Abstract
Ether lipids are an emerging class of lipids which have so far not been investigated and understood in every detail. They have important roles as membrane components of e.g. lens, brain and testis, and as mediators such as platelet-activating factor. The metabolic enzymes for biosynthesis and degradation have been investigated to some extent. As most involved enzymes are integral membrane proteins they are tricky to handle in biochemical protocols. The sequence of some ether lipid metabolising enzymes has only recently been reported and other sequences still remain obscure. Defined enzymes without assigned sequence are known as orphan enzymes. One of these enzymes with uncharacterised sequence is plasmanylethanolamine desaturase, a key enzyme for the biosynthesis of one of the most abundant phospholipids in our body, the plasmalogens. This review aims to briefly summarise known functions of ether lipids, give an overview on their metabolism including the most prominent members, platelet-activating factor and the plasmalogens. A special focus is set on the description of orphan enzymes in ether lipid metabolism and on the successful strategies how four previous orphans have recently been assigned a sequence. Only one of these four was characterised by classical protein purification and sequencing, whereas the other three required alternative strategies such as bioinformatic candidate gene selection and recombinant expression or development of an inhibitor and multidimensional metabolic profiling.
Keywords: Ether lipid, Alkylglycerol, Plasmalogen, Platelet-activating factor, Sequence assignment, Orphan enzyme
Highlights
► An actual overview on the metabolism of ether lipids is presented. ► We describe enzymes of ether lipid metabolism with unknown sequence. ► We discuss how recent sequence assignments have been achieved.
1. Ether lipids
1.1. Chemical nature and function of ether lipids
Ether lipids (alkyl and alkenyl glycerols) have an alkyl chain of mostly 16 or 18 carbon atoms linked to the sn-1 position of the backbone glycerol by an ether bond. This ether bond, in contrast to the ester bond of the better described acyl lipids, provides this lipid class with a higher metabolic stability. Ether lipids are present in organisms ranging from bacteria, protozoa, fungi, higher plants to mammals including humans [1–3]. Most prevalent are these ether bonded side chains in phospholipids, here again saturated alkyl side chains are predominantly found in phosphatidylcholines and single unsaturated alkyl side chains (alk-1′-enyl) in phosphatidylethanolamines [4]. Alk-1′-enyl phospholipids have a vinyl ether bond and are termed plasmalogens. It has been shown that the amount of ether lipids in human and animal tumours is higher in neoplastic than in healthy cells [5,6]. Other studies have confirmed the increased presence of ether lipids in cancerous cells [7–10]. Encouraged by these findings, there were efforts trying to establish ether lipids as tumour markers in medical cancer diagnostics [11], however, as follow-up investigations found unaltered or even decreased amounts of ether lipids this path had to be abandoned [12,13].
Ether lipids in the diet are thought to have general beneficial effects on health. Shark oil which is especially rich in these lipids is used for wound healing and against gastric ulcers, colon inflammation and arthritis [14]. Ether lipids have been reported to be immunostimulatory by activation of macrophages and to confer an anti-angiogenic effect [15]. Their activity spectrum also includes antibacterial [16] and antifungal [17] properties and they can increase permeability of the blood brain barrier thereby enabling delivery of drugs to the brain [18–20]. Another study reported an increase in sperm motility in pigs and elevated fertility after treatment with these molecules [21]. In Madin–Darby canine kidney cells, alkylglycerols have been shown to inhibit protein kinase C (PKC) and thereby mediate cell-density dependence of proliferation in vitro [22].
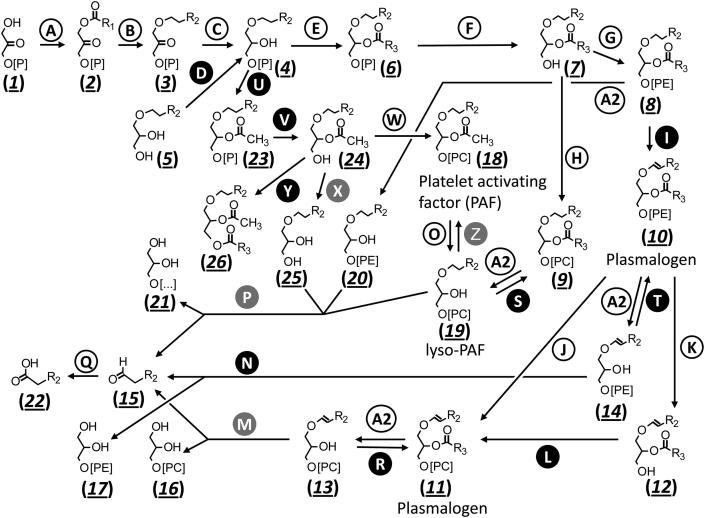
A well-studied alkylglycerol is the platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, (18) in Fig. 1). It is involved in physiological processes such as e.g. inflammation, reproduction and blood pressure regulation. It has been reported to play a pathophysiological role in cardiovascular, renal, neuronal, pulmonary, immunological disorders and in shock [23] and was first described in 1972 [24]. PAF is synthesised by two alternative strategies in the body, the de novo and the remodelling pathway. For its role as mediator, the remodelling pathway is of prime importance. This pathway includes cleavage of a precursor lipid by phospholipase A2, acetylation at the sn-2 position to yield its active form, and deacetylation at the sn-2 position to inactivate the molecule (reactions (A2), (Z) and (O)), respectively, Fig. 1, [25].
Fig. 1.
Ether lipid metabolism including biosynthesis and degradation of PAF and plasmalogens. Reactions catalysed by orphan enzymes (i.e. enzymes with unknown sequence) are shown in black circles with white lettering and enzymes with recent sequence assignments in grey circles with white lettering. R, carbon side chain of R1, mostly 15 or 17 atoms (=acyl); R2, 14 or 16 atoms (=alkyl); R3, at least 15 atoms, single- or poly-unsaturated (=acyl). For a description of the enzymatic reactions (capital letters) and the metabolites (Arabic numerals, underlined, italic) see text. ENZYMES (A) glycerone-phosphate O-acyltransferase (E.C. 2.3.1.42), (A2) phospholipase A2 (E.C. 3.1.1.4), (B) alkylglycerone-phosphate synthase (E.C. 2.5.1.26), (C) acylglycerone-phosphate reductase (E.C. 1.1.1.101), (D) alkylglycerol kinase (E.C. 2.7.1.93), (E) alkylglycerolphosphate 2-O-acyltransferase (E.C. 2.3.1.-), (F) phosphatidate phosphatase (E.C. 3.1.3.4), (G) ethanolamine-phosphotransferase (E.C. 2.7.8.1), (H) diacylglycerol cholinephosphotransferase (E.C. 2.7.8.2), (I) plasmanylethanolamine desaturase (E.C. 1.14.99.19), (J) a not further characterised transferase (E.C. 2.6.-.-), (K) phospholipase C (E.C. 3.1.4.3), (L) 1-alkylglycerophosphocholine O-acyltransferase (E.C. 2.7.8.22), (M) alkenylglycerophosphocholine hydrolase (E.C. 3.3.2.2), (N) alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5), (O) 1-O-alkyl-2-acetylglycerophosphocholine esterase (E.C. 3.1.1.47), (P) alkylglycerol monooxygenase (E.C. 1.14.16.5), (Q) long-chain-aldehyde dehydrogenase (E.C. 1.2.1.48), (R) 1-alkenylglycerophosphocholine O-acyltransferase (E.C. 2.3.1.104), (S) 1-alkylglycerophosphocholine O-acyltransferase (E.C. 2.3.1.63), (T) 1-alkenylglycerophosphoethanolamine O-acyltransferase (E.C. 2.3.1.121), (U) alkylglycerophosphate 2-O-acetyltransferase (E.C. 2.3.1.105), (V) alkylacetylglycerophosphatase (E.C. 3.1.3.59), (W) diacylglycerol cholinephosphotransferase (E.C. 2.7.8.2), (X) acetylalkylglycerol acetylhydrolase (E.C. 3.1.1.71), (Y) 1-alkyl-2-acetylglycerol O-acyltransferase (E.C. 2.3.1.125), (Z) alkylglycerophosphocholine O-acetyltransferase (E.C. 2.3.1.67). METABOLITES (1) glycerone phosphate (dihydroxyacetone phosphate), (2) 1-acyl-glycerone 3-phosphate, (3) 1-O-alkyl-glycerone 3-phosphate, (4) 1-O-alkyl-sn-glycero-3-phosphate, (5) 1-O-alkyl-sn-glycerol, (6) 1-O-alkyl-2-acyl-sn-glycero-3-phosphate, (7) 1-O-alkyl-2-acyl-sn-glycerol, (8) 1-O-alkyl-2-acyl-sn-glycero-3-phosphoethanolamine (plasmanylethanolamine), (9) 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine (plasmanylcholine), (10) 1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphoethanolamine (plasmenylethanolamine), (11) 1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholine (plasmenylcholine), (12) 1-O-alkenyl-2-acyl-sn-glycerol, (13) 1-O-alk-1′-enyl-sn-glycero-3-phosphocholine (lysoplasmenylcholine), (14) 1-O-alk-1′-enyl-sn-glycero-3-phosphoethanolamine (lysoplasmenylethanolamine), (15) fatty aldehyde, (16) sn-glycero-3-phosphocholine, (17) sn-glycero-3-phosphoethanolamine, (18) 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF), (19) 1-O-alkyl-sn-glycero-3-phosphocholine (lyso-PAF), (20) 1-O-alkyl-sn-glycero-3-phosphoethanolamine, (21) glycerol or sn-glycero-3-phosphocholine or sn-glycero-3-phosphoethanolamine, (22) long-chain fatty acid, (23) 1-O-alkyl-2-acetyl-sn-glycero-3-phosphate, (24) 1-O-alkyl-2-acetyl-sn-glycerol, (25) 1-O-alkyl-sn-glycerol, (26) 1-O-alkyl-2-acetyl-3-acyl-sn-glycerol.
Plasmalogens, a subclass of ether phospholipids which harbours a vinyl double bond in the alkyl side chain ((10) and (11) in Fig. 1), are found ubiquitously in animal cells and form 18% of the total phospholipid mass in humans [26]. The sn-2 position of glycerol in plasmalogens is very commonly acylated by poly-unsaturated fatty acids of the omega-3 and omega-6 class (e.g. arachidonic acid) [4], the sn-3 position carries either a phosphocholine or a phosphoethanolamine residue. Plasmalogens seem to have implications in protection against oxidative stress, however, their function still remains somewhat obscure (for review see [27]). Plasmalogen contents are highest in brain and spermatozoa and lowest in liver [4]. Another ether lipid species especially enriched in human spermatozoa is seminolipid, a sulfogalactolipid (for review see [28]).
Ether lipids are a constituent of glycosylphosphatidylinositol (GPI) anchors which link proteins to membranes via posttranslational modification. GPI anchor synthesis has been shown to be indispensable for the germline development of the nematode Caenorhabditis elegance [29]. The lipid part of GPI anchors is frequently 1-O-alkyl-2-acyl-sn-glycerol.
1.2. Ether lipid metabolism
The biosynthetic routes to ether lipids including plasmalogens involve many integral membrane enzymes not all of which have been characterised in detail. However, most enzymatic steps are known. A short overview of the central part of ether lipid metabolism is given in Fig. 1, the respective enzyme names and their E.C. numbers can be found in Table 1. For more detailed information the reader is referred to a recent review [30].
Table 1.
Overview of enzymes shown in Fig. 1. All enzymes are listed according to their letter code used in Fig. 1 together with their full name and E.C. number. Enzymes described in Sections 2.1. and 2.2. are listed with the respective references mentioned in the main text.
| Letter | Full name | E.C. number | Reference |
|---|---|---|---|
| (A) | Glycerone-phosphate O-acyltransferase | 2.3.1.42 | |
| (A2) | Phospholipase A2 | 3.1.1.4 | |
| (B) | Alkylglycerone-phosphate synthase | 2.5.1.26 | |
| (C) | Acylglycerone-phosphate reductase | 1.1.1.101 | |
| (D) | Alkylglycerol kinase | 2.7.1.93 | [50] |
| (E) | Alkylglycerolphosphate 2-O-acyltransferase | 2.3.1.- | |
| (F) | Phosphatidate phosphatase | 3.1.3.4 | |
| (G) | Ethanolamine-phosphotransferase | 2.7.8.1 | |
| (H) | Diacylglycerol cholinephosphotransferase | 2.7.8.2 | |
| (I) | Plasmanylethanolamine desaturase | 1.14.99.19 | [39,52] |
| (J) | Transferase | 2.6.-.- | |
| (K) | Phospholipase C | 3.1.4.3 | |
| (L) | 1-Alkylglycerophosphocholine O-acyltransferase | 2.7.8.22 | [59] |
| (M) | Alkenylglycerophosphocholine hydrolase | 3.3.2.2 | [33,42] |
| (N) | Alkenylglycerophosphoethanolamine hydrolase | 3.3.2.5 | [42,55] |
| (O) | 1-O-Alkyl-2-acetylglycerophosphocholine esterase | 3.1.1.47 | |
| (P) | Alkylglycerol monooxygenase | 1.14.16.5 | [43–47] |
| (Q) | Long-chain-aldehyde dehydrogenase | 1.2.1.48 | |
| (R) | 1-Alkenylglycerophosphocholine O-acyltransferase | 2.3.1.104 | [56] |
| (S) | 1-Alkylglycerophosphocholine O-acyltransferase | 2.3.1.63 | [49,57] |
| (T) | 1-Alkenylglycerophosphoethanolamine O-acyltransferase | 2.3.1.121 | [57,58] |
| (U) | Alkylglycerophosphate 2-O-acetyltransferase | 2.3.1.105 | [53] |
| (V) | Alkylacetylglycerophosphatase | 3.1.3.59 | [53,54] |
| (W) | Diacylglycerol cholinephosphotransferase | 2.7.8.2 | |
| (X) | Acetylalkylglycerol acetylhydrolase | 3.1.1.71 | [48] |
| (Y) | 1-Alkyl-2-acetylglycerol O-acyltransferase | 2.3.1.125 | [60] |
| (Z) | Alkylglycerophosphocholine O-acetyltransferase | 2.3.1.67 | [49,53] |
The first step in ether lipid synthesis is catalysed by glycerone-phosphate O-acyltransferase (E.C. 2.3.1.42) (A), a peroxisomal enzyme that acylates glycerone phosphate (dihydroxyacetone phosphate) (1) with a long-chain acyl-CoA ester. The acyl side chain of the resulting 1-acyl-glycerone 3-phosphate (2) is then substituted by a fatty alcohol yielding 1-O-alkyl-glycerone 3-phosphate (3) in a complex reaction catalysed by alkylglycerone-phosphate synthase (E.C. 2.5.1.26) (B) [31]. This peroxisomal enzyme uses FAD as cofactor.
The first glycerol based intermediate 1-O-alkyl-sn-glycero-3-phosphate (4) is obtained by acylglycerone-phosphate reductase (E.C. 1.1.1.101) (C) by reduction of the keto group at C2 at the expense of one molecule NADPH. 1-O-Alkyl-sn-glycero-3-phosphate (4) is an important branching point in ether lipid synthesis either being the starting point for PAF biosynthesis or for all plasmanyl/plasmenyl species. According to a widely used nomenclature, ether phospholipids without a vinyl double bond are termed plasmanyl species (e.g. 1-O-alkyl-2-acyl-sn-glycero-3-phosphoethanolamine (8), is termed plasmanylethanolamine), whereas ether phospholipids with the vinyl double bond are called plasmenyl species (e.g. 1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphoethanolamine (10), a plasmalogen, is termed plasmenylethanolamine). 1-O-Alkyl-sn-glycero-3-phosphate (4) can also be generated in the body by alkylglycerol kinase (E.C. 2.7.1.93) (D), a microsomal enzyme that phosphorylates dietary alkylglycerols (5) with ATP.
1-O-Alkyl-sn-glycero-3-phosphate (4) is transported to the endoplasmic reticulum [32] and is esterified with acyl-CoA at the sn-2 position of glycerol by a not further characterised acyltransferase, i.e. alkylglycerolphosphate 2-O-acyltransferase (E.C. 2.3.1.-) (E) yielding 1-O-alkyl-2-acyl-sn-glycero-3-phosphate (6), the phosphate of which is removed by phosphatidate phosphatase (E.C. 3.1.3.4) (F). The resulting 1-O-alkyl-2-acyl-sn-glycerol (7) can then either be converted to 1-O-alkyl-2-acyl-sn-glycero-3-phosphoethanolamine (plasmanylethanolamine (8)) with cytidine-diphosphate-ethanolamine via ethanolamine-phosphotransferase (E.C. 2.7.8.1) (G) or to 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine (plasmanylcholine (9)) by diacylglycerol cholinephosphotransferase (E.C. 2.7.8.2) (H). The plasmanylethanolamine (8) is oxidised by plasmanylethanolamine desaturase (E.C. 1.14.99.19) (I) to yield the vinyl double bond in plasmenylethanolamine (1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphoethanolamine (10), a plasmalogen) in a reaction involving cytochrome b5 reductase, molecular oxygen and a reduction equivalent. Plasmanylcholines (9) are no substrates for plasmanylethanolamine desaturase (I). Plasmenylcholines ((11), 1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholines) therefore are produced from plasmenylethanolamines (10), possibly by a not further characterised transferase (E.C. 2.6.-.-) (J). Alternatively, plasmenylethanolamines (10) are cleaved by phospholipase C (E.C. 3.1.4.3) (K) to 1-O-alkenyl-2-acyl-sn-glycerol (12) and then transformed to plasmenylcholine (11) by 1-alkylglycerophosphocholine O-acyltransferase (E.C. 2.7.8.22) (L). Phospholipase A2 (E.C. 3.1.1.4) (A2) hydrolyses the ester bond at sn-2 of plasmenylcholines (11) to the resulting 1-O-alk-1′-enyl-sn-glycero-3-phosphocholine (lysoplasmenylcholines (13)), and similarly, the ester bond at sn-2 of plasmenylethanolamines (10) to 1-O-alk-1′-enyl-sn-glycero-3-phosphoethanolamine (lysoplasmenylethanolamines (14)). Phospholipids with a free hydroxy group at the sn-2 position are known as lysolipids due to their membrane-dissolving properties. Lysoplasmenylcholines (13) are degraded to fatty aldehyde (15) and sn-glycero-3-phosphocholine (16) by alkenylglycerophosphocholine hydrolase (E.C. 3.3.2.2) (M) [33]. Likewise, lysoplasmenylethanolamines (14) are degraded to fatty aldehyde (15) and sn-glycero-3-phosphoethanolamine (17) by alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5) (N).
Deacetylation at the sn-2 glycerol position of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor, PAF, (18)) by a specific acetylhydrolase (1-O-alkyl-2-acetylglycerophosphocholine esterase (E.C. 3.1.1.47) (O)), and deacylation of plasmanylcholines (9) or plasmanylethanolamines (8) by phospholipases A2 (E.C. 3.1.1.4) (A2) leads to production of 1-O-alkyl-2-lysoglycerols ((19) and (20)), the ether bond of which is cleaved in a tetrahydrobiopterin-dependent manner by alkylglycerol monooxygenase (E.C. 1.14.16.5) (P) yielding the corresponding glycerol derivatives (21) and again a long-chain fatty aldehyde (15) which is further oxidised by long-chain-aldehyde dehydrogenase (E.C. 1.2.1.48) (Q) in an NAD-dependent manner to the long-chain fatty acid (22). As an alternative to degradation, lysolipids may be acylated back by specific acyltransferases. Such reactions in Fig. 1 are, in addition to the reaction (E) mentioned above, 1-alkenylglycerophosphocholine O-acyltransferase (E.C. 2.3.1.104) (R), 1-alkylglycerophosphocholine O-acyltransferase (E.C. 2.3.1.63) (S) and 1-alkenylglycerophosphoethanolamine O-acyltransferase (E.C. 2.3.1.121) (T).
The PAF de novo pathway starts at the important branching point 1-O-alkyl-sn-glycero-3-phosphate (4) where alkylglycerophosphate 2-O-acetyltransferase (E.C. 2.3.1.105) (U) acetylates at the sn-2 hydroxy group yielding 1-O-alkyl-2-acetyl-sn-glycero-3-phosphate (23). Alkylacetylglycerophosphatase (E.C. 3.1.3.59) (V) removes the phosphate to generate 1-O-alkyl-2-acetyl-sn-glycerol (24). The last step in PAF de novo synthesis is catalysed by diacylglycerol cholinephosphotransferase (E.C. 2.7.8.2) (W) which transfers a phosphocholine residue to the sn-3 position. 1-O-Alkyl-2-acetyl-sn-glycerol (24), the PAF precursor, can be deacetylated to 1-O-alkyl-sn-glycerol (25) in a similar reaction like PAF itself (reaction (O)) by acetylalkylglycerol acetylhydrolase (E.C. 3.1.1.71) (X). The resulting 1-O-alkyl-sn-glycerols (25) are identical in their chemical structure with the dietary alkylglycerols (5) mentioned above, and are substrates of alkylglycerol monooxygenase (P). Another detour from the last step of PAF synthesis is the acylation of 1-O-alkyl-2-acetyl-sn-glycerol (24) at the sn-3 position with 1-alkyl-2-acetylglycerol O-acyltransferase (E.C. 2.3.1.125) (Y) to yield 1-O-alkyl-2-acetyl-3-acyl-sn-glycerol (26).
A more important route to PAF, however, is the remodelling pathway which in a first step consists in deacylation of the acyl residue, predominantly arachidonate, at the sn-2 position of 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine (9) to yield 1-O-alkyl-sn-glycero-3-phosphocholine (lyso-PAF, (19)) by a phospholipase A2 (A2). Acetylation of lyso-PAF (19) with acetyl-CoA by alkylglycerophosphocholine O-acetyltransferase (E.C. 2.3.1.67) (Z) then yields PAF (18) in this remodelling pathway. Deactivation of PAF is mostly accomplished by 1-O-alkyl-2-acetylglycerophosphocholine esterase (O) as mentioned above.
1.3. Ether lipid deficiency
Deficiencies in ether lipids are most commonly caused by defects in peroxisomal assembly (e.g. Zellweger syndrome, rhizomelic chondrodysplasia punctata) because the first two biosynthetic enzymes, glycerone-phosphate O-acyltransferase (A) and alkylglycerone-phosphate synthase (B) are localised to these organelles. A good tool to study such a deficiency is a mouse model. Indeed, two models are available to date which have deletions in one of the first two enzymes of ether lipid synthesis and therefore completely lack ether lipids.
Knockouts in glycerone-phosphate O-acyltransferase (dihydroxyacetone phosphate acyltransferase, DHAPAT, E.C. 2.3.1.42) (A) display hypomyelination of central nerves, structural alterations in testis accompanied by male infertility and early postnatal bilateral cataractogenesis due to impaired lens organisation leading to blindness of aged animals [34–36]. Isolated nerve terminals from ether lipid deficient mice showed impaired neurotransmission, reduced respiration and a lowered ATP/ADP ratio. Although ether lipid deficient tissue contains less lipid peroxidation products, fibroblasts lacking ether lipids are more susceptible to oxidative stress [37]. Blind sterile 2 mice were shown to have a mutation in alkylglycerone-phosphate synthase (AGPS, E.C. 2.5.1.26) (B). These mice also have a very similar phenotype with reduced ether lipid quantities, male infertility due to absence of mature sperm and a severely affected lens causing cataract and blindness [38].
Another possibility to study ether lipid function is ether lipid deficient cell lines. These were either isolated from patients with inherited metabolic diseases in ether lipid biosynthetic enzymes or have been generated by random mutagenesis and sophisticated clone selection techniques [4]. By this a murine macrophage cell line with a combined defect in enzymes (A) and (I) could be generated [39]. Investigations in these cells showed that ether lipid levels can be selectively restored by using different metabolic precursors and that the plasmalogen vinyl double bond is important for protection against oxidants [40].
2. Orphan enzymes in ether lipid metabolism
Orphan proteins have known functions but no corresponding sequence. The ORENZA database (a database of ORphan ENZyme Activities, http://www.orenza.u-psud.fr [41]) keeps an actual list of all orphan enzymes. These enzymes have well defined reaction characteristics described by an Enzyme Commission number (E.C.) but no sequence information has been made available for them. To date there are 1187 orphan enzymes listed in ORENZA (version 2_34 of 03-May-2011), 384 of these are associated to enzymes integrated in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Twelve ORENZA orphan enzymes are members of the KEGG ether lipid metabolism pathway (KEGG release 61.1, February 1, 2012).
Due to the lipophilic nature of the substrates, many enzymes involved in the ether lipid biosynthetic pathway are integral membrane enzymes. These enzymes are often especially sensitive towards biochemical purification protocols and need strictly optimised solubilisation conditions to retain activity. For some of these enzymes purification in active form has never been achieved. This explains the difficulty in assigning a sequence to some of these enzymes. The following Section 2.1. describes four enzymes of ether lipid metabolism with recently assigned sequence (grey circles with white lettering in Fig. 1). The methods applied to find the sequences of these four enzymes will be briefly mentioned. Section 2.2. will then describe enzymes in ether lipid metabolism with unknown sequence (black circles with white lettering in Fig. 1). This includes e.g. plasmanylethanolamine desaturase (I), the key enzyme for the synthesis of one of the most abundant lipid classes in our body, the plasmalogens.
2.1. Enzymes in ether lipid metabolism with recently assigned sequence
Alkenylglycerophosphocholine hydrolase (lysoplasmalogenase (E.C. 3.3.2.2) (M)) catalyses cleavage of the vinyl ether bond of lysoplasmenylcholines (13) yielding a fatty aldehyde (15) and the glycerol derivative (16). This enzyme, though still reported as orphan enzyme in ORENZA, has recently been cloned and characterised by Wu and co-workers [42]. They succeeded in 500-fold purification of alkenylglycerophosphocholine hydrolase from rat liver microsomes after solubilisation with 2% octyl glucoside and four chromatographical steps. The enriched protein had a protein mass of 19 kDa on an SDS gel. The band was excised and the sequence determined by mass spectrometry. The identified peptides corresponded to TMEM86b, a protein with previously unknown function. Identity was confirmed by transfection of tagged protein into mammalian cells and Escherichia coli and testing of activity using an alkenylglycerophosphocholine hydrolase assay and quantification of protein expression by Western blotting against the epitope tag. Transmembrane prediction tools revealed that alkenylglycerophosphocholine hydrolase contains six transmembrane regions in its 226 amino acid residues. The authors recombinantly expressed the protein and demonstrated that it also accepts lysoplasmenylethanolamines (14) as substrates. Thus TMEM86b displays two enzymatic activities, alkenylglycerophosphocholine hydrolase (E.C. 3.3.2.2) (M) and alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5) (N) [42]. As mentioned in Section 2.2. below, a protein associated exclusively with alkenylglycerophosphoethanolamine hydrolase activity (E.C. 3.3.2.5) (N) but not with alkenylglycerophosphocholine hydrolase (EC 3.3.2.2) (M) with so far unknown sequence may be additionally encoded in mammalian genomes.
Alkylglycerol monooxygenase (E.C. 1.14.16.5) (P) is a tetrahydrobiopterin-dependent enzyme which cleaves the ether bond of 1-O-alkyl-sn-glycerols (19, 20, 25) via a hydroxylation and subsequent rearrangement of the resulting hemiacetal into a fatty aldehyde (15) and a glycerol derivative (21). It accepts alkylglycerols which are unsubstituted at sn-3 or which carry a wide range of common phospholipid substitutions at sn-3, except the phosphate group alone as in (4), which is no substrate of the enzyme [43]. The enzyme requires, however, a free hydroxy group at sn-2 and cannot cleave lipids which carry a vinyl ether bond. Thus, only lysoalkylglycerols and lysoalkylglycerol phospholipids are substrates. Alkylglycerol monooxygenase was first described already in 1964 by Tietz and co-workers [44] but only little advance has been made in its characterisation in the following decades. Only recently, we were able to assign a sequence to this enzyme by bioinformatic selection of candidate clones, transfection into mammalian cells and screening for enzymatic activity [45]. An important prerequisite for this was a reliable and very sensitive assay for alkylglycerol monooxygenase which uses a fluorescent alkylglycerol as substrate and subsequent HPLC separation with fluorescence detection [46]. The assigned sequence, a protein with unknown function formerly named TMEM195, belongs to the family of fatty acid hydroxylases, a protein class of hydroxylases and desaturases which contain a di-iron centre for catalysis and are extremely labile towards purification protocols. No member of this enzyme family has ever been purified to homogeneity. Alkylglycerol monooxygenase is located in the membrane of endoplasmic reticulum. Upon analysis of its primary sequence using prediction tools, nine transmembrane domains were identified in its 445 amino acid residues [47].
Acetylalkylglycerol acetylhydrolase (alkylacetylglycerol acetylhydrolase (E.C. 3.1.1.71) (X)) is responsible for hydrolysis of the acetyl moiety of 1-O-alkyl-2-acetyl-sn-glycerol (24), the direct precursor of PAF in the de novo pathway. In 2006, the predicted protein KIAA1363 was identified as gene coding for this enzymatic activity by using a multidimensional profiling strategy [48]. The authors had previously found high expression of KIAA1363 in cancer cell lines. By taking advantage of a specific inhibitor against the enzyme they were able to detect a significantly downregulated metabolite in inhibitor-treated cell lines by liquid chromatography–mass spectrometry. This metabolite was then identified to be 1-O-hexadecylglycerol. From the information of equal regulation in enzyme activity and metabolite quantities, it could be concluded that 1-O-hexadecylglycerol was the product of the reaction. To test whether the substrate consisted of the 2-acetylated form of 1-O-hexadecylglycerol, mammalian cells were transfected with KIAA1363 and checked for acetylalkylglycerol acetylhydrolase activity which was indeed strongly elevated above background.
Alkylglycerophosphocholine O-acetyltransferase (lyso-PAF acetyltransferase (E.C. 2.3.1.67) (Z)), the sequence of which was termed LPCAT2 and published in 2006, is the PAF biosynthetic enzyme in the remodelling pathway and is induced by the inflammatory stimulus lipopolysaccharide [49]. Interestingly, this enzyme also displays a lysophosphatidylcholine acyltransferase activity because under resting conditions in a cell it is able to transfer an arachidonoyl moiety to glycerophospholipids, thereby synthesising a PAF precursor. Assignment of the gene coding for this enzyme has been achieved by expression of a putative open reading frame with sequence homology to another previously reported lysophosphatidylcholine transferase and subsequent analysis of its tissue and subcellular localisation, substrate specificity and identity of the product [49].
2.2. Enzymes in ether lipid metabolism without known sequence (orphans)
Alkylglycerol kinase (ATP:alkylglycerol phosphotransferase (E.C. 2.7.1.93) (D)) is a microsomal enzyme that uses 1-O-alkyl-sn-glycerols (5) as substrates and phosphorylates them to 1-O-alkyl-sn-glycero-3-phosphate (4) which is an important branch point in ether lipid metabolism [50]. This enzyme is responsible for the use of dietary supplements such as (5) in order to bypass the first two, peroxisomally located, reactions of the de novo ether lipid biosynthesis (A) and (B) [51]. So far no sequence of this enzyme has been described.
Plasmanylethanolamine desaturase (Δ1′-alkyl desaturase (E.C. 1.14.99.19) (I)) is the enzyme that converts plasmanylethanolamine (8) into plasmenylethanolamine (a plasmalogen, (10)) by introduction of the vinyl double bond. This desaturase is membrane-bound and requires cytochrome b5, molecular oxygen and either NADPH or NADH as cofactors for functional catalysis [52]. If this enzyme is disrupted, no ether lipids with a vinyl double bond (i.e. no plasmalogens) can be formed in the cell [39]. Sequence information for plasmanylethanolamine desaturase is still missing.
Alkylglycerophosphate 2-O-acetyltransferase (acetyl-CoA:alkyl-lysoglycerophosphate acetyltransferase (E.C. 2.3.1.105) (U)) is a microsomal enzyme which has been shown to have a different substrate specificity than alkylglycerophosphocholine O-acetyltransferase (E.C. 2.3.1.67) (Z) [53]. This enzyme is expressed in significant amounts in spleen, but also brain, heart, lung and liver [53]. No sequence has been reported to date.
Alkylacetylglycerophosphatase (alkylacetylglycerophosphate phosphohydrolase (E.C. 3.1.3.59) (V)) is responsible together with alkylglycerophosphate 2-O-acetyltransferase (U) for formation of 1-O-alkyl-2-acetyl-sn-glycerol (24), the direct precursor of PAF in the de novo pathway. This enzyme is also found in the microsomal fraction and is inhibited by reduced assay temperature (23 °C), sodium vanadate and sodium fluoride [53]. A detailed characterisation of this orphan enzyme is still missing, however, Lee and co-workers have shown that it is predominantly expressed in kidney medulla. Smaller amounts of activity were found in brain, spleen, kidney cortex and lung [54]. This enzyme accepts both carbon chain lengths of 16 and 18 atoms at the sn-1 position at glycerol, the sn-2 position must be acetylated [54]. Its sequence is still unknown.
Alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5) (N): As mentioned above, ORENZA version 2_34 lists twelve orphan enzymes in the KEGG pathway of ether lipid metabolism. In the last year, however, alkenylglycerophosphocholine hydrolase (lysoplasmalogenase (E.C. 3.3.2.2) (M)), has been identified to be encoded by TMEM86b ([42], see Section 2.1. above). The recombinant protein cleaves both lysoplasmenylcholine (13) and lysoplasmenylethanolamine (14), thus displays alkenylglycerophosphocholine hydrolase (EC 3.3.2.2) (M) and alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5) (N) enzymatic activity. Biochemical evidence suggests the occurrence of an additional specific alkenylglycerophosphoethanolamine hydrolase (E.C. 3.3.2.5) (N) which cleaves lysoplasmenylethanolamine (14) but not lysoplasmenylcholine (13) [55]. So far no sequence for this enzyme has been found.
1-Alkenylglycerophosphocholine O-acyltransferase (E.C. 2.3.1.104) (R) reacylates lysoplasmenylcholine (13) to plasmenylcholine (11). The activity of this enzyme is high e.g. in human erythrocytes, which contain a considerable amount of plasmalogens [56]. An identical reaction is also described as plasmalogen synthase (E.C. 2.3.1.25), an additional orphan enzyme. No sequences corresponding to these two E.C. numbers have been described yet.
1-Alkylglycerophosphocholine O-acyltransferase (lyso-PAF acyltransferase, E.C. 2.3.1.63) (S) reacylates lysoplasmanylcholine (19), which is also termed lyso-PAF. The protein encoded by the gene identified for lyso-PAF acetyltransferase (Z), LPCAT2, does also accept acyl-CoA in addition to acetyl-CoA as co-substrate. Thus it exhibits 1-alkylglycerophosphocholine O-acyltransferase (S) activity [49]. Surprisingly, however, the inflammatory stimulant lipopolysaccharide augmented only lyso-PAF acetyltransferase (Z) but not lyso-PAF acyltransferase (S) activity [49]. Thus, although the LPCAT2 gene product also displays 1-alkylglycerophosphocholine O-acyltransferase (S) activity in vitro, it remains open whether this is the gene responsible for this activity inside the cells. In addition, a novel mammalian brain isoform of acyl-CoA-lysophospholipid acyltransferase, LPEAT2, has been described which also displayed 1-alkylglycerophosphocholine O-acyltransferase (S) activity [57]. Further work is needed to unequivocally assign a sequence to this enzymatic activity.
1-Alkenylglycerophosphoethanolamine O-acyltransferase (E.C. 2.3.1.121) (T) has been shown to occur in guinea pig heart. Based on acyl specificities, pH profiles and their responses to heat inactivation and thiol reagents it has been shown that this enzyme is different from the enzyme acylating the corresponding ester lipid, i.e. 1-acylglycerophosphoethanolamine acyltransferase [58]. On the other hand, it has been described that the LPEAT2 gene product displays acetyltransferase activity to both, 1-acyl and 1-alkenylglycerophosphoethanolamines [57]. Again it will be crucial to demonstrate which of the gene products displaying this activity are responsible for the respective metabolic reaction in the various tissues.
1-Alkylglycerophosphocholine O-acyltransferase (E.C. 2.7.8.22) (L) is another orphan enzyme listed in ether lipid metabolism. In this case biochemical evidence indicates that this enzyme may be similar to diacylglycerol cholinephosphotransferase (E.C. 2.7.8.2) (H) [59].
1-Alkyl-2-acetylglycerol O-acyltransferase (E.C. 2.3.1.125) (Y) is an enzyme acylating the hydroxy group at sn-3 of glycerol in 1-O-alkyl-2-acetyl-sn-glycerol (24) to form 1-O-alkyl-2-acetyl-3-acyl-sn-glycerol (26). This enzyme has been observed in HL-60 promyelocytic leukaemia cells and was shown to differ biochemically from diacylglycerol O-acyltransferase (E.C. 2.3.1.20) [60].
Taken together, many enzymes important in ether lipid metabolism still lack an equivocal annotation to a gene responsible for the corresponding metabolic activity inside the cells. Recent sequence assignments of ether lipid metabolising enzymes had been achieved only in one case, i.e. alkenylglycerophosphocholine hydrolase (M), by classical protein purification, sequencing of the purified protein and cloning of the gene [42]. In the three further cases, the protein could not be purified. Alternative strategies used were development of a selective inhibitor and multidimensional metabolic profiling in the case of acetylalkylglycerol acetylhydrolase (X) [48], bioinformatic candidate gene selection combined with recombinant expression in cells for alkylglycerol monooxygenase (P) [45] and expression of an open reading frame homologous to a recently described acyltransferase for alkylglycerophosphocholine O-acetyltransferase (Z) [49]. As indicated in Section 2.2., several important enzymes still await the assignment of a sequence. This includes plasmanylethanolamine desaturase (I), the key enzyme of the biosynthesis of one of the most abundant phospholipid classes in our body, the plasmalogens.
Acknowledgement
The experimental work of the authors on ether lipids was supported by the Austrian Science Funds (FWF), project 22406.
References
- 1.Hallgren B., Niklasson A., Stallberg G., Thorin H. On the occurrence of 1-O-(2-methoxyalkyl)glycerols and l-O-phytanylglycerol in marine animals. Acta Chem. Scand. B. 1974;28:1035–1040. doi: 10.3891/acta.chem.scand.28b-1035. [DOI] [PubMed] [Google Scholar]
- 2.Hallgren B., Niklasson A., Stallberg G., Thorin H. On the occurrence of 1-O-alkylglycerols and 1-O-(2-methoxyalkyl)glycerols in human colostrum, human milk, cow's milk, sheep's milk, human red bone marrow, red cells, blood plasma and a uterine carcinoma. Acta Chem. Scand. B. 1974;28:1029–1034. doi: 10.3891/acta.chem.scand.28b-1029. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi K., Takagi T. Comparative studies on the ether-linked lipids of ratfish and shark. 5. Characteristics of methoxy-glyceryl ethers from some cartilaginous fish liver lipids. Bull. Jpn. Soc. Sci. Fish. 1982;48:1345–1351. [Google Scholar]
- 4.Nagan N., Zoeller R.A. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 2001;40(3):199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 5.Snyder F., Wood R. The occurrence and metabolism of alkyl and alk-1-enyl ethers of glycerol in transplantable rat and mouse tumors. Cancer Res. 1968;28:972–978. [PubMed] [Google Scholar]
- 6.Snyder F., Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969;29:251–257. [PubMed] [Google Scholar]
- 7.Howard B.V., Morris H.P., Bailey J.M. Ether lipids and α-glycerol phosphate dehydrogenase and growth rate in tumors and cell cultures. Cancer Res. 1972;32:1533–1538. [PubMed] [Google Scholar]
- 8.Albert D.H., Anderson C.E. Ether-linked glycerolipids in human-brain tumors. Lipids. 1977;12:188–192. doi: 10.1007/BF02533292. [DOI] [PubMed] [Google Scholar]
- 9.Lin H.J., Ho F.C.S., Lee C.L.H. Abnormal distribution of O-alkyl groups in the neutral glycerolipids from human hepatocellular carcinomas. Cancer Res. 1978;38:946–949. [PubMed] [Google Scholar]
- 10.Merchant T.E., Meneses P., Gierke L.W., Den Otter W., Glonek T. 31P magnetic resonance phospholipid profiles of neoplastic human breast tissues. Br. J. Cancer. 1991;63:693–698. doi: 10.1038/bjc.1991.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H.J., Wu P.C., Ho J.C. The ether lipid tumour marker in human liver with hepatocellular carcinoma. Br. J. Cancer. 1980;41(2):320–324. doi: 10.1038/bjc.1980.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H.J., Ng W.L., Tung-Ma L., Lee C.L. Absence of the ether lipid tumour marker in diethylnitrosamine-induced rat liver cell cancer. Cancer Lett. 1981;11(3):231–237. doi: 10.1016/0304-3835(81)90113-0. [DOI] [PubMed] [Google Scholar]
- 13.Chabot M.C., Greene D.G., Brockschmidt J.K., Capizzi R.L., Wykle R.L. Etherlinked phosphoglyceride content of human leukemia cells. Cancer Res. 1990;50:7174–7178. [PubMed] [Google Scholar]
- 14.Solomon N., Passwater R., Joelsson I., Haimes L. first ed. Kensington Books; New York: 1997. Shark Liver Oil: Nature's Amazing Healer. p. 175. [Google Scholar]
- 15.Pedrono F., Martin B., Leduc C., Le Lan J., Saiag B., Legrand P., Moulinoux J.P., Legrand A.B. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr. Cancer. 2004;48:64–69. doi: 10.1207/s15327914nc4801_9. [DOI] [PubMed] [Google Scholar]
- 16.Ved H.S., Gustow E., Mahadevan V., Pieringer R.A. Dodecylglycerol. A new type of antibacterial agent which stimulates autolysin activity in Streptococcus faecium ATCC 9790. J. Biol. Chem. 1984;259:8115–8121. [PubMed] [Google Scholar]
- 17.Haynes M.P., Buckley H.R., Higgins M.L., Pieringer R.A. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agents Chemother. 1994;38:1523–1529. doi: 10.1128/aac.38.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdlenbruch B., Jendrossek V., Eibl H., Lakomek M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp. Brain Res. 2000;135:417–422. doi: 10.1007/s002210000553. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath D., Ravi D., Rao B.R., Apte S.S., Rambhau D. 1-O-alkylglycerol vesicles (Algosomes): their formation and characterization. Int. J. Pharm. 2002;246:187–197. doi: 10.1016/s0378-5173(02)00397-6. [DOI] [PubMed] [Google Scholar]
- 20.Erdlenbruch B., Alipour M., Fricker G., Miller D.S., Kugler W., Eibl H., Lakomek M. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharmacol. 2003;140:1201–1210. doi: 10.1038/sj.bjp.0705554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheminade C., Gautier V., Hichamim A., Allaume P., Le Lannou D., Legrand A.B. 1-O-alkylglycerols improve boar sperm motility and fertility. Biol. Reprod. 2002;66(2):421–428. doi: 10.1095/biolreprod66.2.421. [DOI] [PubMed] [Google Scholar]
- 22.Warne T.R., Buchanan F.G., Robinson M. Growth-dependent accumulation of monoalkylglycerol in Madin-Darby canine kidney cells. Evidence for a role in the regulation of Protein kinase C. J. Biol. Chem. 1995;270:11147–11154. doi: 10.1074/jbc.270.19.11147. [DOI] [PubMed] [Google Scholar]
- 23.Centemeri C., Colli S., Tosarello D., Ciceri P., Nicosia S. Heterogeneous platelet-activating factor (PAF) receptors and calcium increase in platelets and macrophages. Biochem. Pharmacol. 1999;57:263–271. doi: 10.1016/s0006-2952(98)00294-9. [DOI] [PubMed] [Google Scholar]
- 24.Benveniste J., Henson P.M., Cochrane C.G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J. Exp. Med. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott S.M., Zimmerman G.A., Stafforini D.M., McIntyre T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 26.Mangold H.K., Weber N. Biosynthesis and biotransformation of ether lipids. Lipids. 1987;22:789–799. doi: 10.1007/BF02535533. [DOI] [PubMed] [Google Scholar]
- 27.Wallner S., Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids. 2011;164(6):573–589. doi: 10.1016/j.chemphyslip.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Honke K., Zhang Y., Cheng X., Kotani N., Taniguchi N. Biological roles of sulfoglycolipids and pathophysiology of their deficiency. Glycoconj. J. 2004;21(1–2):59–62. doi: 10.1023/B:GLYC.0000043749.06556.3d. [DOI] [PubMed] [Google Scholar]
- 29.Murata D., Nomura K.H., Dejima K., Mizuguchi S., Kawasaki N., Matsuishi-Nakajima Y., Ito S., Gengyo-Ando K., Kage-Nakadai E., Mitani S., Nomura K. GPI-anchor synthesis is indispensable for the germline development of the nematode Caenorhabditis elegans. Mol. Biol. Cell. 2012;23(6):982–995. doi: 10.1091/mbc.E10-10-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnusson C.D., Haraldsson G.G. Ether lipids. Chem. Phys. Lipids. 2011;164(5):315–340. doi: 10.1016/j.chemphyslip.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Brown A.J., Snyder F. Alkyldihydroxyacetone-P synthase. Solubilization, partial purification, new assay method, and evidence for a ping-pong mechanism. J. Biol. Chem. 1982;257(15):8835–8839. [PubMed] [Google Scholar]
- 32.Das A.K., Horie S., Hajra A.K. Biosynthesis of glycerolipid precursors in rat liver peroxisomes and their transport and conversion to phosphatidate in the endoplasmic reticulum. J. Biol. Chem. 1992;267(14):9724–9730. [PubMed] [Google Scholar]
- 33.Jurkowitz M.S., Horrocks L.A., Litsky M.L. Identification and characterization of alkenyl hydrolase (lysoplasmalogenase) in microsomes and identification of a plasmalogen-active phospholipase A2 in cytosol of small intestinal epithelium. Biochim. Biophys. Acta. 1999;1437(2):142–156. doi: 10.1016/s1388-1981(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 34.Gorgas K., Teigler A., Komljenovic D., Just W.W. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim. Biophys. Acta. 2006;1763(12):1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Teigler A., Komljenovic D., Draguhn A., Gorgas K., Just W.W. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum. Mol. Genet. 2009;18(11):1897–1908. doi: 10.1093/hmg/ddp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komljenovic D., Sandhoff R., Teigler A., Heid H., Just W.W., Gorgas K. Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice. Cell. Tissue Res. 2009;337(2):281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- 37.Brodde A., Teigler A., Brugger B., Lehmann W.D., Wieland F., Berger J., Just W.W. Impaired neurotransmission in ether lipid-deficient nerve terminals. Hum. Mol. Genet. 2012;21(12):2713–2724. doi: 10.1093/hmg/dds097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liegel R., Chang B., Dubielzig R., Sidjanin D.J. Blind sterile 2 (bs2), a hypomorphic mutation in Agps, results in cataracts and male sterility in mice. Mol. Genet. Metab. 2011;103(1):51–59. doi: 10.1016/j.ymgme.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoeller R.A., Rangaswamy S., Herscovitz H., Rizzo W.B., Hajra A.K., Das A.K., Moser H.W., Moser A., Lazarow P.B., Santos M.J. Mutants in a macrophage-like cell line are defective in plasmalogen biosynthesis, but contain functional peroxisomes. J. Biol. Chem. 1992;267(12):8299–8306. [PubMed] [Google Scholar]
- 40.Zoeller R.A., Lake A.C., Nagan N., Gaposchkin D.P., Legner M.A., Lieberthal W. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 1999;338:769–776. [PMC free article] [PubMed] [Google Scholar]
- 41.Lespinet O., Labedan B. ORENZA: a web resource for studying ORphan ENZyme activities. BMC Bioinformatics. 2006;7:436. doi: 10.1186/1471-2105-7-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L.C., Pfeiffer D.R., Calhoon E.A., Madiai F., Marcucci G., Liu S., Jurkowitz M.S. Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J. Biol. Chem. 2011;286(28):24916–24930. doi: 10.1074/jbc.M111.247163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder F., Malone B., Piantadosi C. Tetrahydropteridine-dependent cleavage enzyme for O-alkyl lipids: substrate specificity. Biochim. Biophys. Acta. 1973;316(2):259–265. doi: 10.1016/0005-2760(73)90018-0. [DOI] [PubMed] [Google Scholar]
- 44.Tietz A., Lindberg M., Kennedy E.P. A new pteridine-requiring enzyme system for the oxidation of glyceryl ethers. J. Biol. Chem. 1964;239:4081–4090. [PubMed] [Google Scholar]
- 45.Watschinger K., Keller M.A., Golderer G., Hermann M., Maglione M., Sarg B., Lindner H.H., Hermetter A., Werner-Felmayer G., Konrat R., Hulo N., Werner E.R. Identification of the gene encoding alkylglycerol monooxygenase defines a third class of tetrahydrobiopterin-dependent enzymes. Proc. Natl. Acad. Sci. U. S. A. 2010;107(31):13672–13677. doi: 10.1073/pnas.1002404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner E.R., Hermetter A., Prast H., Golderer G., Werner-Felmayer G. Widespread occurrence of glyceryl ether monooxygenase activity in rat tissues detected by a novel assay. J. Lipid. Res. 2007;48(6):1422–1427. doi: 10.1194/jlr.D600042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watschinger K., Fuchs J.E., Yarov-Yarovoy V., Keller M.A., Golderer G., Hermetter A., Werner-Felmayer G., Hulo N., Werner E.R. Catalytic residues and a predicted structure of tetrahydrobiopterin-dependent alkylglycerol monooxygenase. Biochem. J. 2012;443(1):279–286. doi: 10.1042/BJ20111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang K.P., Niessen S., Saghatelian A., Cravatt B.F. An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling. Chem. Biol. 2006;13(10):1041–1050. doi: 10.1016/j.chembiol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Shindou H., Hishikawa D., Nakanishi H., Harayama T., Ishii S., Taguchi R., Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:lyso-PAF acetyltransferase. J. Biol. Chem. 2006;282(9):6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- 50.Snyder F. Alkylglycerol phosphotransferase. Methods Enzymol. 1992;209:211–215. doi: 10.1016/0076-6879(92)09025-x. [DOI] [PubMed] [Google Scholar]
- 51.Brites P., Ferreira A.S., da Silva T.F., Sousa V.F., Madheiro A.R., Duran M., Waterham H.R., Baes M., Wanders R.J. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6(12):e28539. doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blank M.L., Snyder F. Plasmanylethanolamine delta 1-desaturase. Methods Enzymol. 1992;209:390–396. doi: 10.1016/0076-6879(92)09048-8. [DOI] [PubMed] [Google Scholar]
- 53.Lee T.C., Malone B., Snyder F. New de novo pathway for the formation of 1-Alkyl-2-acetyl-snglycerols, precursors of platelet activating factor. J. Biol. Chem. 1986;261(12):5373–5377. [PubMed] [Google Scholar]
- 54.Lee T.C., Malone B., Snyder F. Formation of 1-alkyl-2-acetyl-sn-glycerols via the de novo biosynthetic pathway for platelet-activating factor. Characterization of 1-alkyl-2-acetyl-sn-glycero-3-phosphate phosphohydrolase in rat spleens. J. Biol. Chem. 1988;263(4):1755–1760. [PubMed] [Google Scholar]
- 55.Arthur G., Page L., Mock T., Choy P.C. The catabolism of plasmenylcholine in the guinea pig heart. Biochem. J. 1986;236(2):475–480. doi: 10.1042/bj2360475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waku K., Lands W.E. Acyl coenzyme A:1-alkenyl-glycero-3-phosphorylcholine acyltransferase action in plasmalogen biosynthesis. J. Biol. Chem. 1968;243:2654–2659. [PubMed] [Google Scholar]
- 57.Cao J., Shan D., Revett T., Li D., Wu L., Liu W., Tobinand J.F., Gimeno R.E. Molecular identification of a novel mammalian brain isoform of acyl-CoA:lysophospholipid acyltransferase with prominent ethanolamine lysophospholipid acylating activity, LPEAT2. J. Biol. Chem. 2008;283:19049–19057. doi: 10.1074/jbc.M800364200. [DOI] [PubMed] [Google Scholar]
- 58.Arthur G., Page L., Choy P.C. Acylation of 1-alkenylglycerophosphoethanolamine and 1-acylglycerophosphoethanolamine in guinea-pig heart microsomes. Biochim. Biophys. Acta. 1987;921(2):259–265. doi: 10.1016/0005-2760(87)90026-9. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y.F., O K., Choy P.C. Plasmenylcholine (1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholine) biosynthesis in guinea-pig heart and liver: cholinephosphotransferase is a bifunctional enzyme for the synthesis of phosphatidylcholine and plasmenylcholine. Biochem. J. 1994;301(Pt1):131–137. doi: 10.1042/bj3010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawasaki T., Snyder F. Synthesis of a novel acetylated neutral lipid related to platelet-activating factor by acyl-CoA:1-O-alkyl-2-acetyl-sn-glycerol acyltransferase in HL-60 cells. J. Biol. Chem. 1988;263:2593–2596. [PubMed] [Google Scholar]