony formation assay, 1 × 104 U87MG or A549 cells, pretreated for 3 hours either with GL21.T or with the unrelated aptamer, were plated in 60 mm dishes in a solution containing DMEM 2× (Sigma-Aldrich), Tryptose phosphate broth and 1.25% of Noble Agar (Difco; BD, Franklin Lakes, NJ). Cells were left grown for 2 weeks in presence of each aptamer (200 nmol/l-final concentration) renewing the treatment each 3 days.
Spheroid formation assay. To generate U87MG cell spheroids, 1 × 104 cells left either untreated or treated for 3 hours with 200 nmol/l GL21.T or, alternatively, with unrelated aptamer, were grown in DMEM-F12 supplemented with 1% B-27, human recombinant basic fibroblast growth factor (10 ng/ml), and epidermal growth factor (20 ng/ml), both from Sigma-Aldrich, in 60 mm low-adherent plate. Sphere were left growing for 10 days either in the absence or in the presence of each aptamer (renewing the treatment each 3 days). Spheroids average ~200 µm in diameter were seeded in complete grown medium onto 24-well plates and allowed to adhere and migrate for 24 hours. Anti-CD133 antibodies (Cell Signaling Technology) staining was performed following the provider indications.
In vivo experiments. Athymic CD-1 nude mice (nu/nu) were housed in a highly controlled microbiological environment, thus to guarantee specific pathogen free conditions. To assess the GL21.T aptamer ability to inhibit in vivo tumor growth, mice were injected subcutaneously with 3 × 106 (in 100 µl) in vitro propagated A549. Sixteen non-necrotic tumors of ~0.5 cm in diameter were randomly divided into two groups of eight mice as follows: group 1, unrelated aptamer-treated; group 2, GL21.T-treated.
Aptamers (200 pmol/injection) were injected intratumorally in 100-µl volumes three times a week for 22 days. During the study mice were daily monitored to avoid any sign of suffering. Tumors were measured every 2 days with calipers and tumor volume was calculated as follows: VT = (WXLXH) × 0.5236 (W, the shortest dimension; L, the longest dimension; H, the intermediate dimension). The growth curves are plotted as the means tumor volume ± SEM. Alternatively, A549-luc cells (5 × 106 in 100 µl) were injected into mice, tumors were left growing until they were palpable and then treated with the aptamers (for intratumor treatment, a single 200-pmol injection; for retro-orbital intravenous treatment, 1,600 pmol/day for the first 4 days plus a single dose of 1,600 pmol at day 8). Growth inhibition of tumors was measured as bioluminescence intensity by CALIPER IVIS Spectrum.
For the in vivo evaluation of aptamer binding specificity, six (nu/nu) immunodeficient mice each bearing a double tumor xenograft were utilised. To this end, each mouse was injected subcutaneously with 3 × 106 A549-Luc cells and 6 × 106 MCF7-Luc cells on the right and left sides of the animal, respectively. Aptamers, GL21.T and the unrelated aptamer, have been internal-labeled with the fluorescent probe Alexa Fluor 647 following the provider indications (Invitrogen) and then administered by retro-orbital injection. Three mice were injected with 1,600 pmol of GL21.T and three mice with 1,600 pmol of the unrelated aptamer. Results are shown in Figure 6 for a single couple of mice and were found reproducible for all cases. For the in vivo imaging analysis it has been used the CALIPER IVIS Spectrum. The system allows a noninvasive monitoring in living animals by using optical imaging technology to acquire bioluminescences and fluorescence images. The images were processed by using “Caliper living image software 4.1.” Unmixing algorithm has been used to reduce the tissue and ingested food background, in order to enhanced the fluorescence images.
Histology and immunohistochemistry. Formalin-fixed, paraffin embedded tissues from the tumors were selected. Representative slides of each tumor were stained with hematoxylin and eosin to confirm the diagnosis of the tumors. To evaluate the proliferative activity of the neoplastic cells, 4-µm serial sections from representative blocks were cut, mounted on poly-L-lysine coated glass slides and used for the immunohistochemical staining of the Ki-67 antigen. Ki-67 is a proliferative marker and its cutoff value of 10% is commonly used in several types of malignant tumors. Representative sections were incubated overnight at 4 °C with the primary antibodies. Subsequently, the slides were incubated with biotinylated secondary antibodies, peroxidase-labeled streptavidin (DAKO LSAB kit HRP; DAKO, Carpinteria, CA) and chromogenic substrate diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) for the development of the peroxidase activity. Slides were counterstained with hematoxylin, dehydrated and cover-slipped with a synthetic mounting medium (Entellan, Merck, Germany).
Ethics statement. All the experimental procedures were approved by the Ethical Committee for the Animal Use (CESA) of the Istituto di Ricerche Genetiche Gaetano Salvatore (IRGS) and where communicated to the national authorities accordingly with national and European rules (permit numbers 1551, 1564).
SUPPLEMENTARY MATERIAL Figure S1. GL2.T aptamer. Figure S2. GL2.T inhibits serum-dependent Axl phosphorylation.
Acknowledgments
We wish to thank Dr L. Baraldi for technical assistance, G. Condorelli and S. Catuogno for suggestions, comments, and for critically reading the manuscript. The patent request no. PCT/EP2011/067624 describing the GL21.T aptamer has been filed. This work was supported by funds from CNR, from AICR No 11-0075 (L.C.), MIUR grant, MERIT RBNE08YFN3_001 (VdF), AIRC No 11781 (L.C.), supported in part by the Compagnia di San Paolo and from the Italian Ministry of Economy and Finance to the CNR for the Project FaReBio di Qualità. C.L.E. is recipient of a FIRC fellowship; A.R is recipient of an AIRC/Marie Curie fellowship. The authors declared no conflict of interest.
Supplementary Material
GL2.T aptamer.
GL2.T inhibits serum-dependent Axl phosphorylation.
REFERENCES
- Ohashi K, Nagata K, Toshima J, Nakano T, Arita H, Tsuda H.et al. (1995Stimulation of sky receptor tyrosine kinase by the product of growth arrest-specific gene 6 J Biol Chem 27022681–22684. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C.et al. (1995The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases Cell 80661–670. [DOI] [PubMed] [Google Scholar]
- Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW.et al. (1995Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6 Nature 373623–626. [DOI] [PubMed] [Google Scholar]
- Mark MR, Chen J, Hammonds RG, Sadick M., and, Godowsk PJ. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271:9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- Hall MO, Obin MS, Heeb MJ, Burgess BL., and, Abrams TA. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Uehara H., and, Shacter E. Auto-oxidation and oligomerization of protein S on the apoptotic cell surface is required for Mer tyrosine kinase-mediated phagocytosis of apoptotic cells. J Immunol. 2008;180:2522–2530. doi: 10.4049/jimmunol.180.4.2522. [DOI] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P.et al. (2006TAM receptor function in the retinal pigment epithelium Mol Cell Neurosci 3396–108. [DOI] [PubMed] [Google Scholar]
- Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS.et al. (2005Expression of axl in lung adenocarcinoma and correlation with tumor progression Neoplasia 71058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P., and, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L.et al. (2008AXL is a potential target for therapeutic intervention in breast cancer progression Cancer Res 681905–1915. [DOI] [PubMed] [Google Scholar]
- Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS.et al. (2002Clinical significance of AXL kinase family in gastric cancer Anticancer Res 222B1071–1078. [PubMed] [Google Scholar]
- Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ.et al. (2009The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target Cancer Biol Ther 8618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BI, Malkowicz SB, Nguyen TB, Libertino JA., and, McGarvey TW. Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol. 2003;22:533–540. doi: 10.1089/10445490360708946. [DOI] [PubMed] [Google Scholar]
- Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N.et al. (2008Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme Clin Cancer Res 14130–138. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H.et al. (2007Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer Cell 1311190–1203. [DOI] [PubMed] [Google Scholar]
- Avilla E, Guarino V, Visciano C, Liotti F, Svelto M, Krishnamoorthy G.et al. (2011Activation of TYRO3/AXL tyrosine kinase receptors in thyroid cancer Cancer Res 711792–1804. [DOI] [PubMed] [Google Scholar]
- Sensi M, Catani M, Castellano G, Nicolini G, Alciato F, Tragni G.et al. (2011Human cutaneous melanomas lacking MITF and melanocyte differentiation antigens express a functional Axl receptor kinase J Invest Dermatol 1312448–2457. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D.et al. (2011The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy Blood 1171928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT.et al. (2008Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia Cancer Lett 268314–324. [DOI] [PubMed] [Google Scholar]
- Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R.et al. (2009Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL Cancer Res 696871–6878. [DOI] [PubMed] [Google Scholar]
- Sayan AE, Stanford R, Vickery R, Grigorenko E, Diesch J, Kulbicki K.et al. (2012Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL Oncogene 311493–1503. [DOI] [PubMed] [Google Scholar]
- Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J.et al. (2011AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma Oncogene 301229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger RM, Keating AK, Earp HS., and, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Warner SL, Vankayalapati H, Bearss DJ., and, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W.et al. (2010R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer Cancer Res 701544–1554. [DOI] [PubMed] [Google Scholar]
- Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D.et al. (2010An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies Oncogene 295254–5264. [DOI] [PubMed] [Google Scholar]
- Cerchia L., and, de Franciscis V. Nucleic acid aptamers against protein kinases. Curr Med Chem. 2011;18:4152–4158. doi: 10.2174/092986711797189592. [DOI] [PubMed] [Google Scholar]
- Esposito CL, Catuogno S, de Franciscis V., and, Cerchia L. New insight into clinical development of nucleic acid aptamers. Discov Med. 2011;11:487–496. [PubMed] [Google Scholar]
- Cerchia L., and, de Franciscis V. Nucleic acid-based aptamers as promising therapeutics in neoplastic diseases. Methods Mol Biol. 2007;361:187–200. doi: 10.1385/1-59745-208-4:187. [DOI] [PubMed] [Google Scholar]
- Cerchia L., and, de Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010;28:517–525. doi: 10.1016/j.tibtech.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Cerchia L, Esposito CL, Jacobs AH, Tavitian B., and, de Franciscis V. Differential SELEX in human glioma cell lines. PLoS ONE. 2009;4:e7971. doi: 10.1371/journal.pone.0007971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rockey WM, Hernandez FJ, Huang SY, Cao S, Howell CA, Thomas GS.et al. (2011Rational truncation of an RNA aptamer to prostate-specific membrane antigen using computational structural modeling Nucleic Acid Ther 21299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., and, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A.et al. (1997Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site Oncogene 142619–2631. [DOI] [PubMed] [Google Scholar]
- Esposito CL, Passaro D, Longobardo I, Condorelli G, Marotta P, Affuso A.et al. (2011A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death PLoS ONE 6e24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell YW, Villa J, Jr, Attar EC., and, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J Biol Chem. 1998;273:7123–7126. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H.et al. (2006Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival Proc Natl Acad Sci USA 1035799–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE.et al. (2005Multiple roles for the receptor tyrosine kinase axl in tumor formation Cancer Res 659294–9303. [DOI] [PubMed] [Google Scholar]
- Reddy KB, Nabha SM., and, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T.et al. (2010Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival Proc Natl Acad Sci USA 1071124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J.et al. (2003Identification of a cancer stem cell in human brain tumors Cancer Res 635821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T.et al. (2004Identification of human brain tumour initiating cells Nature 432396–401. [DOI] [PubMed] [Google Scholar]
- Yeh CY, Shin SM, Yeh HH, Wu TJ, Shin JW, Chang TY.et al. (2011Transcriptional activation of the Axl and PDGFR-a by c-Met through a ras- and Src-independent mechanism in human bladder cancer BMC Cancer 11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Ben-Batalla I, Schultze A., and, Loges S. Macrophage-tumor crosstalk: role of TAMR tyrosine kinase receptors and of their ligands. Cell Mol Life Sci. 2012;69:1391–1414. doi: 10.1007/s00018-011-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe AD, Pai S., and, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR.et al. (2009Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors Nat Biotechnol 27839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KW., and, Giangrande PH. Intracellular delivery of RNA-based therapeutics using aptamers. Ther Deliv. 2010;1:849–861. doi: 10.4155/tde.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., and, Rossi JJ. Aptamer-targeted cell-specific RNA interference. Silence. 2010;1:4. doi: 10.1186/1758-907X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GL2.T aptamer.
GL2.T inhibits serum-dependent Axl phosphorylation.