Abstract
Previous efforts to derive lung progenitor cells from human embryonic stem (hES) cells using embryoid body formation or stromal feeder cocultures had been limited by low efficiencies. Here, we report a step-wise differentiation method to drive both hES and induced pluripotent stem (iPS) cells toward the lung lineage. Our data demonstrated a 30% efficiency in generating lung epithelial cells (LECs) that expresses various distal lung markers. Further enrichment of lung progenitor cells using a stem cell marker, CD166 before transplantation into bleomycin-injured NOD/SCID mice resulted in enhanced survivability of mice and improved lung pulmonary functions. Immunohistochemistry of lung sections from surviving mice further confirmed the specific engraftment of transplanted cells in the damaged lung. These cells were shown to express surfactant protein C, a specific marker for distal lung progenitor in the alveoli. Our study has therefore demonstrated the proof-of-concept of using iPS cells for the repair of acute lung injury, demonstrating the potential usefulness of using patient's own iPS cells to prevent immune rejection which arise from allogenic transplantation.
Introduction
Acute and chronic lung injuries incur significant morbidity and mortality. The choice of efficacious medical therapy is limited and in advanced cases where transplantation is the final option, donor organ availability remains a severe limiting factor. A potentially viable alternative to lung transplantation is the transplantation of airway progenitor cells to enhance lung regeneration. Physiologically, the alveoli, which are the functional units of the lung, are made up of two cell types, namely the type I and type II pneumocytes. While type I pneumocytes tend to be large flattened cells responsible for gaseous exchange, the type II pneumocytes are smaller cuboidal cells that secrete surfactant proteins which play important roles in reducing surface tension, thus preventing the alveoli from collapsing. As the lung is constantly exposed to foreign pathogens and toxins that can harm the alveolar epithelium, the ability to elicit self repair and regeneration of the damaged tissue is critical for maintaining normal pulmonary function. Developmentally, type II pneumocytes are considered to be the progenitors for type I alveoli epithelial cells.1 Although the type II pneumocytes can proliferate and differentiate into type I epithelial cells, this endogenous repair mechanism of the alveolar epithelium is often inadequate to reconstitute damaged alveoli in several life threatening pulmonary diseases such as acute lung injury and chronic obstructive pulmonary disease.
Several studies have reported the derivation of type II pneumocytes from both mouse and human ES cells either through embryoid body formation or using differentiation medium designed for the maintenance of mature small airway epithelium.2,3,4,5,6,7,8,9,10 Such differentiation processes require relatively long differentiation period (~15–30 days) and were often achieved with low efficiencies (~5%). Since the lung is derived embryologically from the endoderm lineage, we speculated that a step-wise differentiation process, initially toward the endodermal lineage, would favor the differentiation of pluripotent stem cells toward the lung lineage. At the same time, recent advances in somatic cell reprogramming to induced pluripotent stem (iPS) cells have greatly increased the possibilities of generating patient-specific stem cells. Therefore, as a proof-of-concept and to test the potential usefulness of iPS cells, we generated human iPS clones from human MRC5 and BJ cell lines using the Yamanaka factors.11 Through this differentiation method, a heterogeneous population of differentiated cells was obtained, of which ~30% was found to express surfactant protein A in vitro.
Despite the ability to generate lung epithelial cells (LECs) reproducibly from pluripotent stem cells using the step-wise differentiation protocol, the cells obtained were still unsuitable for transplantation due to its heterogeneity. In this study, we report the use of magnetic-activated cell sorting (MACS) on the heterogeneous pool of differentiating LECs, and identified CD166 as a potential marker, capable of enriching a subpopulation of cells that were able to engraft and function in vivo. The functionality of these sorted LECs that were derived from both human embryonic stem (hES) and hiPS cells were demonstrated to integrate into the lung alveoli of bleomycin-injured NOD/SCID mice, thereby improving lung pulmonary functions and enhancing the survivability of the mice.
Results
Generation of human iPS cell lines
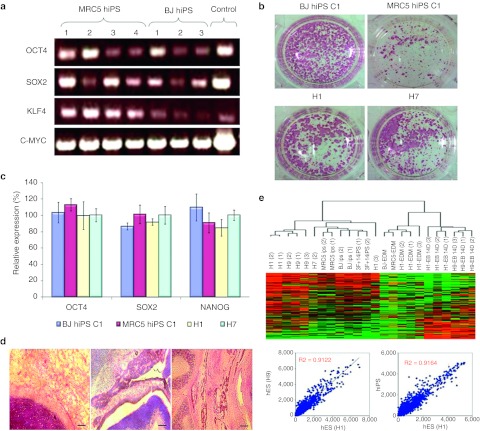
Human iPS clones generated from human MRC5 and BJ cell lines were shown to express all the four transgenes (OCT4, SOX2, KLF4, and C-MYC) that were introduced into these fibroblasts to effect the reprogramming process (Figure 1a). The G-band karyotype analysis of these clones revealed normal karyotype (data not shown). To test the stemness of these clones, alkaline phosphatase staining test was performed and the results illustrated comparable expression of this enzyme in both human iPS and wild-type hES cells (Figure 1b). Furthermore, quantitative-PCR also revealed similar expression of endogenous pluripotency markers (OCT4, SOX2, and NANOG) in these iPS clones as well as wild-type hES cells (Figure 1c). In order to examine the differentiation potential of these human iPS clones, 1 × 106 pluripotent stem cells were injected subcutaneously behind the hind limbs into NOD/SCID mice. Typical teratoma formation comprising of tissues belonging to the three lineages, namely the endoderm, mesoderm, and ectoderm was observed (Figure 1d). Furthermore, microarray analysis aimed to examine the global transcriptome profiles of the hiPS clones and wild-type hES cells revealed that the hiPS clones are highly similar to wild-type hES cells (R value >0.9) (Figure 1e). Overall, these data showed that the hiPS clones obtained from the MRC5 and BJ cell lines behave similarly to wild-type hES cells in terms of its pluripotency status and differentiation capability.
Figure 1.
Generation of human induced pluripotent stem (hiPS) cell lines. (a) PCR showing the presence of transgenes (OCT4, SOX2, KLF4, and C-MYC) in hiPS clones generated from human MRC5 and BJ fibroblast cell lines. (b) Alkaline phosphatase staining of hiPS (BJ and MRC5) and wild-type human ES (H1 and H7) colonies. (c) Graphical representation of relative gene expression of pluripotency markers (OCT4, SOX2, and NANOG) from both hiPS (BJ and MRC5) and wild-type human embryonic stem (hES) (H1 nd H7) cell lines. Bars, standard error of n = 3 experiments. (d) Hematoxylin and eosin (HE) staining of teratoma sections obtained from mice 4–6 weeks following subcutaneous injection with 1 × 106 hiPS cells. These sections revealed tissues belonging to the three germ layers (endoderm, mesoderm and ectoderm). Bar = 500 µm. (e) Microarray analysis illustrating closeness between the various wild-type hES and hiPS samples. The correlation coefficient between different wild-type hES cells (H1 and H9) is shown to be 0.9122, which is similar to the correlation coefficient of 0.9164 between hiPS and hES lines.
Efficient differentiation of human ES and iPS cells toward the lung lineage
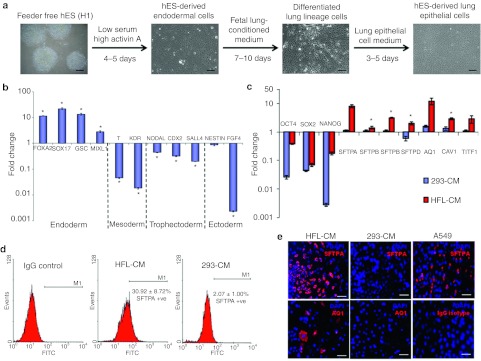
The step-wise differentiation process adopted in this study is summarized in Figure 2a. Differentiation to definitive endoderm was achieved using earlier published protocol with the cells showing expression of specific endoderm markers such as SOX17, FOXA2, GSC, and MIXL112 (Figure 2b). In contrast, other lineage markers such as T-BRACHYURY, KDR (mesoderm), NODAL, CDX2, SALL4 (trophectoderm), and NESTIN, FGF4 (ectoderm) were shown to be downregulated. FACS analysis for the expression of FOXA2 and SOX17 revealed near homogeneity of the endodermal cells obtained, with >95% of the cells expressing these proteins, which was further demonstrated by immunocytochemistry (Supplementary Figure S1).
Figure 2.
In vitro differentiation of human embryonic stem (ES) cells toward lung epithelial cells via definitive endoderm. (a) Schematic diagrams summarizing the differentiation protocol adopted in this study. The various media used and representative images of the cellular phenotypes at different stages of differentiation were as shown. Scale = 200 µm. (b) Graphical representation illustrating that human ES cells cultured in low serum, high Activin A medium expressed high levels of endodermal markers after 5 days. Quantitative-PCR on cells after treatment revealed high expressions of endodermal markers such as SOX17, FOXA2, and GSC, but downregulation of other lineage markers. The fold change in gene expression values were normalized to ES cells that were cultured in mouse embryonic fibroblast conditioned medium (MEF-CM). Bars, SD of n = 3 experiments. *P < 0.05 for Kruskal–Wallis one-way analysis of variance compared to control (ES cells cultured in MEF-CM). (c) Quantitative-PCR comparing gene expression of lung specific genes in human embryonic stem (hES)-derived lung epithelial cells (hES-LEC) that were differentiated with either human fetal lung conditioned medium (HFL-CM) or nonspecific medium (293-CM). The fold change in gene expression values were normalized to ES cells that were cultured in MEF-CM. Bars, SD of n = 3 experiments. *P < 0.05 for Kruskal–Wallis one-way analysis of variance compared to control. (d) Flow cytometric analysis of surfactant protein A (SFTPA) positive cells differentiated with either HFL-CM or 293-CM. Human ES cells that have been subjected to the step-wise differentiation process using either HFL-CM or 293-CM was stained with rabbit IgG primary antibody (Isotype control) or rabbit anti-surfactant protein A primary antibody, followed by goat anti-rabbit IgG Alexa 488 (FITC). The percentages of SFTPA-positive cells were as shown. SD of n = 4 experiments. (e) Immunocytochemistry of lung epithelial cells (LECs) differentiated with either HFL-CM or 293-CM. Representative fluorescent images of SFTPA (red) staining of A549 cell line (positive control) and differentiated cells were as shown. Cells differentiated with HFL-CM were also shown to be positive for aquaporin 1 (AQ1). SFTPA (red) was demonstrated to be a cytoplasmic protein with rabbit anti-surfactant protein A primary antibody from Chemicon International, while AQ1 was revealed with rabbit anti-aquaporin 1 (Chemicon International) primary antibody. Cell nuclei (blue) were stained with 4′, 6-diamidino-2-phenylindole (DAPI). Bar = 100 µm.
Once the pluripotent stem cells had been differentiated to the endoderm lineage, we hypothesized that given the appropriate inductive signals, these cells would further differentiate into cells contributing to liver, lung, pancreas, thyroid, or other endodermal tissues. Henceforth, we postulated that further manipulation of endodermal cells toward the lung lineage may be achieved by exposure to medium that has been conditioned by culture of human fetal lung cells. The underlying hypothesis is that the fetal lung tissue secretes signaling molecules that may simulate the environment in vivo during early organogenesis of the lung, thereby inducing multipotent cells to further differentiate toward the lung lineage. Figure 2c shows the gene expression of the differentiated cells after 7 days culture in human fetal lung conditioned medium (HFL-CM). Strikingly, the differentiated cells expressed lung specific makers such as SFTPA, SFTPB, AQ1, TITF1, and CAV1 compared to cells that were cultured in a nonspecific medium (293-CM). Through FACS analysis, we estimated that treatment with HFL-CM is ~15 times more efficient than nonspecific medium (293-CM) in generating distal lung cells that are expressing surfactant protein A (Figure 2d). Immunocytochemistry further confirmed the expression of these lung specific proteins (Figure 2e and Supplementary Figure S2).
In addition, the specificity of the HFL-CM-mediated induction of cellular differentiation toward the lung lineage was further demonstrated by using the surfactant protein C promoter-DsRed (SPC-DsRed) reporter hES cell line (Supplementary Figure S3b) and SPC-GFP reporter mES cell line (a kind gift from Hogan B.L. lab) (Supplementary Figure S3c). Evidently, the HFL-CM is capable of driving further differentiation of definitive endodermal cells to distal LECs with ~30% and 10% of the stem cell-derived LECs expressing surfactant protein A and C, respectively (Figure 2d and Supplementary Figure S3b). Apart from generating lung lineage cell types, ~15% of the differentiated cells cultured in HFL-CM were also shown to express mesenchymal lineage marker, CD105 (Supplementary Figure S3d).
As the human lung is largely generating conducting airways in the 11–22-week gestation period, one would envisage that conducting airway epithelium would therefore be favored by the differentiation protocol. Indeed, the use of conditioned medium obtained from early fetal lung samples also resulted in conducting airway differentiation as K18 positive cells were also observed (Supplementary Figure S2). However, results from flow cytometric analysis of differentiated cells seemed to suggest that the differentiation protocol favors the derivation of distal lung epithelium (Supplementary Figure S3). This could possibly be due to the use of lung epithelial medium following HFL-CM induction during the differentiation process.
Transplantation of human ES/iPS-derived LECs rescue mice from acute lung injury
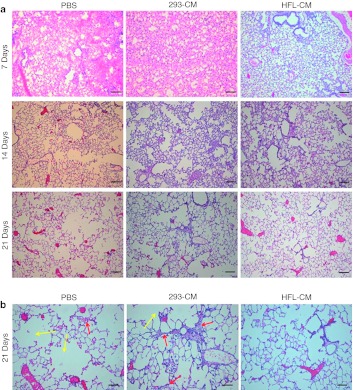
To assess the functional capacity of these pluripotent stem cell-derived LECs, we performed transplantation of hES-LECs and hiPS-LECs in immunecompromised mice. In order to facilitate tracking of cells in vivo, we generated stable hES and hiPS cell lines harboring the CSII-EF-MCS-IRES2-Venus construct. The green fluorescent protein (GFP)-positive clones selected for our study were shown to possess characteristics of wild-type hES cells (Supplementary Figure S4). Transplantation of hES-LECs and hiPS-LECs was performed on NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID) mice as these animals have been shown to be better recipients for engraftment of cross-species host cells.13,14 Since diapedesis of differentiated LECs into the lung parenchyma and eventual integration is an extremely inefficient process in the absence of lung injury, acute lung injury was induced by intratracheal injection of 5 U/kg bleomycin 3 days before the transplantation of cells. The anti-neoplastic drug bleomycin primarily targets the pulmonary epithelium as its mode of action depends largely on the availability of oxygen, which is abundant in the lung. Lung injury was typically observed in the initial 48–72 hours following intratracheal introduction of bleomycin and was characterized histologically by perivascular oedema, capillary congestion, and alveolar wall thickening.15 By day 14 after bleomycin treatment, majority of the lung epithelium was severely damaged, depicted by the interstitial thickening, collapse of the alveolar wall and enlargement of the cystic air spaces (Figure 3a). Transplantation of stem cell-derived LECs greatly reduced the extent of lung damage inflicted by bleomycin. Tracking of the lung recovery process over 3 weeks at 7 days interval showed reduction in capillary congestion and areas of thickened alveolar walls in lungs of animals transplanted with stem cell-derived LECs (Figure 3a). Histological analysis of lungs 21 days after transplantation showed that the alveolar architecture of animals transplanted with stem cell-derived LECs were closest to normal lung compared to lungs of animals transplanted with phosphate-buffered saline (PBS) or 293-CM differentiated cells (controls) (Figure 3a). Large air spaces and thickened alveolar walls were still evident in both controls 21 days post-transplantation (Figure 3b), suggesting that the transplanted LECs were able to integrate and reconstitute damaged alveoli.
Figure 3.
Time-course study showing lung repair in mice. A total of 27 mice, with 9 mice in each group, were transplanted with phosphate-buffered saline (PBS), 293-CM differentiated cells or human fetal lung conditioned medium (HFL-CM)-differentiated cells, which were derived from human embryonic stem (hES) cells. The recovery process was traced for 21 days at 7 days interval. Lungs from three mice were harvested for analysis at each time point. However, for mice that were transplanted with PBS or with cells that were differentiated with 293-CM, due to availability of surviving mice, only 1 animal was harvested on day 14 and day 21, respectively. (a) Hematoxylin and eosin (HE) staining of lung sections. Lung sections obtained from mice transplanted with PBS, 293-CM differentiated cells and HFL-CM differentiated cells after bleomycin (BLM)-induced lung injury. Note the enlarged air spaces in the lung sections obtained from PBS and 293-CM controls after 21 days. Bar = 200 µm. (b) High magnification of HE staining of lung sections 21 days after cellular transplantation. Enlarged air spaces (yellow arrows) were observed in both PBS control and experimental group transplanted with 293-CM differentiated cells. Thickened alveoli walls were also observed in animal transplanted with 293-CM differentiated cells (red arrows) compared to animals transplanted with HFL-CM differentiated cells. Bar = 400 µm.
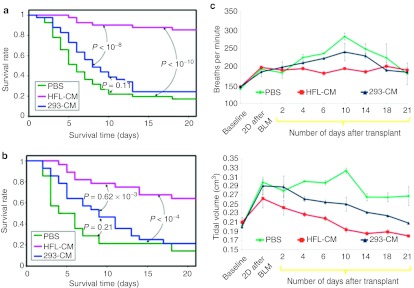
To assess whether transplanting hES-LECs and hiPS-LECS support long-term recovery, we assessed a total of 120 NOD/SCID mice in five separate experiments. Animals transplanted with PBS alone or hES cells treated with 293-CM (controls) were compared with animals transplanted with HFL-CM-treated hES or hiPS cells. The survival rates of the bleomycin-injured mice over 3 weeks are summarized in Figure 4a. It was noted that mice from both control groups started to die from day 3 and the survival rate began to stabilize by day 14, with ~20% (PBS-treated) and 25% (293-CM-treated endodermal cells) of the mice surviving. In contrast, mice that had been transplanted with human hES-LECs showed low mortality throughout the time-course, with >80% of the mice surviving after 3 weeks. Thus far, mice transplanted with stem cell-derived LECs followed over at least 12 months have not shown any incidence of tumor formation (data not shown). Although mice transplanted with hiPS-LECs did not result in similar percentage of survival compared to hES-LECs, significant rescue (~70%) was recorded in two independent experiments (Figure 4b).
Figure 4.
Human ES and induced pluripotent stem (iPS) cells differentiated with human fetal lung conditioned medium resulted in enhanced survivability and improved lung physiological functions in bleomycin lung injury model. (a) Kaplan–Meier curve showing survival rate of mice transplanted with phosphate-buffered saline (PBS), 293-CM differentiated or human fetal lung conditioned medium (HFL-CM) differentiated human ES cells over a 3 weeks period after bleomycin-induced lung injury. Transplantation study was performed five times with eight mice in each experimental group using human embryonic stem (hES) cells differentiated with HFL-CM, PBS, or 293-CM differentiated cells as controls. The P values between the experimental groups are as shown. (b) Kaplan–Meier curve showing survival rate of mice transplanted with hiPS cells differentiated with HFL-CM, PBS, or 293-CM differentiated cells into bleomycin-injured mice. The experiment was performed twice with five mice in each of the experimental groups. The survival analysis was performed using Kaplan–Meier estimator. The P values between the experimental groups are as shown. (c) Measurement of lung functions by whole-body plethysmography of mice transplanted with hES cells differentiated with HFL-CM, PBS, or 293-CM differentiated cells. Graphical representation showing breathing rates and tidal volumes of the three groups of mice measured over a 3-week period. Error bars represent SD obtained from remaining mice at each time point.
To obtain a better and more quantitative correlation between survival rate of the mice and lung pulmonary functions, we performed whole-body plethysmography of mice transplanted with hES-LECs. Examination of two important aspects of lung functions, breathing rates and tidal volume, revealed that the time point of greatest difference in pulmonary functions coincided with the time point where high mortalities were observed in the control mice. Between days 4–14 post-transplantation, the breathing rates of mice in the two control groups remained high (between 200 and 280 breaths/minute) compared to the relatively low breathing rates of <200 breaths/minute observed in mice transplanted with hES-LECs (Figure 4c). Furthermore, tidal volumes in the two control groups rose to between 0.23 and 0.31 cm3 despite transplantation with either PBS or cells differentiated with 293-CM. In contrast, the tidal volume of mice transplanted with hES-LECs began to decrease from ~0.27 cm3 2 days after transplantation and eventually registered a relatively constant volume of ~0.19 cm3 (Figure 4c). The improved and better physiological parameters in animals transplanted with hES-LECs differentiated with HFL-CM provided strong evidence that these cells, despite their heterogeneity, supported the repair of distal lung epithelia and the restoration of overall functional surface area for gaseous exchange.
CD166pos subpopulation from hES and hiPS derived LECs is responsible for repair in acute lung injury
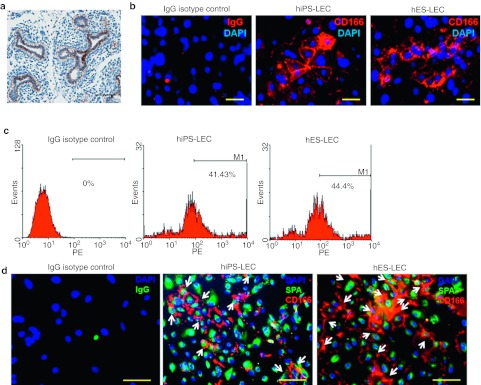
To further identify the subset of stem cell-derived LECs that were responsible for the rescue observed in the transplantation experiments, we sought for surface cell markers that could serve in cell sorting. By screening through some of the known surface markers for epithelial progenitor cells (CD44, CD133, CD166, and EpCAM),16,17,18,19,20 an examination of early human fetal lung at the pseudoglandular stage (week 7–16) revealed that all the epithelial cells lining the budding lung express significant levels of the surface antigen CD166 (Figure 5a), and these CD166pos cells are likely to be those that self-renew and contribute to all epithelial cell lineages in the lung. Further analysis revealed that ~40–45% of the differentiated cells in hES-LECs and hiPS-LECs expressed CD166 antigen (Figure 5b–c), of which ~60% of the CD166pos cells were also shown to express surfactant protein A, a distal lung marker (Figure 5d and Supplementary Figure S5).
Figure 5.
In vitro characterization of CD166pos lung epithelial cells (LECs)-derived from both human embryonic stem (hES) and human induced pluripotent stem (hiPS) cells. (a) Representative image of CD166 expression in the pseudoglandular stage during normal human lung development. Bar = 200 µm. (b) Fluorescent images showing CD166 expression in hES (H1) and hiPS (BJ)-derived LECs. IgG isotype negative control is as shown. Cell nuclei (blue) were stained with 4, 6-diamindino-2-phenylindole (DAPI). Bar = 50 µm. (c) The percentages of cells expressing CD166 in either hES- or hiPS-derived LECs were determined by flow cytometric analysis. Typically, between 40 and 50% of the cells express CD166 antigen. (d) Representative fluorescent images of both hiPS-LEC and hES-LEC demonstrated the presence of surfactant protein A (green) and CD166 (red) dual-positive cells. IgG isotype negative control is as shown. Cell nuclei (blue) were stained with DAPI. Bar = 50 µm.
As several recent studies reported CD166 to be a prognostic biomarker for cancer,19,20,21 we attempted to characterize the expression of CD166 during the differentiation of hES and hiPS, at several time points, to determine the progression of CD166 expression. Using adult human lung tissue sections to represent the “end state ” of CD166 expression levels in normal lung development, we demonstrated fairly low levels of CD166 expression in the terminal bronchioles of adult lung as compared to during the pseudograndular phase (Supplementary Figure S6). Thus, it seems that the expression of CD166 increases in the early embryonic stages of lung development and subsequently decreases in normal adult lung. Consistent with this observation, in vitro experiments performed to recapitulate lung progenitor cells derivation from hES and hiPS cells demonstrated progressive increase in CD166 expressions after definitive endoderm stage. The expressions of CD166 subsequently decrease in mature cultures of the hES- and hiPS-derived LECs (Supplementary Figure S6). Henceforth, we hypothesized that the cell type responsible for the repair of acute lung injury in bleomycin-treated mice to be the CD166pos cells.
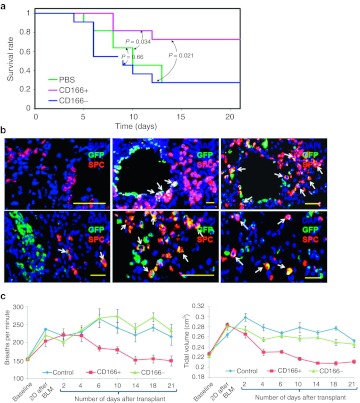
To confirm this, we transplanted CD166pos or CD166neg LECs from differentiated stem cell populations. Mice treated with bleomycin were given either 5 × 105 CD166pos LECs or CD166neg LECs. Strikingly, we consistently observed high survival rate (~75%; P < 0.05) after 3 weeks in mice transplanted with CD166pos cells compared to the control mice that were injected with either PBS or CD166neg populations, which showed ~25% survival (Figure 6a). To unambiguously determine whether these CD166pos LECs were truly functional in vivo, immunofluorescent staining was performed using anti-GFP (green) and anti-prosurfactant protein C (red) primary antibodies to determine the localization of these cells. Clusters of GFP-positive cells were found to be located near damaged areas in the left lung. Notably, most of the labeled cells were found either in the alveoli or in the terminal bronchioles (Figure 6b). Immunofluorescent staining for prosurfactant protein C also revealed that a subpopulation of the integrated GFP-positive cells was also positive for SPC, a marker for type II pneumocyte. Consistently, improvements in lung physiological functions were observed in mice transplanted with CD166pos LECs as compared to those transplanted with CD166neg population, which performed similarly to the PBS control (Figure 6c). Further investigation to demonstrate specific engraftment of the cells in the surviving mice 3 weeks post-transplantation revealed the presence of GFPpos LECs in the lung but not in the other organs such as the heart, spleen, and liver. These results confirmed the specific integration of CD166pos stem cell-derived LECs in the lungs of surviving mice (Supplementary Figure S7).
Figure 6.
CD166pos lung epithelial cells (LECs) were responsible for the repair of acute lung injury. (a) Kaplan–Meier curve showing survival rates of mice transplanted with phosphate-buffered saline (PBS), CD166pos or CD166neg LECs derived from human embryonic stem (hES) over a 3 weeks period after bleomycin-induced lung injury. The experiment was performed twice with eight mice in each of the experimental groups. The survival analysis was performed using Kaplan–Meier estimator. The P values between the experimental groups are as shown. (b) Immunocytochemistry of lung section from surviving mice transplanted with CD166pos LECs. Top left panel represents control lung section obtained from surviving mice that were treated with bleomycin, but without cellular transplantation. For mice that were transplanted with CD166pos LECs, the cells were present at the terminal bronchioles and appeared as clusters in the alveolar regions of damaged areas. Some of the green fluorescent protein (GFP)-positive cells were shown to be positive for SPC, a specific marker for type II pneumocyte (white arrow). Clusters of GFPpos CD166pos LECs eventually spread out to form new alveoli. Cell nuclei (blue) were stained with 4, 6-diamindino-2-phenylindole (DAPI). Sections were scanned with a high-resolution MIRAX MIDI system (Carl Zeiss) equipped with both bright field and fluorescence illumination. Images were then analyzed by the software Miraxviewer. Bar = 50 µm. (c) Measurement of lung pulmonary functions was performed by whole body plethysmography of mice transplanted with PBS, CD166pos or CD166neg lung epithelial cells (LECs). The breathing rates and tidal volumes of the three groups of mice were monitored over a 3 weeks period. Error bars represent standard deviation obtained from surviving mice at each time point.
Discussion
Previous studies have shown that pulmonary mesenchyme is required for normal lung development and that small molecules secreted by the embryonic mesenchyme have profound effects on the growth, patterning, and differentiation of the developing lung epithelium.22,23 Similar work employing the coculture approach to drive ES cells differentiation has been recently reported. Soto-Gutierrez and colleagues demonstrated that coculture of murine ES cells with liver nonparenchymal cell lines induced high percentage of differentiation of ES cells to hepatocytes that can metabolize almost as well as primary mouse hepatocytes.24 Here, we demonstrated that by first specifying endodermal lineage differentiation followed by treatment with medium that has been conditioned by human fetal lung fibroblasts, both hES and hiPS cells can be differentiated efficiently toward cells of the pulmonary tree. This approach highlights the increasing evidence that the use of tissue-specific conditioned medium may be a very useful compendium in the technology of tissue regeneration.25,26,27,28,29
At present, due to a lack of efficacious medical therapy for both acute and chronic lung diseases, cell based therapy seems to be a viable option for lung repair. Previous study has reported that transplantation of primary type II pneumocytes was able to reverse lung injury in rats that were subjected to bleomycin treatment,30 suggesting that endogenous lung progenitor cells may prove useful in repairing damaged lung tissue. However, it is extremely challenging to purify sufficient human primary type II pneumocytes for treatment of lung injuries, let alone complications that could possibly arise due to immune rejection. Wang et al. recently reported that transplantation of hES-derived alveolar epithelial type II pneumocytes were able to repair lung injury in mice that were treated with bleomycin.5 However, it should be noted that the isolation of type II pneumocytes was done using genetically modified human ES cells that harbor the SPC reporter.31 Although such study is valuable to assess the therapeutic potential of hES-derived type II pneumocytes in acute lung injury, it nonetheless poses potential complication due to genetic modification of the transplanted cells in the long term.
In contrast, our study has demonstrated a specific and efficient method to derive distal LECs using HFL-CM. Although transplantation of stem cell-derived LECs rescued mice from acute lung injury, it is unclear which cell type is responsible for the repair due to the heterogeneity of the cells after differentiation. Attempts to identify the subpopulation of differentiated cells which are responsible for lung repair have revealed CD166 as a useful surface marker to select for lung regenerative cells, thus overcoming complications that could possible arise from genetic manipulation of cells. Through magnetic-activated cell sorting and transplantation study, we confirmed the specific cell population responsible for the survival of mice following bleomycin injury to be CD166-positive. More importantly, the high survival rate observed in mice transplanted with CD166pos LECs was accompanied with corresponding improvements in lung pulmonary functions. This clearly demonstrated that CD166pos LECs play a major role in repairing or preventing tissue damage caused by bleomycin. As bleomycin-induced lung injury typically shows three phases in disease development, namely (i) neutrophil/macrophage-dependent acute inflammation, (ii) lymphocytes accumulation, and then (iii) fibrosis development, we hypothesize that apart from engrafting and functioning as a type II pneumocyte in the lung, the CD166pos LECs, to a larger extent, might have immunomodulating effects in this injury model. This is because from our data, CD166pos LECs also express surfactant protein A (SFTPA), and that SFTPA was previously shown to modulate the immunologic environment of the lung so as to protect the host from potential pathogens and, at the same time, modulate an overzealous inflammatory response that could potentially damage the lung thereby impairing gas exchange.32,33 Consistent with this, we observed less extensive interstitial infiltration of inflammatory cells in lung sections 7 days after bleomycin induction in mice that were transplanted with stem cells that were differentiated with HFL-CM.
Notably, mice transplanted with hiPS-LECs did not perform as well compared to hES-LECs in the survival studies despite significant rescue (~70%). This observation could possibly be due to the epigenetic memory of the iPS cells, having retained the transcriptional memory of the original cells.34,35 In addition, hES and hiPS cells genome-wide profiling study conducted by Chin et al. recently reported the unique expression signature of hiPS cells, suggesting that reprogrammed cells might not have efficiently silenced the gene expression pattern of somatic cell from which they were derived.36 These inherent genetic and epigenetic differences in hiPS cells might have caused the reprogrammed cells to be less efficient in differentiating to other somatic cell types besides the original cell type they were derived from.
Previous study by Alvaez-Dolado et al. demonstrated fusion of bone marrow derived cells in vivo with hepatocytes in liver, Purkinje neurons in the brain and cardiac muscle in the heart, resulting in the formation of multinucleated cells, suggesting the possibility that cell fusion might have contributed to the development and maintenance of key cell types, hence aiding in repair and regeneration processes.37 In our study, several lines of evidence indicated that the chances of cell fusion to be very unlikely. Firstly, engrafted LECs are mononucleated. This can be seen from the immunocytochemistry of GFP-positive cells, having a single nucleus in the lung sections (Figure 6b). Secondly, applying the same side-scatter and forward-scatter gating for flow cytometric analysis 3 weeks post-transplantation, GFP-positive cell population was found in whole lung single cell suspension obtained from transplanted animals, indicating that the engrafted GFP-positive LECs are of similar sizes with endogenous lung cells. This indicates that cell fusion is unlikely because fused multinucleated cells tend to be of a larger size. Thirdly, cell fusion occasionally may lead to aneuploidy and potentially cancer.38 In our study, mice transplanted with stem cell-derived LECs followed over at least 12-month period have not shown any incidence of tumor formation.
Although FACS analysis on various organs demonstrated the specific engraftment of CD166pos LECs in the lung, only a small percentage of the whole organ comprises of GFP-positive cells (~1%), which is consistent with several similar studies.39,40,41 Possible explanation for this observation include (i) loss of GFP expression in the hES or hiPS cells, (ii) the engrafted LECs were outgrown and replaced by endogenous progenitor cells, (iii) involvement of secreted paracrine factors by engrafted LECs in lung repair. Indeed, at present, a large body of evidence indicates that the beneficial effects of cell therapy are related to the secretion of soluble factors acting in a paracrine manner.42 These secreted paracrine factors may play crucial role to elicit repair and regeneration mechanisms during lung injury.
In summary, our study has shown enhanced efficiency in deriving distal LECs from both hES and hiPS cells using a two-step differentiation methodology. The functionality of these stem cell-derived LECs were further demonstrated in transplantation experiments where mice treated with lethal dosage of bleomycin survived with improved lung pulmonary functions. In addition, we further identified CD166 as a suitable cell surface marker to enrich for early lung progenitor cells without the need for genetic manipulation. Although low percentage of engrafted cells were present 3 weeks post-transplantation, it is unequivocal that their presence in damaged lung tissue provided therapeutic benefits, either in the form of cellular effects or paracrine effects, to mice subjected to bleomycin acute lung injury. Lastly, it is noteworthy that the therapeutic effects of the transplanted CD166pos LECs were long lasting and without teratoma formation.
Materials and Methods
Cell culture and transduction of human ES cells. The human ES cell line, H1 (Wicell, Madison, WI) was cultured in feeder-free condition on Matrigel (BD, Franklin Lakes, NJ). The cells were maintained in mouse embryonic fibroblast conditioned medium (MEF-CM). The MEF-CM was prepared according to Xu et al.43 Preparation of the MEF-CM involves the conditioning of hES medium (80% Dulbecco's modified Eagle's medium F12 (DMEM F12), 20% knockout serum replacement, 1 mmol/l L-glutamine, 0.1 mmol/l β-mercaptoethanol, 1% nonessential amino acids, and 4 ng/ml human basic fibroblast growth factor (all from Invitrogen) on MEF overnight with an additional 8 ng/ml of basic fibroblast growth factor before feeding human ES cells. For culture of MEF and HFL, medium consisting of 90% Dulbecco's modified Eagle medium high glucose, 10% fetal bovine serum, 2 mmol/l L-glutamine, 50 µg/ml Pen-Strep (all from Invitrogen, Carlsbad, CA) was used. The cell growth was arrested by treating with 10 µg/ml mitomycin-C (Kyowa Hakko Kogyo, Tokyo, Japan). Collection of fetal lung tissues was done on aborted fetuses from week 11 of the gestation period, 12–24 hours after insertion of the pessary. Individuals gave written, informed consent and the samples were collected from the National University Hospital of Singapore. Lung samples were washed with PBS several times to remove blood, after which the connective tissues and blood vessels were removed using forceps and scissor. The samples were further cut into small, fine pieces in the presence of enzymes such as trypsin and collagenase, and incubated at 37 °C for ~15–20 minutes. A 5-ml syringe (Fisher Scientific, Pittsburgh, PA) may be used to further break up the clumps. The primary culture was cultured up to passage 5.
Transduction of hES cells was performed under feeder-free culture condition. Briefly, hES cells were either incubated in suspension with 1 × 107 viral particles harboring the CSII-EF-MCS-IRES2-Venus (a kind gift from H. Miyoshi, RIKEN Tsukuba Institute, Ibaraki, Japan) or SPC-DsRED-pLenti6 construct, and 8 µg/ml polybrene for 1 hour at 37 °C. The cells were then replated and cultured feeder-free as described. GFP-positive human ES clones were selected by performing colony forming assay as described by Soh et al.44 These clones were subsequently expanded under feeder-free conditions.
Generation of human iPS cell lines. GP2-293 packaging cells were plated into four T75 flasks 1 day before such that they were 70–80% confluent for transfection. The cells were transfected with retroviral pMXs vectors containing human OCT4, SOX2, KLF4, and C-MYC genes together with VSV-G (a plasmid express viral envelope protein) by Lipofectamine2000. Forty-eight hours post-transfection, the virus-containing supernatants were collected and filtered through a 0.45-µm pore-size filter. The virus-containing supernatants were then concentrated by ultracentrifugation (ultracentrifugation using rotor SW28, spin at 25,000 rpm, 4 °C for 2 hours). 1 × 105 human fibroblast cells (BJ or MRC5 cell line) were infected in a 35 mm dish. Six day post-transduction, fibroblasts were replated at 1 × 105 cells per 100-mm dish on 1.2 × 106 inactivated MEF. The medium was replaced with Primate ES cell medium supplemented with 4 ng/ml basic fibroblast growth factor the following day. The culture medium was changed every day. Within 2 weeks, iPS-like colonies emerged.
Step-wise hES/hiPS cell differentiation protocol. Human ES cells (H1 cell line) and hiPS lines (BJ and MRC5 hiPS) were differentiated toward definitive endoderm by culturing the cells in low serum, high Activin A medium as described by D'Amour et al.12 The definitive endodermal cells were subsequently cultured in HFL-CM for an additional 7 days. As a comparison to demonstrate specific differentiation capability of the HFL-CM, control cells were treated with 293T-CM for the subsequent 7 days instead. The differentiated cells were subsequently cultured in lung epithelial medium to enable expansion of lung progenitor cells prior to transplantation.45
Flow cytometry. Flow cytometric analysis was performed on in vitro differentiated hES cells following treatment with HFL-CM. Briefly, 1 × 106 cells were either stained with rabbit IgG primary antibody (Isotype control) or rabbit anti-surfactant protein A primary antibody at 1:500 dilution (Chemicon, Temecula, CA), followed by goat anti-rabbit IgG Alexa 488 at 1:1,000 dilution (FITC) (Invitrogen). Samples were analyzed on a FACSCalibur flow cytometry (Becton Dickinson, Bohemia, NY). For endodermal cells, goat anti-SOX17 (R&D Systems, Minneapolis, MN) and Rabbit anti-FOXA2 (Upstate Biotechnology, Lake Placid, NY) primary antibodies were used at 1:500 dilutions followed by goat anti-rabbit IgG Alexa 488 or Donkey anti-goat IgG Alexa 488 at 1:1,000 dilutions. PE-conjugated anti-CD166 antibody (R&D) was used at 1:500 dilutions to quantitate CD166 cell population.
RNA extraction and cRNA synthesis. For cultured cell samples, 2 × 106 cells were harvested and lysed in TRIzol reagent (Invitrogen) and purified with the RNeasy Mini Kit (Qiagen, Singapore, Singapore) according to the manufacturer's recommendations. Five hundred nanogram of total RNA from each sample were used to generate cRNA using the Illumina Totalprep RNA Amplification Kit (Ambion, Austin, TX) according to the manufacturer's recommendations.
Microarray analysis. Microarray study was conducted using Human Ref8 v3 Sentrix BeadChip (Illumina, San Diego, CA). Hybridization and scanning of the Sentrix BeadChip using the Illumina BeadArray Reader were performed according to manufacturer's recommendations. All gene expression data were first subtracted from the background, and then normalized using the cross-correlation.46 Hierarchical clustering was performed for the whole normalized data set, showing similarity/dissimilarity of arrays. Heatmaps using log2 transformed intensities subtracted by the mean of the means of all cell types/lines were then produced for all genes. Correlation between two arrays was done using the normalized expression intensities.
Immunocytochemistry. Immunocytochemical analysis was performed using rabbit anti-aquaporin 1 (Chemicon), mouse anti-caveolin 1 (Upstate Cell Signaling Solution) and rabbit anti-surfactant protein A (Chemicon) primary antibodies at 1:200 dilutions. Cells were first harvested and washed once with PBS. Fixation of cells was achieved with 4% paraformaldehyde for 30 minutes. The cells were then blocked with PBS containing 5% fetal bovine serum and 1% BSA for 30 minutes at room temperature. Primary antibodies were added at recommended dilutions and incubated for 1 hour at room temperature. After washing, the cells were incubated for another 45 minutes at room temperature without light exposure with either 1:500 diluted Alexa Fluor 488 or FITC-labeled secondary antibodies. Nuclei were counterstained with 4, 6-diamindino-2-phenylindole (DAPI). The cells were observed under a fluorescent microscope (Carl Zeiss Axio Observer D1; Carl Zeiss, Jena, Germany).
Labeling and MACS of hES/hips-derived LECs. Single cell suspensions of CD166pos LECs were magnetically labeled using the MACS cell separation system according to manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). Briefly, the cell pellet was resolved in MACS buffer (Dulbecco's PBS + 2 mmol/l EDTA + 0.5% fetal bovine serum) and incubated with PE-conjugated anti-CD166 antibody (R&D; 1:20 dilution) for 15 minutes on ice under constant shaking. Five microliter of MACS buffer was then added to the cells and the cell suspension was centrifuged for 10 minutes at 200g at 4 °C. The supernatant was discarded and the cells were labeled with anti-PE MicroBeads (Miltenyi Biotech) according to the manufacturer's recommendations. Cells were incubated on ice for 15 minutes on a shaker. Thereafter, 5 ml of MACS buffer was added to stop the incubation, following which the cell suspension was centrifuged for 10 minutes at 200g at 4 °C. Large volume separation columns (Miltenyi Biotech) were placed in the magnetic field and washed once with 3-ml degassed MACS buffer. The labeled cell suspensions were resuspended in 2-ml degassed MACS buffer and filled into the reservoir of the columns. The effluent was collected and combined with the effluents from three washes. This suspension was designated the CD166neg LEC fraction and stored on ice until further use. The CD166pos LEC fraction was eluted by flushing the columns with 5-ml MACS buffer after removing the column from the magnetic field.
Transplantation of hES-derived LECs into bleomycin-injured mouse. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID) mouse strain harboring a null mutation of the common cytokine receptor γ chain (Il2rgtm1Wjl) (NOD/SCID/IL2rγnull) from Jackson Laboratory (Bar Harbor, ME) was used in our lung injury model. The protocol for the lung injury model was approved by the National University Institutional Review Board (NUS-IRB). All procedures were performed on mice between 8 and 10 weeks of age. Briefly, mice were anesthetized with 2.5% avertin and 5 U/kg bleomycin sulfate (Sigma-Aldrich, St Louis, MO) dissolved in 75 µl of sterile PBS was instilled intratracheally. Three days after bleomycin-induced injury, the mice were transplanted intravenously via tail vein with either 300 µl of PBS (control) or 5 × 105 cells that have undergone differentiation. The mice were observed for up to 3 weeks post-transplantation.
Whole-body plethysmography of mice. Mice were retrieved at designated time points for evaluation of lung function. In vivo respiratory patterns were measured using a whole-body plethysmography system as previously described (Buxco Electronics, Sharon, CT).47 Briefly, unrestrained, conscious mice were placed individually into plethysmograph chambers, allowed to acclimatize for 30 minutes and the pressure difference was compared against a reference chamber to measure airway physiological parameters. After chamber calibration, signals were recorded, and various respiratory variables were calculated using the manufacturer software. Mean respiratory variables were then calculated from 15 minutes recordings of continuous respiratory behavior. Resulting changes in pressure/volume readings in inspiration and expiration were taken as an indication of pulmonary function.
Histopathology and immunochemical staining. Mice were sacrificed 3 weeks post-transplantation and lung tissues were harvested and fixed in 10% neutral buffered formalin solution (Sigma-Aldrich) for 24 hours. The tissues were processed with Tissue Processor (Leica Microsystems, Buffalo Grove, IL) and embedded in paraffin. Sections were cut at a 5-µm thickness, mounted on polylysine coated slides (Thermal Fisher Scientific, Pittsburgh, PA), de-waxed, rehydrated, and processed for hematoxylin and eosin stain staining according to a standard protocol. For immunofluorescent staining, antigen retrieval was carried out by digesting the slides in proteinase K solution (Sigma-Aldrich, 20 mg/ml, in 50 mmol/l Tris–HCl, 1 mmol/l EDTA, pH 8.0) at 37 °C for 30 minutes. Sections were then immersed for 1 hour in blocking buffer (3% BSA, 0.2% Triton X-100 in PBS), then incubated in primary antibody (made up in blocking buffer) at 4 °C overnight, followed by incubation in secondary antibody at 4 °C for 1 hour. Goat anti-SPC (Santa Cruz Biotechnology, sc-7706) rabbit anti-GFP (Abcam; ab290) were used at a 1:50 dilution. Secondary antibodies, donkey anti-rabbit or anti-goat with different Alexa Fluor conjugations were purchased from Invitrogen and used at a 1:200 dilution. All lung sections were counter stained with DAPI (blue). Sections were mounted with antifade reagent (Invitrogen) and then scanned with a high-resolution MIRAX MIDI system (Carl Zeiss) equipped with both bright field and fluorescence illumination. Images were analyzed by the software Miraxviewer.
Statistical analysis. The error bars in all graphs refer to standard error of means of stated replicates. A nonparametric test (Kruskal–Wallis one-way analysis of variance) was used to assess whether the mean values of the experimental groups could be considered to be significantly different compared to the control group at 95% confidence level. For the survival study, analysis was performed using Kaplan–Meier estimator.
SUPPLEMENTARY MATERIAL Figure S1. In vitro differentiation of human ES cells toward lung epithelial cells via definitive endoderm. Figure S2. In vitro characterization of hES and hiPS cells differentiated with HFL-CM. Figure S3. Human fetal lung conditioned medium drove differentiation towards the lung lineage. Figure S4. Generation of GFP-positive human ES (H1) and hiPS (BJ and MRC5) cell lines. Figure S5. Quantification of CD166 and SFTPA dual-positive cells. Figure S6. Characterization of CD166 expression in developing human lung and differentiating hES- and hiPS-derived LECs. Figure S7. Specific engraftment and quantification of hES- and hiPS-derived CD166pos LECs in lung tissue.
Acknowledgments
We thank all members of the Lim lab for their support and helpful discussions. This work is supported by the Agency for Science, Technology and Research (Singapore) and by a grant from the Defense Advanced Research Projects Agency (DARPA; Project N66001-09-2121). This work is also partially supported by Biomedical Research Council grant to E.H.L (BMRC /05/1/21/19/391). B.S.S. is a recipient of the A*STAR graduate scholarship. The authors declared no conflict of interest.
Supplementary Material
In vitro differentiation of human ES cells toward lung epithelial cells via definitive endoderm.
In vitro characterization of hES and hiPS cells differentiated with HFL-CM.
Human fetal lung conditioned medium drove differentiation towards the lung lineage.
Generation of GFP-positive human ES (H1) and hiPS (BJ and MRC5) cell lines.
Quantification of CD166 and SFTPA dual-positive cells.
Characterization of CD166 expression in developing human lung and differentiating hES- and hiPS-derived LECs.
Specific engraftment and quantification of hES- and hiPS-derived CD166pos LECs in lung tissue.
REFERENCES
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11 Suppl:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM.et al. (2002Derivation of type II alveolar epithelial cells from murine embryonic stem cells Tissue Eng 8541–550. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC.et al. (2006Derivation of distal airway epithelium from human embryonic stem cells Tissue Eng 12867–875. [DOI] [PubMed] [Google Scholar]
- Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH.et al. (2009Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application Tissue Eng Part A 153351–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Morales JE, Calame DG, Alcorn JL., and, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon HJ, Ali NN, Polak JM., and, Bishop AE. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M., and, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G.et al. (2010Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation Tissue Eng Part A 162565–2580. [DOI] [PubMed] [Google Scholar]
- Lin YM, Zhang A, Rippon HJ, Bismarck A., and, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16:1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- Banerjee ER, Laflamme MA, Papayannopoulou T, Kahn M, Murry CE., and, Henderson WR., Jr Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS ONE. 2012;7:e33165. doi: 10.1371/journal.pone.0033165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E., and, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W.et al. (2011Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma©−/− mice improves engraftment of human hematopoietic cells in humanized mice Proc Natl Acad Sci USA 10813218–13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber AO, Fraga D, Kotb M, Lo A, Sabek O., and, Latif K. Human islet graft function in NOD-SCID mice predicts clinical response in islet transplant recipients. Transplant Proc. 2004;36:1108–1110. doi: 10.1016/j.transproceed.2004.04.055. [DOI] [PubMed] [Google Scholar]
- Hay J, Shahzeidi S., and, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ., and, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117 Pt 16:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Horst D, Kriegl L, Engel J, Kirchner T., and, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 2009;27:844–850. doi: 10.1080/07357900902744502. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM.et al. (2008Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo J Cell Physiol 214413–421. [DOI] [PubMed] [Google Scholar]
- Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC.et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract Gastroenterology 1392072–2082. e2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S.et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis Cell 148259–72. [DOI] [PubMed] [Google Scholar]
- Tachezy M, Zander H, Marx AH, Gebauer F, Rawnaq T, Kaifi JT.et al. (2011ALCAM (CD166) expression as novel prognostic biomarker for pancreatic neuroendocrine tumor patients J Surg Res 170226–232. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Pan T, Nielsen LD, Edeen KE., and, Mason RJ. Lung fibroblasts improve differentiation of rat type II cells in primary culture. Am J Respir Cell Mol Biol. 2001;24:235–244. doi: 10.1165/ajrcmb.24.3.4302. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wang F., and, Ornitz DM. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development. 2011;138:3169–3177. doi: 10.1242/dev.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gutiérrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N.et al. (2007Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines Nat Protoc 2347–356. [DOI] [PubMed] [Google Scholar]
- Korecki CL, Taboas JM, Tuan RS., and, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze A., and, Stolzing A. Differentiation of mouse bone marrow derived stem cells toward microglia-like cells. BMC Cell Biol. 2011;12:35. doi: 10.1186/1471-2121-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe E, Tanaka H, Ishimi Y, Miyaura C, Hayashi T, Nagasawa H.et al. (1986Differentiation-inducing factor purified from conditioned medium of mitogen-treated spleen cell cultures stimulates bone resorption Proc Natl Acad Sci USA 835958–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacham-Kaplan O, Chy H., and, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- Chen SS, Revoltella RP, Papini S, Michelini M, Fitzgerald W, Zimmerberg J.et al. (2003Multilineage differentiation of rhesus monkey embryonic stem cells in three-dimensional culture systems Stem Cells 21281–295. [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A., and, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E., and, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastva AM, Wright JR., and, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122:861–79; quiz 880. doi: 10.1016/j.jaci.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T.et al. (2011Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells Nat Cell Biol 13541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yi BA, Wu H, Bock C, Gu H, Lui KO.et al. (2012Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature Cell Res 22142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C.et al. (2009Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures Cell Stem Cell 5111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K.et al. (2003Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes Nature 425968–973. [DOI] [PubMed] [Google Scholar]
- Lu X., and, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N.et al. (2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects Proc Natl Acad Sci USA 1008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J.et al. (2005Bone marrow-derived mesenchymal stem cells in repair of the injured lung Am J Respir Cell Mol Biol 33145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT., and, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A., and, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD.et al. (2001Feeder-free growth of undifferentiated human embryonic stem cells Nat Biotechnol 19971–974. [DOI] [PubMed] [Google Scholar]
- Soh BS, Song CM, Vallier L, Li P, Choong C, Yeo BH.et al. (2007Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells Stem Cells 253029–3037. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., and, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SW, Vijayakumar P, Nissom PM, Yam CY, Wong VV., and, Yang H. A novel normalization method for effective removal of systematic variation in microarray data. Nucleic Acids Res. 2006;34:e38. doi: 10.1093/nar/gkl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sio SW, Moochhala S, Lu J., and, Bhatia M. Early protection from burn-induced acute lung injury by deletion of preprotachykinin-A gene. Am J Respir Crit Care Med. 2010;181:36–46. doi: 10.1164/rccm.200907-1073OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro differentiation of human ES cells toward lung epithelial cells via definitive endoderm.
In vitro characterization of hES and hiPS cells differentiated with HFL-CM.
Human fetal lung conditioned medium drove differentiation towards the lung lineage.
Generation of GFP-positive human ES (H1) and hiPS (BJ and MRC5) cell lines.
Quantification of CD166 and SFTPA dual-positive cells.
Characterization of CD166 expression in developing human lung and differentiating hES- and hiPS-derived LECs.
Specific engraftment and quantification of hES- and hiPS-derived CD166pos LECs in lung tissue.