Highlights
► High odour emission from food waste compost was correlated to low pH. ► Microbes in high-odour samples included Lactic acid bacteria and Clostridia. ► For odour prevention, try high initial aeration rate and recycled compost as additive.
Keywords: Lactic acid bacteria, Olfactometry, Microarray, Organic acids
Abstract
A major problem for composting plants is odour emission. Slow decomposition during prolonged low-pH conditions is a frequent process problem in food waste composting. The aim was to investigate correlations between low pH, odour and microbial composition during food waste composting. Samples from laboratory composting experiments and two large scale composting plants were analysed for odour by olfactometry, as well as physico-chemical and microbial composition. There was large variation in odour, and samples clustered in two groups, one with low odour and high pH (above 6.5), the other with high odour and low pH (below 6.0). The low-odour samples were significantly drier, had lower nitrate and TVOC concentrations and no detectable organic acids. Samples of both groups were dominated by Bacillales or Actinobacteria, organisms which are often indicative of well-functioning composting processes, but the high-odour group DNA sequences were similar to those of anaerobic or facultatively anaerobic species, not to typical thermophilic composting species. High-odour samples also contained Lactobacteria and Clostridia, known to produce odorous substances. A proposed odour reduction strategy is to rapidly overcome the low pH phase, through high initial aeration rates and the use of additives such as recycled compost.
1. Introduction
Composting is a waste treatment technology which facilitates reduction of waste to landfill and recycling of plant nutrients and organic matter from biological waste to cultivated soils. A major problem facing many composting plants is odour emission into the surrounding environment. There have been many investigations screening odours from various composts, and hundreds of odorous substances have been identified in compost gases (Miller, 1993; Pöhle and Kliche, 1996; Smet et al., 1999). It is known that the quantity and quality of odour from composting vary, depending on substrate composition and process conditions. However, very little is known about how variations in process conditions cause variations in odour.
In the composting of food waste, there is often a low-pH phase at the start of the process. In Scandinavia, slow decomposition during prolonged low-pH conditions is a frequent process problem in food-waste composting (Sundberg and Jönsson, 2008). The dominating acids are lactic and acetic acid, but many other acids have been found at lower concentrations (Sundberg and Jönsson, 2005). Organic acids are known to be odourous, especially longer chain acids (Brinton, 1998). It is therefore possible that the odour problems observed at food waste composting plants in Scandinavia are linked to the low pH conditions. Organic acids have received relatively little attention in compost odour research. Nevertheless, acids have been reported in research on odour from mushroom composting (Noble et al., 2001) and composting of food waste in combinations with other substrates (Gallego et al., 2012; Krzymien et al., 1999; Komilis et al., 2004; Mao et al., 2006; Tsai et al., 2008). None of these authors, however, reported acids to be a major component of odour, and unfortunately they did not report pH or organic acid concentrations in compost samples.
Odour can be quantified by dynamic olfactometry, a standardised method (EN 13725) whereby a group of persons evaluate the odour of gas samples that are diluted with nitrogen at different concentrations. This method is costly and time consuming, and is thus not optimal for the frequent measurements of odour at a composting plant. Instruments based on separation methods (chromatography) are commonly used to measure the concentrations of individual substances. However, to be of value in quantification of odours, prior knowledge is required as to which substances are the most dominant causes for odour.
The low pH typically found in Scandinavian wastes has been correlated with a high concentration of lactic acid bacteria (LAB) in incoming wastes and decomposing material during the early phases of the composting process (Sundberg et al., 2011; Partanen et al., 2010). In well functioning processes, the number of LAB rapidly decrease during the start-up phase as pH and temperature rise (Partanen et al., 2010; Kurola et al., 2011).
The main aim of this work was to test the hypotheses that prolonged acidic conditions during the composting phase is a main cause for odour problems during food waste composting. A further aim was to identify parameters that can be used as odour indicators in food waste composting. We analysed samples from laboratory composting experiments as well as from two large scale composting plants for odour, physico-chemical and microbial characteristics. The data analysis focused on relationships between these characteristics in the compost samples. Based on odour and pH, the samples clustered in two groups (high odour-low pH and low odour-high pH) and the differences between the two groups were described and discussed. From the analysis of the results, recommendations on odour reduction strategies were developed.
2. Methods
2.1. Composting processes and sampling
Samples for analysis of odour, chemical and microbial composition were taken from a laboratory composting reactor and from two large scale composting plants. Both large scale plants had enclosed main processes with forced aeration, in tunnels at NSR, where two batches were investigated (Helsingborg, Sweden), and in horizontal plug-flow channels at IVAR (Sandnes, Norway) where three batches were investigated. Temperature was monitored on-line and aeration rates were measured throughout the processes. Composts were sampled at the end of the main process (after 30 days at IVAR and after 33 at NSR; samples NSR1a and 2a). At NSR, samples were also collected during curing, 90 days after the start of composting (NSR1b and 2b). During unloading of the compost, cone-shaped heaps of 2–3 m height were formed. After letting the heaps sit for about 2 h, gas samples were extracted with a pump at a rate of 1 L/min from the pore volume of the heaps at a height of 1–1.5 m and depth of 0.5 m. Since these pore gas samples were warm (47, 44, 38 and 42 °C in NSR samples 1a, 1b, 2a and 2b respectively, and about 50 °C in IVAR samples) and moist (relative humidity 100%), they were diluted with an equal volume of nitrogen gas in order to avoid condensation in the bags. The substrate at IVAR was food waste mixed with various woody structure materials including reused structure materials and its characteristics are presented in Table 1. At NSR, the substrate consisted of food waste and mixed household waste which had been screened to remove pieces larger than 100 mm and its characteristics are described in Table 1. The substrates have been described in more detail by Sundberg et al. (2011).
Table 1.
Waste substrates before composting.
| DM (% of fresh weight) | pH | C/N-ratio | Total acid in material (mmol/kg compost) | |
|---|---|---|---|---|
| IVAR | 48 ± 2 | 6.0 | 20 | 28 ± 3 |
| NSR1 | 42 ± 7 | 4.9 | 18 | 242 ± 60 |
| NSR2 | 42 ± 7 | 5.0 | 22 | 131 ± 84 |
| Laboratory reactor (Cool and Heat) | 44.9 ± 0.6 | 5.7 | 27 | 18 ± 6 |
In the laboratory reactor experiment, samples were extracted from five 16-day compost trials (Table 2) carried out in a 200-L compost reactor in which the temperature and oxygen content were controlled independently (Smårs et al., 2001). In order to maintain constant oxygen content, new air was added at a rate matching the oxygen consumption of the process. Substrate mixture, water content and sampling intervals were the same in all runs. The compost substrate (Table 1) was a mixture of thawed source separated food waste and two fractions of crushed wood waste (fine: <3 mm and coarse: 3–19 mm) used as a structure material. The wet weight proportions of the compost substrate were 10:4:1 (food waste:coarse structure:fine structure). Mature compost and water (each 5% weight of the weight of food waste) were also added. The substrate components were thoroughly mixed, which included mincing through a screen plate with approximately 20 mm holes.
Table 2.
Temperature and oxygen concentration settings in the five laboratory reactor trials.
| Name | Start-phase | Later temperature | O2 concentration (%) |
|---|---|---|---|
| Cool-1% | Coolinga | 55 °C | 1 |
| Cool-16% | Coolinga | 55 °C | 16 |
| Cool-16%(70°) | Coolinga | 55 °C; 70 °C on day 9–16 | 16 |
| Heat-16% | Self-heatinga | 55 °C | 16 |
| Heat-16%(70°) | Self-heatinga | 55 °C; 70 °C on day 9–16 | 16 |
See explanation in text.
The compost material was mixed daily for the duration of the experiments by turning the reactor. Sampling (in triplicate) was conducted during mixing, with the reactor being turned between each sampling. On days 3, 8 and 16, samples of material were taken for chemical and microbial analysis, and samples of gas were taken for olfactometric odour analysis. Temperature, oxygen (O2), carbon dioxide (CO2), TVOC and gas flow were measured automatically every 5 min during the trials. The gas samples consisted of process gas, which had been extracted and cooled to approximately 14 °C and heated to approximately 21 °C before extraction for analysis. During cooling, some condensation occurred, and condensate was collected for analysis.
Two different temperature strategies were used in the laboratory reactor trials (Table 2). In the self-heating strategy (HEAT), the substrate was allowed to self-heat and when self-heating decreased, the material was externally heated to 55 °C. The aim of the self-heating strategy was to reproduce the heating process in large-scale composting with constant gas flow, where the temperature often rapidly reaches approximately 55 °C. In one run, the temperature was further increased to 70 °C from day 9. In the cooling strategy (COOL), the substrate was allowed to self-heat to 38 °C, and was then cooled to maintain a temperature just below 40 °C until the pH of the condensate increased above 6. The temperature was then allowed to rise to 55 °C. This strategy has been shown to result in a quick transfer from acidic to neutral/alkaline conditions and rapid decomposition (Smårs et al., 2002). Five runs were performed; two with each start-up strategy at 16% O2 concentration and one run with the COOL strategy at 1% O2 concentration (Table 2).
2.2. Gas analysis
Compost gases for olfactometry were collected in Tedlar bags (approx. 10 L), and sent to Molab (Oslo) for olfactometric analysis according to EN 13725 within 24 h. The TVOC of the compost gas was measured by an online Photoionisation (PID) instrument (TVOC®, Ion Science, Cambridge, UK) at the laboratory reactor, while at the composting plants, it was measured with a portable PID instrument (Phocheck 1000, Ion Science, Cambridge, UK).
Odour and TVOC were measured on samples of pore gas, i.e. the gases within the compost mass. This means that the results presented are not odour emissions from the composts. Instead the odour concentration in pore gas can be seen as a measure of odour potential, i.e. the risk of odour emissions in the further handling of the material.
2.3. Chemical analysis
For the analysis of pH and organic acids, waste was mixed with de-ionised water in the proportions 1:5. The mixture was stirred and allowed to stand for 1 hour, after which time pH was measured in the liquid phase. Organic acids present in the filtrate after passage through a 0.45 μm PVDF (polyvinylidene difluoride) filter (acetic acid, lactic acid, butyric acid and propionic acid) were analysed using HPLC (high-pressure liquid chromatography) using an ion-exchange column (HC-75, Hamilton, USA) at 60 °C and refractive index detection at 40 °C. Undiluted condensate from the laboratory reactor was analysed for organic acids and pH. Dry matter was determined by drying at 105 °C for 22–24 h. Volatile solids (VSs) were determined by ignition at 550 °C for 4 h. Total carbon (Tot-C) and total nitrogen (Tot-N) were measured in dried (50 °C) and milled samples using a Leco CNS-2000 analyser. Ammonium and nitrate nitrogen (NH4–N and NO3–N) were analysed colorimetrically using a TRAACS 800 AutoAnalyser (Seal Analytical, UK). Samples were milled to 5–10 mm, shaken in 2 M KCl overnight and centrifuged. The supernatant was subjected to colorimetric analysis.
2.4. Microbiological analyses
Analysis of the microbial composition of samples was carried out by two methods: (i) sequencing PCR amplified and cloned 16S rRNA gene fragments as previously described (Sundberg et al., 2011), and (ii) hybridization to the COMPOCHIP DNA microarray as described by Franke-Whittle et al. (2009) and Sundberg et al. (2011).
The inserts from the clones to be sequenced were reamplified with vector-specific primers and sent for sequencing to the University of Helsinki, Institute of Biotechnology. Forty cloned fragments were sequenced from each sample. Homology searches were conducted using the FASTA EMBL database and the NCBI Blast programme. Species membership was determined by homology values of 97% or higher.
Microarray analysis was also conducted in order to determine the microorganisms present in the different samples. The array is spotted with 369 probes targeting compost-relevant microorganisms, including plant, animal and human pathogens, and bacteria related to plant disease suppression. Fluorescence labelling of target DNA, hybridization, scanning of arrays and image analysis were conducted as described by Franke-Whittle et al. (2005, 2009).
2.5. Data analysis
Odour and physico-chemical characteristics data for all samples were analysed graphically, which revealed that the samples clustered in two groups (with the exception of one outlier) as explained in Section 3. For these data, the statistical significance of the difference between the two groups was analysed by the two sample Wilcoxon rank sum test. Furthermore, the correlation between odour and some of the physico-chemical characteristics were analysed by linear regression. The statistical software Minitab 15 was used for these analyses. Standard deviation is used throughout in tables as a measure of variation.
The microarray data was analysed by principal component analysis (PCA) using the signal to noise ratio (SNR) values of probes for which at least one compost sample gave a SNR value >3.
In the statistical analysis, data were viewed as individual samples. However, samples came from composting processes (up to three samples per process) that developed over time. For some processes more data were collected (i.e. continuous temperature measurements). These aspects of the samples are discussed, but was not analysed statistically.
3. Results and discussion
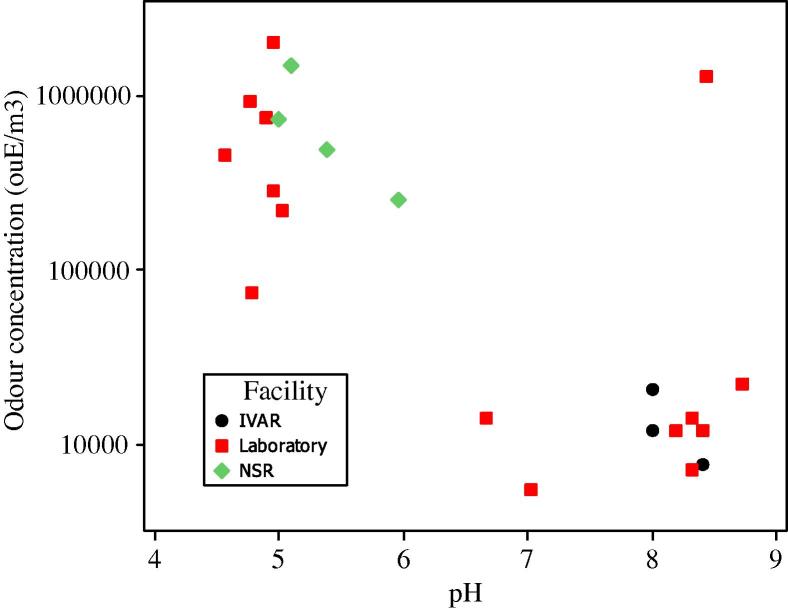
A large variation in odour concentration in samples from the different composts was found, with values ranging from 5500 to 2 million ouE/m3 (Table 3). Odour concentration in samples was higher at low pH (Fig. 1). Samples clustered into two significantly different (p < 0.005) groups, one group (A) with pH values <6.0 and odour concentrations higher than 70,000 ouE/m3, and the other (group B) with pH values >6.6 and odour concentrations <41,000 ouE/m3 (Table 4). One sample (Cool-1%:8) was found to be an anomaly, it had a high odour concentration (1,300,000 ouE/m3), and also a high pH value of 8.4 and was not included in any of the groups (Table 3). The two groups differed significantly also in TVOC and acid concentration, with higher TVOC and acid concentrations in the group with higher odour concentration (Tables 3 and 4). Moreover, nitrate concentrations differed significantly between groups; nitrate values in Group A were close to those in the waste substrates (98 and 128 mg/kg DM at IVAR and in the laboratory reactor respectively) while group B had lower nitrate concentrations (Table 4). There was thus a significant difference between the two groups in terms of odour, pH, acids, TVOC and nitrate, but this was not associated to any strong linear correlation between odour and the other variables. Within group A, odour was positively correlated to TVOC (p < 0.1, R2 = 0.54) but not to any other parameter. Within group B, odour was positively correlated to DM (p ⩽ 0.05, R2 = 0.40) but not to any other parameter.
Table 3.
Compost sample characteristics.
| Composting time (days) | DM (% of fresh weight) | pH | C/N-ratio | Odour concentration (ouE/m3) | Total acid in material (mmol/kg compost) | TVOC concentration (ppm) | Microbial analysis | Group | |
|---|---|---|---|---|---|---|---|---|---|
| Cool-1%:3 | 3 | 44.1 | 4.6 | 29.2 | 450,000 | 201 | 549 | Yes | A |
| Cool-1%:8 | 8 | 51.5 | 8.4 | 23.8 | 1,300,000 | b.d.a | 741 | Yes | – |
| Cool-1%:16 | 16 | 44.9 | 8.7 | 25.5 | 22,000 | b.d.a | 44 | Yes | B |
| Cool-16%:3 | 3 | 48.6 | 6.7 | 24.3 | 14,000 | b.d.a | 162 | Yes | B |
| Cool-16%:8 | 8 | 46.0 | 8.3 | 20.6 | 7100 | b.d.a | 16 | Yes | B |
| Cool-16%:16 | 16 | 48.9 | 8.3 | 22.8 | 14,000 | b.d.a | 2 | Yes | B |
| Cool-16%(70):3 | 3 | 47.2 | 7.0 | 21.8 | 5500 | b.d.a | 154 | Yes | B |
| Cool-16%(70):8 | 8 | 50.6 | 8.4 | 20.7 | 12,000 | b.d.a | 12 | Yes | B |
| Cool-16%(70):16 | 16 | 49.1 | 8.2 | 23.4 | 12,000 | b.d.a | 6 | Yes | B |
| Heat-16%:3 | 3 | 42.3 | 4.8 | 27.0 | 74,000 | 139 | 495 | No DNA | A |
| Heat-16%:8 | 8 | 45.0 | 4.95 | 27.9 | 280,000 | 120 | 554 | No DNA | A |
| Heat-16%:16 | 16 | 44.9 | 5.0 | 26.8 | 220,000 | 147 | 358 | No DNA | A |
| Heat-16%(70°):3 | 3 | 42.3 | 4.8 | 25.5 | 930,000 | 136 | 751 | Yes | A |
| Heat-16%(70°):8 | 8 | 43.5 | 4.9 | 28.5 | 740,000 | 129 | 1018 | No DNA | A |
| Heat-16%(70°):16 | 16 | 46.8 | 5.0 | 26.3 | 2,000,000 | 156 | 684 | No DNA | A |
| IVAR 1 | 30 | 61.0 | 8.4 | 14.7 | 15,200 | b.d.a | 7 | Yes | B |
| IVAR 2 | 30 | 57.1 | 8.0 | 14.7 | 23,600 | b.d.a | 19 | Yes | B |
| IVAR 3 | 30 | 62.4 | 8.0 | 12.7 | 41,000 | b.d.a | 10 | Yes | B |
| NSR 1a | 33 | 46.7 | 5.0 | 17.2 | 730,000 | 259 | – | Yes | A |
| NSR 1b | 90 | 54.6 | 6.0 | 18.8 | 250,000 | 218 | – | No | A |
| NSR 2a | 33 | 46.4 | 5.1 | 18.4 | 1,500,000 | 257 | – | Yes | A |
| NSR 2b | 90 | 52.5 | 5.4 | 17.5 | 490,000 | 329 | – | No | A |
Below the detection limit of 9, 6, 8 and 6 mmol/kg compost for acetic, lactic, propionic and butyric acid respectively.
Fig. 1.
Odour concentration as a function of pH at IVAR, NSR and in the laboratory reactor, with group A samples clustering in the lower right and group B samples in the upper left.
Table 4.
Comparison of samples with odour concentration of 74,000 ouE/m3 and higher (Group A) and 41,000 ouE/m3 or lower (Group B). For variables marked with ∗, Group A and Group B were significantly different at 95% confidence level.
| Group A | Group B | |
|---|---|---|
| No of samples | 11 | 10 |
| Odour concentration (ouE/m3)∗ | 566,000 ± 425,000 | 16,600 ± 10,200 |
| DM (% of fresh weight)∗ | 46.3 ± 3.9 | 51.6 ± 6.3 |
| pH∗ | 5.0 ± 0.4 | 8.0 ± 0.7 |
| Tot-C (% of DM) | 40.8 ± 5.4 | 37.3 ± 9.3 |
| Tot-N (% of DM) | 1.73 ± 0.16 | 1.84 ± 0.16 |
| NH4–N (g/kg DM) | 1.3 ± 0.6 | 1.3 ± 0.8 |
| NO3–N (mg/kg DM)∗ | 93 ± 58 | 8 ± 15 |
| C/N-ratio | 23.9 ± 4.8 | 20.1 ± 4.5 |
| TVOC∗,a | 630b ± 213 | 43 ± 62 |
| Acetic acid in material (mmol/kg compost)∗ | 46 ± 47 | 0b |
| Lactic acid in material (mmol/kg compost)∗ | 131 ± 28 | 0b |
| Total acid in material (mmol/kg compost)∗ | 190 ± 68 | 0b |
Not measured at NSR, only 7 samples in Group A.
All samples were below the detection limit of 9 mmol/kg compost for acetic acid and 6 mmol/kg compost for lactic acid.
Odour and TVOC were measured on samples of pore gas (gases within the compost mass). The odour of the gas emitted from compost at the composting plants was not measured. In the laboratory reactor, however, due to large internal air circulation, pore gas and emitted gas are the same. We regard the odour concentration in pore gas as a measure of odour potential, i.e. the risk of odour emissions from further handling of the compost material.
At the composting plants, the samples were taken from pore gas that had been extracted from a single point in a large compost heap. The representativity of this sampling method was tested at IVAR, where two samples were taken on the same occasion but at different places in each heap (data not shown). For two heaps the differences were 15% or lower and for the third heap, one value was three times higher than the other. Considering that the differences between samples was three orders of magnitude, such a sampling error was considered acceptable.
3.1. High-odour samples
The high-odour group contained samples from the laboratory reactor (HEAT and COOL strategy) and from one of the large scale plants (NSR). In the laboratory reactor, the samples from the HEAT strategy all belonged to the high odour group (Table 3). With this strategy, when the temperature exceeded 40 °C, the microbial activity, measured by CO2 production, declined to almost zero and the pH remained low (approximately 5) during the rest of the trial, confirming earlier findings that a combination of thermophilic conditions and low pH is detrimental to the composting process (Smårs et al., 2002; Sundberg et al., 2004). It was not possible to extract DNA from the HEAT samples on days 8 and 16, most likely because of lower microbial numbers.
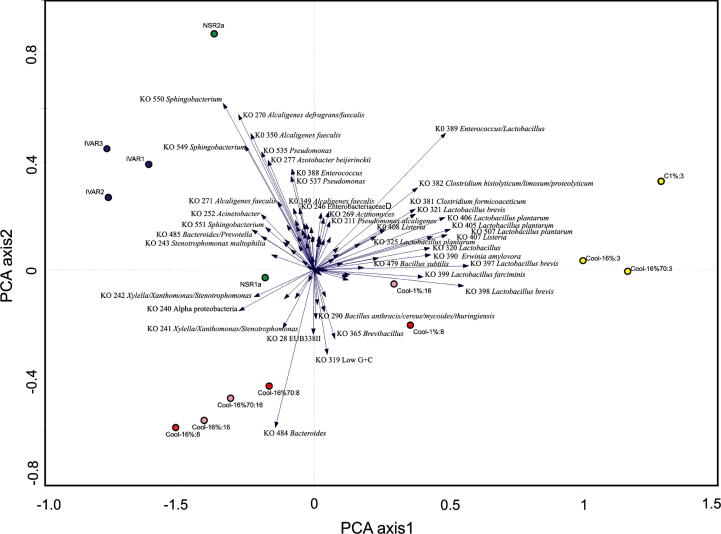
Only two of the COOL strategy samples gave high odour concentrations. These were the Cool 1% run samples collected on days 3 and 8. The odour level decreased by day 16 (Table 4). The Cool 1% run samples were found by the COMPOCHIP microarray to have microbial communities not so different from those found in the low odour COOL strategy samples, as can be seen in Fig. 2. Signals for many of the Lactobacillus probes were detected in these samples (L. farciminis, L. brevis, L. plantarum), as were higher signals for Listeria (KO 407, KO 408) and Clostridium formicoaceticum (KO 381 and KO 382). Sequencing results indicated that the Cool-1%:3 sample was totally dominated by the order Lactobacillales, while the order Bacillales was the most common in Cool-1%:8.
Fig. 2.
Principal component analysis loading plot depicting the organisms responsible for community differences amongst the samples. The lengths of the arrows indicate the significance for compost differentiation. Arrows of probes point in the direction of samples with above average signal.
The odour concentration in NSR samples was high, as was the acid concentration, and the pH was low (Table 3). This plant had the highest acid concentration of the incoming substrate (Table 1) and it remained high (Table 3). The temperature during the process increased very slowly (Table 5).
Table 5.
Characteristics of processes at large scale composting plants.
| Process | Average aeration rate (m3/h, tonin) | Average temperature (°C) | Maximum temperature (°C) |
|---|---|---|---|
| IVAR 1 | 24 ± 5 | 55 | 69 |
| IVAR 2 | 26 ± 5 | 60 | 71 |
| IVAR 3 | 25 ± 5 | 60 | 71 |
| NSR 1 | 3.0 ± 0.5 | 40 | 51 |
| NSR 2 | 1.5 ± 0.5 | 32 | 46 |
Fewer signals and signals of lower intensity were detected in NSR samples upon application of the COMPOCHIP microarray when compared with the majority of the other samples studied. Fig. 2 shows an ordination graph: the two relevant axes explained 63.8% of the variance (first axis 46.5%). Interestingly, the two NSR samples did not cluster together. Both NSR samples were dominated by Bacillales (sequences with a similarity above 97% to e.g. Bacillus infernus and B. sporotermodurans) and Actinobacteridae (sequences with a similarity above 99% to e.g. Corynebacterium aurimucosum). Together these phyla represented over 50% of the sequences, but the species represented were atypical for the compost environments.
3.2. Low-odour samples and processes
The low-odour group (group B) contained samples from the laboratory reactor COOL strategy (all samples at 16% O2 and one sample at 1% O2) and from the IVAR composting plant.
In all group B samples, except Cool-1%:16, the Bacillales plus Actinobacteridae – phyla, were found to dominate, and represented more than 50% of the sequences. The most commonly found representative of Bacillales was Bacillus thermoamylovorans (similarity > 97%), and of Actinobacteriadeae, Thermomonospora chromogena (similarity 99%). Also Gammaproteobacteria such as Escherichia coli, Pseudoxanthomonas taiwanensis and Pseudomonas cellulosa, were found in most group B samples, but since these bacterial groups were found also in group A samples, their presence does not have a predictive value.
Fig. 2 shows the COOL strategy samples to be relatively spread out in the PCA generated from the microarray data. The COOL samples collected after 3 days were found to group together, while the samples collected after 8 and 16 days also grouped loosely together. There did not however appear to be any similarities in microbial community between the COOL strategy samples in group A and group B.
At IVAR, the pH increased during the process and after the process the concentrations of odour, TVOC and organic acids were all low (Table 3). Conditions for good composting, which were fulfilled during the trials, were: good air supply (Table 5), weekly mixing and addition of water to maintain uniform water content.
3.3. Acids, pH and odour
The samples were found to cluster in two groups, which were significantly different in odour, pH and acid concentration (Fig. 1, Table 4). However, there was no correlation between odour and pH or acid concentration within the groups. For the low-odour group this was expected; acid concentrations were low, thus, other substances causing odour must have been present, and there are numerous other substances that form odour in compost (Gallego et al., 2012; Miller, 1993). For the high-odour group, where odour seems to be linked to low pH conditions and high acid concentrations, the lack of correlation requires an explanation. One reason is that different organic acids have different odour intensity.
Lactic and acetic acid were found to be the dominant acids present in our samples. These acids have much higher odour thresholds than butyric and valeric acid (Rosenfeld et al., 2007) which were detected in low concentrations in some samples. In our experiments however, the only odorous compounds that were analysed and identified were organic acids, but other substances may have contributed to the odour under low-pH conditions. This is another possible reason for the observed lack of correlation between pH and odour within the high-odour group. Anaerobic and oxygen limited conditions, as is the situation in the active stage of composting (Hamelers, 2001) often produce highly malodorous volatile substances, resulting from the degradation of protein and/or lipid-rich substrates. Under low pH conditions, Lactobacillus (87% of the Lactobacillales sequences, data not shown) and Acetobacter (35% of the Alphaproteobacteria sequences, data not shown)-type bacteria, organisms typically found in the very early phases of composting (Alfreider et al., 2002, Partanen et al., 2010, Kurola et al., 2011) produce large amounts of lactic acid and acetic acid, respectively. These bacteria may also form malodorous short-chain organic acids such as butyric acid and propionic acid as fatty acids are degraded (Imhof and Bosset, 1994). In the samples from the laboratory trial with only 1% O2, lower pH and higher concentrations of Lactobacilli were found, compared to samples from the 16% O2 trials. If oxygen limited conditions persist during the thermophilic, high pH phase, strictly anaerobic bacteria from the Bacteroidetes phylum (Bacteroides) and Gram positive bacteria from the Firmicutes phylum (e.g. Clostridia) can produce compounds with an extremely low olfactory threshold and offensive odour (Rappert and Müller, 2005; Wardwell et al., 2001). This is a possible explanation for the anomalous sample Cool-1%:8 (originating from the laboratory composting reactor operated under oxygen limited conditions), where high pH and high odour was found (Fig. 1 and Table 3). Even small quantities of substances such as butanedione, acetoin, or various sulphuric compounds in the emissions can significantly affect the perception of malodours from a composting facility (Romantschuk et al., 2004; Kurola et al., 2011).
Rosenfeld et al. (2004) found that the most important malodorous compounds in the initial phases of the composting of park and garden waste were acetaldehyde and various organic acids, including butyric acid and valeric acid. This adds support to our hypothesis that it was organic acids, in combination with other substances, which caused most of the odour produced during the composting of food waste in our study, even though the acids present in the highest concentrations only contributed a small part to the total odour. This is also in line with recent findings that iso-valeric acid, but not other acids present at higher concentrations, were correlated with odour from stored food waste (Qamaruz-Zaman and Milke, 2012).
The results from this project show that when composting Nordic source separated food waste, pH values can be used as a rough but quick indicator of the risk of odours from the composting process. Measuring pH in condensate from outgoing gas is an easy and reliable method, which has been in use for a long time at our laboratory reactor, although not on-line (Smårs et al., 2002). In the experiments reported here, pH and acetic acid concentration in condensates were consistent with results from the compost.
3.4. Microbial odour indicators
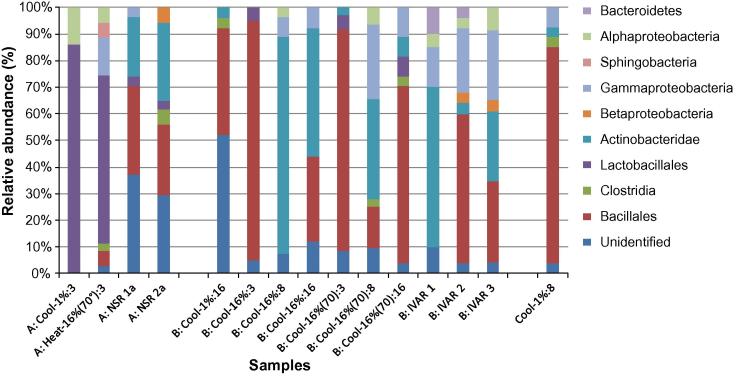
In all high odour (group A) samples, the dominating bacterial phyla/classes were typical composting microbes (Actinobacteria, Bacillales) commonly found in at least some phase in the microbial succession during normal functional composting (Partanen et al., 2010; Kurola et al., 2011). Normally, high proportions of Bacillales and Actinobacteridae is an indication of good composting conditions (Partanen et al., 2010), but here, sequences with close similarity to typical thermophilic composting species were not identified. Instead the observed sequences showed similarity to anaerobic or facultatively anaerobic representatives of the Bacillales (Bacillus thermoamylovorans, Bacillus infernus) and Actinobacteridae (Corynebacterium), indicating low oxygen concentrations. In the NSR sample 2a, the number of Clostridia (Clostridium spp.) represented ca. 6% of the total bacterial diversity (Fig. 3). Clostridium is a genus of strictly anaerobic bacteria with a fermentative metabolism. The end-products from the fermentation of proteins and lipids include noxiously smelling sulphuric compounds and organic acids. Lactobacillus species (L. amylovorus, L. brevis, L. plantarum, L. sanfranciscensis) were also detected in all group A composting plant samples. Although their emissions are not as malodorous, their presence indicates oxygen limitation and a suboptimal temperature. Furthermore, they both indicate and maintain low pH.
Fig. 3.
The bacterial community compositions in high odour (A), low odour (B) and unclassified (Cool-1%:8) food waste composting samples, determined by sequencing cloned 16S rRNA genes. ‘Unidentified’ contains bacterial sequences with no close similarity to sequences in the nucleotide database. ‘Cool’ and ‘Heat’ represent samples from laboratory reactor experiment, and ‘IVAR’ and ‘NSR’ represent samples from large scale plants in Norway and Sweden respectively. The sample numbers corresponds to the numbering in Table 4.
The bacterial community in the low odour samples (group B) were in all cases dominated by Bacillales and Actinobacteria that are typical for well functioning composting processes. One dominant order was Bacillales. Genera and species that indicate aerobic conditions and high temperature Thermoactinomyces, Bacillus pumilus, Thermomonospora (T. chromogena, T. curvata), and Thermobifida fusca, were common in eight of the group B samples (Fig. 3).
All cloned group B samples contained unidentified sequences, a normal finding in compost DNA analyses (Partanen et al., 2010). However, the Cool-1%:16 had an unusually large portion, with over 50% of unidentified OTUs being found. Apparently, due to the low oxygen supply, this sample also contained detectable amounts of Clostridia. Earlier samples from the same process were placed in group A (high odour), and indeed, the bacteria that dominated the day 3 sample were Lactobacillales, typical for low pH and low oxygen situations (Fig. 3). By day 8, it was found that the Lactobacillales had been largely replaced by Bacillaceae in the Cool-1%, sample, indicating an improving process. The high odour may in this case be explained by the presence of Clostridia.
Perhaps unexpected, was the finding of anaerobic Bacteroidetes (e.g. Flavobacterium spp. and Sphingobacterium spp.) in IVAR samples by cloning. This would indicate that although placed in the low-odour group, the IVAR process was not running under optimal conditions, and that some anoxia persisted. Based on the cloning results, Gamma-proteobacteria were among the dominant classes in the day 8 and day 16 samples of the low-odour group B, (except in Cool-1%), as well as in all IVAR samples. In all cases, however, they were outnumbered by the typical composting microbes (Actinobacteria + Bacillales).
The microbial analyses carried out were aimed at roughly determining bacterial diversity, but mainly at identifying dominant bacterial species. The results show a rough distribution between different types of bacteria, but are not quantitative. They merely report relative abundances of the bacteria, and therefore do not show the concentrations of bacteria in the composts.
Nordic biowaste and composting material at early stages of the process are dominated by Lactobacilli and other bacteria that produce organic acids (Partanen et al., 2010 and Sundberg et al., 2011), while a rising pH and temperature correlates with an increase in Actinobacteria and Bacilli. This finding was confirmed by the results presented here. Bacterial analysis can thereby act as support and validation in an evaluation of the composting process. The methods currently available for such analysis are slow and expensive, but DNA chips (microarrays) such as those used in this study (Franke-Whittle et al., 2005, 2009) and under development (Hultman et al., 2008) should allow large progress in this area. For fungal analysis, a similar chip is already in use and has been tested on samples from this project (data not shown). The method is fast, precise and very sensitive, but more qualitative than quantitative, indicating microbial diversity rather than the microbial density.
3.5. TVOC as an odour indicator
PID is a detection method based on the ionisation of organic molecules with UV-light. It produces responses to many different organic compounds present in gas samples (total volatile organic compounds – TVOC), but the response varies greatly between different substances. In general, the sensitivity increases with the number of carbon atoms in the molecule. Despite the fact that TVOC measured with PID does not capture the complexity of odorous volatile substances from compost, TVOC was correlated to odour, both when comparing the high and low-odour groups (Table 4) and within the high-odour group (group A). This is in accordance with previous work on PID and compost odour (Hobbs et al., 1995, Bergersen and Berg, 2001; Berg et al., 2005) and shows that TVOC measured by PID may be a relevant odour indicator for food waste composting. However, this requires further study, since it has been shown that odour concentration can be high even when the TVOC content is relatively low, in cases when the composting process emitted compounds with a very low odour threshold, e.g. acetoin (3-hydroxy-2-butanon) or mercaptan (Rajamäki et al., 2005; Romantschuk et al., 2004).
3.6. Odour reduction strategies
As there is a clear relationship between pH and odour concentration (Fig. 1 and Table 4), an obvious odour reduction strategy for food waste composting is to rapidly overcome the low pH phase and ensure that the composting process is conducted at neutral or alkaline pH values. In the laboratory reactor, a fast transition from low to neutral pH as well as low odour was achieved in the COOL runs at 16% O2 level, where the temperature was kept below 40 °C until the pH increased above 6. When O2 concentration was lower (COOL-1%) or temperature was allowed to rise faster (HEAT), pH and odour remained higher. In large scale composting, keeping a high aeration rate contributes both to cooling and oxygen supply, and is an important step towards a fast pH increase (Sundberg and Jönsson, 2008). At IVAR, where compost with pH above neutral and relatively low odour was obtained after 30 days of composting, aeration rates were five times higher than at NSR, where pH remained low and odour high after 33 and 90 days of composting (Tables 3 and 5).
Moreover, the amendments differed between the two composting plants. At IVAR, wood waste and recycled compost was used. These two amendments promote good structure and aeration. Recycled compost is an especially useful amendment, since it is readily available, it buffers the pH and it inoculates the substrate mixture with appropriate compost microbes (Sundberg et al., 2011).
An additional option is to add other substances that raise the pH during substrate preparation, for example lime, or ash (Kurola et al., 2011). Adding ash or lime will affect the compost quality and it is important to monitor the concentration of metals if ash is used.
Lower total odour emissions can be expected from well functioning composting plants where the pH is raised quickly during the process. This is especially true for plants where the first part of the process occurs in a closed system and curing occurs outdoors. If most of the decomposition, and thus the emissions of odorous compounds occur in the closed system from which the gases are treated, then the odour emissions can be minimised.
4. Conclusions
We analysed the pore space odour and a number of other characteristics of samples from the composting of food waste in a laboratory reactor and at large scale composting plants, and found that the odour was much stronger when the compost was acidic than when it was neutral or alkaline. There was no correlation between odour and pH or acid concentration within the group of high-odour samples. This can be explained by the fact that low pH was mainly caused by acetic and lactic acids, which are not very odorous, and therefore odour was probably mainly caused by other substances present under low pH conditions. Samples of all groups were dominated by Bacillales or Actinobacteria, which are often indicative of well-functioning composting processes, but the high-odour group DNA sequences were similar to those of anaerobic or facultatively anaerobic species, and not to typical thermophilic composting species. Microbes found in high-odour samples included Lactic acid bacteria and Clostridia, which are known to produce odorous substances.
Also, TVOC measured by PID was associated to high odour, so both pH and TVOC are potential indicators of a process causing high odour. Both must however be used with caution, as neither measures odour or odorous substances directly.
An important strategy for reducing odour from food waste composting is to rapidly overcome the initial low-pH phase. This can be obtained by a combination of high aeration rates that provide oxygen and cooling, and additives such as recycled compost.
Acknowledgements
The project was funded by the Nordic Council of Ministers; the Swedish, Norwegian and Finnish Waste Management Associations (Avfall Sverige, Avfall Norge and JLY); three regional waste management companies based in Stavanger (IVAR), Helsingborg (NSR) and Helsinki (YTV); and the Swedish University of Agricultural Sciences. Additional funding was obtained from the Austrian FWF Project P16560 and P20001 and Tekes – the Finnish Funding Agency.
References
- Alfreider A., Peters S., Tebbe C.C., Rangger A., Insam H. Microbial community dynamics during composting of organic matter determined by 16S ribosomal analysis. Compost Sci. Util. 2002;10:303–312. [Google Scholar]
- Berg, B., Kvernheim, A.L., Norgaard, E. Ödegård, K., 2005. Etablering av korrelasjoner mellom TVOC og lukt i komposteringsanlegg. (Establishment of correlations between TVOC and odour in compsting plants). Oslo, SINTEF (in Norwegian).
- Bergersen, O. Berg, B., 2001. Etablere teknikk for indirekte måling av lukt i behandlingsanlegg for organisk avfall ved on-line måling i kompostmassen. (Establishment of technique for indirect measurement of odour in treatment facilities for organic waste through on-line measurement in the compost mass). Oslo, SINTEF: STF66 A01510 (in Norwegian).
- Brinton W.F. Volatile organic acids in compost: production and odorant aspects. Compost Sci. Util. 1998;6:75–82. [Google Scholar]
- Franke-Whittle I.H., Klammer S.H., Insam H. Design and application of an oligonucleotide microarray for the investigation of compost microbial communities. J. Microbiol. Methods. 2005;62:37–56. doi: 10.1016/j.mimet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Franke-Whittle I.H., Knapp B.A., Fuchs J., Kaufmann R., Insam H. Application of COMPOCHIP microarray to investigate the bacterial communities of different composts. Microb. Ecol. 2009;57:510–521. doi: 10.1007/s00248-008-9435-2. [DOI] [PubMed] [Google Scholar]
- Gallego E., Roca F.J., Perales J.F., Sánchez G., Esplugas P. Characterization and determination of the odorous charge in the indoor air of a waste treatment facility through the evaluation of volatile organic compounds (VOCs) using TD-GC/MS. Waste Manage. 2012;32(12):2469–2481. doi: 10.1016/j.wasman.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hamelers, H. V. M., 2001. A Mathematical Model for Composting Kinetics. Doctoral Thesis. Wageningen University, Wageningen, Netherlands.
- Hobbs P.J., Misselbrook T.H., Pain B.F. Assessment of odours from livestock wastes by a photoionization detector, an electronic nose, olfactometry and gas chromatography–mass spectrometry. J. Agric. Eng. Res. 1995;60:137–144. [Google Scholar]
- Hultman J., Ritari J., Romantschuk M., Paulin L., Auvinen P. Universal ligation-detection-reaction microarray applied for compost microbes. BMC Microbiol. 2008;8:237. doi: 10.1186/1471-2180-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof E., Bosset J.O. Relationships between micro-organisms and formation of aroma compounds in fermented dairy products. Z. Lebensm. Unters. Forsch. 1994;198:267–276. [Google Scholar]
- Komilis D.P., Ham R.K., Park J.K. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004;38:1707–1714. doi: 10.1016/j.watres.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Krzymien M., Day K., Shaw R.Mohmad., Sheehan S. The role of feed composition on the composting process. II. Effect on the release of volatile organic compounds and odours. J. Environ. Sci. Health Part A – Environ. Sci. Eng. Toxic Hazard. Subst. Control. 1999;34:1369–1396. [Google Scholar]
- Kurola J.M., Arnold M., Kontro M.H., Talves M., Romantschuk M. Wood ash for application in municipal biowaste composting. Bioresour. Technol. 2011;102:5214–5220. doi: 10.1016/j.biortech.2011.01.092. [DOI] [PubMed] [Google Scholar]
- Mao I.F., Tsai C.J., Shen S.H., Lin T.F., Chen W.K., Chen M.L. Critical components of odors in evaluating the performance of food waste composting plants. Sci. Total Environ. 2006;370:323–329. doi: 10.1016/j.scitotenv.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Miller, F.C., 1993. Minimizing odor generation. In: Hoitink, H.A.J., Keener, H.M. (Eds.), Science and engineering of composting: design, environmental, microbiological and utilization aspects. Wooster, Ohio, The Ohio State University, pp. 219–241.
- Noble R., Hobbs P.J., Dobrovin-Pennington A., Misselbrook T.H., Mead A. Olfactory response to mushroom composting emissions as a function of chemical concentration. J. Environ. Qual. 2001;30:760–767. doi: 10.2134/jeq2001.303760x. [DOI] [PubMed] [Google Scholar]
- Partanen P., Hultman J., Paulin L., Auvinen P., Romantschuk M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010;10:94. doi: 10.1186/1471-2180-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhle H., Kliche R. Emission of odour substances from composting process. Zentralbl. Hyg. Umweltmed. 1996;199:38–50. [PubMed] [Google Scholar]
- Qamaruz-Zaman and Milke, 2012. VFA and ammonia from residential food waste as indicators of odor potential. Waste Manage. 32(12), 2426–2430. [DOI] [PubMed]
- Rajamäki T., Arnold M., Venelampi O., Vikman M., Räsänen J., Itävaara M. An electronic nose and indicator volatiles for monitoring of the composting process. Water Air Soil Pollut. 2005;162:71–87. [Google Scholar]
- Rappert S., Müller R. Odor compounds in waste gas emissions from agricultural operations and food industries. Waste Manage. (Oxford) 2005;25:887–907. doi: 10.1016/j.wasman.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Romantschuk, M., Vasara, T., Arnold, M., 2004. Enhancing composting of biowaste and optimising process control: BIOTEHO. End report for Tekes, Finnish Funding Agency for Technology and Innovation (in Finnish).
- Rosenfeld P.E., Clark J.J.J., Hensley A.R., Suffet I.H. The use of an odour wheel classification for the evaluation of human health risk criteria for compost facilities. Water Sci. Technol. 2007;55:345–357. doi: 10.2166/wst.2007.197. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P.E., Grey M.A., Suffet I.H. Compost odor control using high carbon wood ash. Water Sci. Technol. 2004;49:171–178. [PubMed] [Google Scholar]
- Smet E., Van Langenhove H., De Bo I. The emission of volatile compounds during the aerobic and the combined anaerobic/aerobic composting of biowaste. Atmos. Environ. 1999;33:1295–1303. [Google Scholar]
- Smårs S., Beck-Friis B., Jönsson H., Kirchmann H. An advanced experimental composting reactor for systematic simulation studies. J. Agric. Eng. Res. 2001;78:415–422. [Google Scholar]
- Smårs S., Gustafsson L., Beck-Friis B., Jönsson H. Improvement of the composting time for household waste during an initial low pH phase by mesophilic temperature control. Bioresour. Technol. 2002;84:237–241. doi: 10.1016/s0960-8524(02)00056-1. [DOI] [PubMed] [Google Scholar]
- Sundberg C., Franke-Whittle I., Kauppi S., Yu D., Romantschuk M., Insam H., Jönsson H. Characterisation of source-sorted household waste intended for composting. Bioresour. Technol. 2011;102:2859–2867. doi: 10.1016/j.biortech.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg C., Jönsson H. Process inhibition due to organic acids in fed-batch composting of food waste – influence of starting culture. Biodegradation. 2005;16:205–213. doi: 10.1007/s10532-004-0628-1. [DOI] [PubMed] [Google Scholar]
- Sundberg C., Jönsson H. Higher pH and faster decomposition in biowaste composting by increased aeration. Waste Manage. (Oxford) 2008;28:518–526. doi: 10.1016/j.wasman.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Sundberg C., Smårs S., Jönsson H. Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour. Technol. 2004;95:145–150. doi: 10.1016/j.biortech.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Tsai C.-J., Chen M.-L., Ye A.-D., Chou M.-S., Shen S.-H., Mao I.F. The relationship of odor concentration and the critical components emitted from food waste composting plants. Atmos. Environ. 2008;42:8246–8251. [Google Scholar]
- Wardwell S.A., Yang Y.T., Chang H.Y., San K.Y., Rudolph F.B., Bennet G.N. Expression of Klebsiella pneumonia CG21 acetoin reductase gene in Clostridium acetobutylicum ATCC824. J. Indust. Microbiol. Biotechnol. 2001;27:220–227. doi: 10.1038/sj.jim.7000179. [DOI] [PubMed] [Google Scholar]