Abstract
The established role for Phosphatidylinositol (3,4,5) triphosphate (PI(3,4,5)P3) signalling pathways is to regulate cell metabolism. More recently it has emerged that PI(3,4,5)P3 signalling via mTOR and Foxo transcription factors also controls lymphocyte trafficking by determining the repertoire of adhesion and chemokine receptors expressed by T lymphocytes. In quiescent T cells, non-phosphorylated active Foxos maintain expression of KLF2, a transcription factor that regulates expression of the chemokine receptors CCR7 and S1P1and the adhesion receptor CD62L that together control T cell transmigration into secondary lymphoid tissues. PI(3,4,5)P3 mediated activation of Protein Kinase B phosphorylates and inactivates Foxos thereby terminating expression of KLF2 and its target genes. The correct localization of lymphocytes is essential for effective immune responses and the ability of PI3K and mTOR to regulate expression of chemokine receptor and adhesion molecules puts these signaling molecules at the core of the molecular mechanisms that control lymphocyte trafficking.
Keywords: Phosphatidylinositol (3,4,5) triphosphate; KLF2; CCR7; S1P1; CD62L; lymphocytes
Background
One critical signal transduction pathway in T cells is initiated by the lipid second messenger: Phosphatidylinositol (3,4,5) triphosphate (PI(3,4,5)P3) (Okkenhaug et al., 2004). Intracellular levels of PI-(3,4,5)-P3 are low in quiescent naïve T cells but increase rapidly following triggering of the T cell antigen receptor (TCR) with antigenic peptides presented on major histocompatibility complex (MHC) molecules on the surface of specialized antigen presenting cells (APC)(Costello et al., 2002; Harriague and Bismuth, 2002). T cells can maintain contact with antigen primed APC for many hours and during this time will sustain high levels of PI-(3,4,5)-P3T(Costello et al., 2002).This biochemical response has been visualized using EGFP-tagged high affinity PI-(3,4,5)-P3 lipid binding domains as fluorescent reporters and it is thus known that as T cells respond to antigen, PI(3,4,5)-P3 accumulates relatively uniformly around the T cell plasma membrane creating a signaling platform inside and outside the contact area with the APC(Costello et al., 2002).
The sustained accumulation of PI-(3,4,5)-P3 in T cells undergoing immune activation requires continual engagement of the TCR and continual activation of PI3K(Costello et al., 2002; Huppa et al., 2003). Signals from costimulatory molecules such as CD28 are important during this sustained response (Garcon et al., 2008) although it should be emphasized that costimulatory signals alone are not sufficient to trigger PI-(3,4,5)-P3 production in T cells. Hence T cells that encounter activated dendritic cells do not generate PI-(3,4,5)-P3 unless the APC expresses the cognate peptide/MHC complex(Costello et al., 2002; Garcon et al., 2008). There are however other physiological stimuli such as chemokines and cytokines can directly induce PI-(3,4,5)-P3 production in T cells. For example, antigen primed T cells exposed to Interleukin 2 can sustain high cellular levels of PI-(3,4,5)-P3 over several days(Cornish et al., 2006; Sinclair et al., 2008). Other cytokines such as IL-15 and IL-7 also induce PI-(3,4,5)-P3 accumulation but it should be emphasized that the relative potency of these different cytokines can vary. For example in antigen primed T cells IL-15 can only induce relatively low levels of PI-(3,4,5)-P3 compared to IL-2(Sinclair et al., 2008).
Cellular levels of PI(3,4,5)P3 in T cells are controlled by the balanced activity of class I Phosphatidylinositol 3-kinases (PI3Ks) that phosphorylate the 3′-OH position of the inositol ring of phosphatidylinositol (4,5) biphosphate (PI-(4,5)-P2) and lipid phosphatases, particularily PTEN (phosphatase and tensin homologue deleted on chromosome 10) a lipid phosphatase with specificity for the 3′ position of PI(3,4,5)P3 (Buckler et al., 2006; Hagenbeek et al., 2004; Harris et al., 2007; Suzuki et al., 2001). Class I PI3Ks comprise a 110 kDa catalytic subunit and an adapter regulatory subunit. Four catalytic isoforms exist (α,β,γ,δ) and three adapter subunits, p85α, p85β and p55γ(Cantrell, 2003; Garcon et al., 2008). In mature T cells the majority of PI-(3,4,5)-P3 is produced by the actions of the p110δ PI3K catalytic subunit (Garcon et al., 2008). In simplistic models it is assumed that stimuli such as TCR ligation that cause accumulation of PI(3,4,5)P3 do so because they activates PI3K (Fabre et al., 2005). However, PTEN acts as a critical negative regulator of cellular PI(3,4,5)P3 levels and it is important to note that deletion of PTEN causes an immediate accumulation of PI(3,4,5)P3 indicating that even in quiescent T cells PI3Ks are constitutively active (Hagenbeek et al., 2004; Harris et al., 2007; Suzuki et al., 2001). It thus remains to be determined whether cellular levels of PI(3,4,5)P3 increase in activated T cells solely as a consequence of increased PI3K activity/translocation to the plasma membrane or whether T cells respond to immune activation by deleting PTEN. In this context, the importance of PTEN for T cell pathology has been recognised in a number of studies. Notably, PTEN is down-regulated by Notch1 signaling in T cell acute lymphoblastic leukaemias with a resultant constitutive accumulation of PI(3,4,5)P3 (Palomero et al., 2008).Moreover, tissue specific deletion of PTEN in T cell progenitors in the thymus using Cre-loxP strategies results in constitutive PI(3,4,5)P3 signaling and causes rapid T leukaemogenesis or lymphomagenesis (Hagenbeek and Spits, 2007; Suzuki et al., 2001).
How does PI(3,4,5)P3 signal?
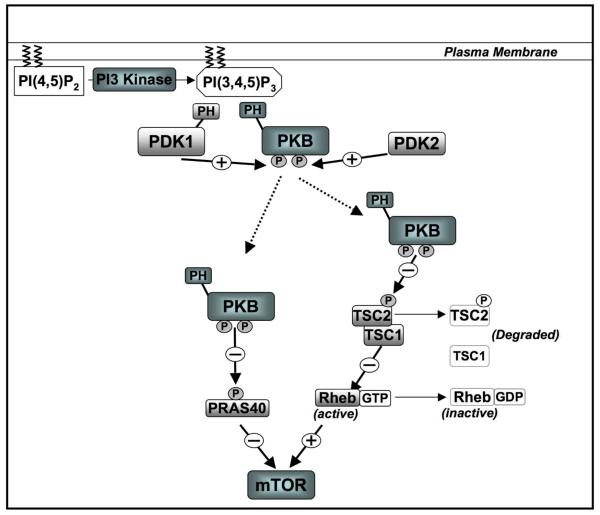
A simple descriptor of the signaling function of PI(3,4,5)P3 is that it binds to the pleckstrin homology (PH) domains of proteins and controls their activity and subcellular localization. One well characterized PI(3,4,5)P3 binding protein is the serine/threonine kinase Protein kinase B (PKB) or Akt (Fig 1). A rate limiting step for PKB activation is phosphorylation of Threonine 308 within the PKB catalytic domain by 3-phosphoinositide-dependent protein kinase-1 (PDK1) (Alessi et al., 1997). PDK1 has a PH domain that binds PI(3,4,5)P3 with high affinity but PDK1 activity is not PI(3,4,5)P3 dependent rather the binding of PI(3,4,5)P3 to the PDK1 PH domain promotes translocation of the enzyme to plasma membrane where it can co-localise with PKB (Bayascas et al., 2008; Currie et al., 1999; McManus et al., 2004). The PI(3,4,5)P3 dependence of PKB activation also reflects that PI(3,4,5)P3 binding to the PKB PH domain causes a conformational change that allows PDK1 to phosphorylate Threonine 308 within the PKB catalytic domain and activate the enzyme (Milburn et al., 2003; Yang et al., 2002). Once activated PKB phosphorylates a number of critical signalling molecules including the transcription factors Foxo1-3 and 4A (Manning and Cantley, 2007). These Foxo family transcription factors are nuclear and active in quiescent cells but when phosphorylated by PKB they exit the nucleus and form a complex with 14-3-3 proteins in the cytosol thereby terminating their transcriptional activity (Burgering, 2008; Coffer and Burgering, 2004). PKB also controls the mTORC1 complex (by phosphorylating and inactivating the Rheb GTPase activating protein TSC2. PKB activation thus results in accumulation of mammalian target of rapamycin complex 1) Rheb-GTP which activates mTORC1 (Manning and Cantley, 2007). PKB also regulates mTORC1 by phosphorylating PRAS40, thereby blocking PRAS40-mediated inhibition of mTORC1 (Manning and Cantley, 2007).
Figure 1. PI3-Kinase dependent activation of PKB and mTOR.
Elevated PI(3,4,5)P3 following PI3-Kinase activation recruits PKB and its upstream activator, PDK1 to the membrane. Full activation of PKB requires the interation of its PH domain with PI(3,4,5)P3 followed by it phosphorylation on two key residues by PDK1 and another kinase, PDK2 which is cell type, context specific. PKB activates mTOR through dual mechanisms: (1) phosphorylation and inactivation of PRAS40, a negative regulator of mTOR and (2) inactivation of the TSC1/TSC2 complex which ihn turn promotes the inactivation of the positive regulator of mTOR, the small GTPase Rheb.
PDK1/Protein Kinase B control of T cell metabolism
One evolutionarily conserved function of PDK1/PKB is to control cell metabolism. In particular, PKB mediated activation of mTORC1 controls translation of a subset of mRNA and controls ribosomal biogenesis. Here it is relevant that the survival and immune function of peripheral T lymphocytes requires that these cells match energy metabolism to energy demands. In particular, activated T cells up-regulate glucose, amino acid and iron uptake and switch to glycolysis in a response that increases cellular energy production and nutrient uptake to support the biosynthetic demands of rapid cell division (Cornish et al., 2006; Greiner et al., 1994; Kelly et al., 2007; Maciver et al., 2008). The signalling pathways that control this facet of T cell metabolism have been best characterised in T cell progenitors in the thymus. In these cells, PDK1/PKB signaling pathways induce and maintain expression of the nutrient receptors CD71 and CD98 which control transferrin and amino acid uptake (Kelly et al., 2007; Mao et al., 2007). PKB also coordinates glucose uptake and survival of thymocytes(Ciofani and Zuniga-Pflucker, 2005). Hence in the absence of PDK1 or PKB thymocytes cannot increase metabolism to match the energy demands of proliferation and they atrophy and fail to develop (Juntilla et al., 2007; Kelly et al., 2007).
One question that has not yet been addressed is whether PI3K/PDK1/PKB signalling has a similar essential role to direct the metabolic program of proliferating peripheral T cells. Certainly antigen receptors, CD28, and the common gamma chain cytokines IL-7, IL-15 and IL2, extrinsic stimuli that control peripheral T cell function, all activate PKB. As well, constitutively active mutants of PKB can substitute for the cytokine IL-7 and stimulate glucose uptake and promote cell growth and survival in mature CD4 T cells(Rathmell et al., 2003). It has also been shown that PI3K inhibitors, which prevent PKB activation prevent increases in glucose uptake following T cell activation (Frauwirth et al., 2002). However, it is now recognized that some of the PI3K inhibitors used to explore T cell metabolism have off target effects. For example Ly294002 can inhibit mTOR(mammalian target of rapamycin) and PIM family serine kinases (Bain et al., 2007). There are also other problems with the concept that PKB is the central regulator of T cell metabolism. For example, constitutively active PKB is effective at inducing survival of CD4 T cells but not CD8 T cells indicating that the signals that control the metabolism of these two different lymphocyte subsets must differ (Saibil et al., 2007). Moreover, in CD4 T cells, deletion of PDK1, which is essential for PKB activation, causes a selective defect in TCR and CD28 activation of NFkB but the ability of IL7 to support survival of CD4 T cells is independent of PDKI(Park et al., 2009). There must therefore be differences between T cell progenitors in the thymus and mature T cell populations in terms of the role of PKB in controlling cell metabolism and survival. This could reflect redundancy between PKB and other AGC kinases and or contribution of Pim serine kinases. Indeed, it has been shown that PIM kinases have a redundant role with mTOR to control survival of T cells (Fox et al., 2005).
PDK1/Protein Kinase B control of T cell trafficking
The role for PI3K and mTOR in the regulation of T cell metabolism is a role these kinases recapitulate in many cell lineages. However, there is emerging evidence for a second crucial function for these signalling molecules in T cells, namely to determine the repertoire of adhesion and chemokine receptors expressed by T lymphocytes and control T cell trafficking(Barbee and Alberola-Ila, 2005; Jarmin et al., 2008; Sinclair et al., 2008; Ward and Marelli-Berg, 2009). In fibroblasts, PI3Ks regulate actin dynamics(Reif et al., 1996) and accordingly there has been a past focus on the role of PI3Ks in regulating leukocyte chemokinesis and chemotaxis and there is indeed a reasonable volume of work showing that PI3Ks can control T cell motility(Ward and Marelli-Berg, 2009). However, there is now very strong data that PI3K and mTOR control T cell recirculation by regulating a Foxo controlled signaling pathway that dictates the membrane expression of receptors that function to home T cells to secondary lymphoid tissue(Fabre et al., 2008; Kerdiles et al., 2009; Ouyang et al., 2009; Sinclair et al., 2008).
The background to this discovery is the fact that naïve T lymphocytes continually recirculate around the body via the blood and lymphatic system and migrate into secondary lymphoid tissues such as lymph nodes by transendothelial migration in specialized high endothelial venules (HEVs). This lymphnode entry process is coordinated by chemokine receptors such as CCR7 and adhesion molecules such as CD62L (L-selectin) and integrins (Arbones et al., 1994; Forster et al., 2008; Galkina et al., 2007). T cells thus move into secondary lymphoid tissue by responding to a gradient of CCR7 ligands and the first step of transmigration is CD62L mediated capture and rolling of naïve lymphocytes on the endothelium of HEVs. CD62L and CCR7 which are constitutively expressed at high levels on naïve T lymphocytes are thus essential for the entry of these cells into peripheral lymph nodes. Importantly, immune activation of T cells induces striking changes in their migratory patterns: Effector T lymphocytes migrate to a greater extent to non-lymphoid tissues and sites of inflammation and have a reduced capacity to home to peripheral lymph nodes compared to naïve and memory T cells(Weninger et al., 2001).
The changes in the trafficking behavior of activated T cells are important for immune responses and are mediated by changes in the expression of chemokine receptors and adhesion molecules. Effector T cells downregulate CCR7 and CD62L but upregulate expression of receptors that facilitate their homing to sites of inflammation such as VLA-4 , P and E-Selectin ligands and inflammatory chemokine receptors such as CXCR3 and CCR5 (Forster et al., 2008; Mora and von Andrian, 2006). The loss of CD62L and CCR7 by activated T cells is an important mechanism that prevents effector T cells re-entering secondary lymphoid organs and allows their redirection to peripheral tissues. Hence ectopic expression of CCR7 in effector T cells causes them to be retained in secondary lymphoid tissues and hence impairs T cell effector function in peripheral tissues(Unsoeld et al., 2005). Similarily, perturbations of the normal cycles of CD62L expression have a significant impact on T cell homing and migration(Galkina et al., 2003; Kadono et al., 2002; Tu et al., 2002; Venturi et al., 2003). Despite the importance of the dynamic changes in CD62L and CCR7 expression that accompany immune activation it is only in the last year that the signal transduction pathways that control these processes have been defined and shown to be regulated by PI3K.
PI3K control of CD62L proteolytic cleavage
The expression of CD62L at the membrane is controlled by a balance of two activities: the rate of CD62L gene transcription and the rate of CD62L proteolytic cleavage. The proteolytic cleavage and shedding of CD62L from the cell surface of T lymphocytes is an acute response to triggering of T cell antigen receptors and takes place proximal to the cellmembrane and is mediated by Tumor necrosis factor (TNF)--convertingenzyme (TACE)/disintegrin and metalloprotease (ADAM) 17 (Galkina et al., 2003; Venturi et al., 2003). One mechanism to control ectodomain shedding of proteins is mediated by ERK1/2 which phosphorylate the metalloprotease TACE/ADAM17 and control its trafficking to the cell surface(Diaz-Rodriguez et al., 2002; Fan and Derynck, 1999; Soond et al., 2005). In T cells, ERKs activation by the TCR is dependent on PI3K particularily, the p110δ PI3K catalytic subunit that produces the PI(3,4,5)P3 that is generated in response to TCR triggering(Okkenhaug et al., 2002). In T cells where homologous recombination has been used to substitute WT p110δ for a catalytically inactive mutant (p110δD910A) TCR triggering no longer activated Erks and no longer induced shedding of CD62L from the T cell membrane (Sinclair et al., 2008). The blocking of CD62L proteolytic cleavage on T cells prevents T cells mediating anti viral T cell responses(Richards et al., 2008). Accordingly, the key role for the p110δ PI3K catalytic subunit for this response would contribute to the necessary role for this signaling molecule for T cell immune responses.
PI3K/mTOR control of CD62L gene transcription
Differentiated effector T cells are typically CD62L low reflecting that these cells have terminated CD62L gene transcription(Chao et al., 1997; Kaech et al., 2002). Indeed the loss of CD62L is often used as a marker to distinguish naïve, memory and effector T cells during immune responses. For example, effector CD8+ cytotoxic T lymphocytes (CTL) are CD62L low and preferentially home to sites of inflammation in peripheral tissues whereas central memory CTL express high levels of CD62L similar to naïve T cells and home to secondary lymphoid tissue (Weninger et al., 2001). Cellular immunologists have not questioned the molecular basis for the loss of CD62L by effector T cells until recently when it became clear that the expression of CD62L reports the PI3K and mTOR signaling status of a T cell and is not simply an epigenetic consequence of immune activation (Sinclair et al., 2008). PI(3,4,5)P3 and mTOR signaling are thus critical negative regulators of CD62L gene expression. Hence, effector T cells that have high levels of PI(3,4,5)P3 and mTOR signaling express low levels of CD62L mRNA and protein. However, treatment of effector T cells with PI3K inhibitors or rapamycin which inhibits the mTORC1 complex restores expression of CD62L mRNA and plasma membrane expression of CD62L. Conversely, in naïve T cells, the production of PI(3,4,5)P3 is sufficient to terminate CD62L gene transcription and protein expression. PI(3,4,5)P3 is normally dephosphorylated by the 3′ phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) to produce PI(4,5)P2. The loss of PTEN in T cells results in accumulation of PI(3,4,5)P3 and in naïve antigen inexperienced T cells this results in loss of CD62L (Sinclair et al., 2008).
How do PI3K and mTOR control expression of CD62L? The answer to this question lies in the roles of these signaling molecules in controlling the expression of the transcription factor KLF2 that directly regulates CD62L gene transcription (Bai et al., 2007; Carlson et al., 2006; Sebzda et al., 2008). KLF2 null naïve T cells thus do not express CD62L and fail to home to secondary lymphid organs and rather migrate to periperal tissues (Carlson et al., 2006; Sebzda et al., 2008). In T cells that express low levels of PI(3,4,5)P3 such as naïve T cells KLF2 levels are very high. In contrast, KLF2 expression is downregulated in effectot T cells that express high levels of PI(3,4,5)P3 This is more than just correlation because effector T cells treated with PI3K inhibitors or mTORc1 inhibitors regain expression of KLF2. Furthermore, the constitutive production of PI(3,4,5)P3 in PTEN null naïve T cells switches off KLF2 expression. In this context it is important to note that KLF2 not only controls CD62L gene transcription but simultaneously controls expression of other key lymphnode homing receptors including CCR7 and the Sphingosine 1 Phosphate receptor (S1P1) which control T cell entry and egress from lymphnodes respectively(Bai et al., 2007; Carlson et al., 2006). The ability of high levels of PI3K signals to downregulate KLF2 thus acts as a switch to terminate expression of a network of important chemokine and adhesion receptors during immune activation (Sinclair et al., 2008).
How do PI3K and mTOR control KLF2 expression?
The answer to this question is incomplete although there is strong evidence that the Foxo family transcription factor Foxo1 is an integral component of the process (Fabre et al., 2008; Kerdiles et al., 2009; Ouyang et al., 2009). Foxo1 is transcriptionally active in the nucleus of quiescent naïve T cells whereas following activation of PI3K there is a PKB mediated phosphorylation of Foxo1 that drives it from the nucleus to cytosol where it forms a complex with 14-3-3 bindng proteins (Fabre et al., 2005). The presence of active Foxo1 in the nucleus of a quiescent T cells but not activated T cells is thus a perfect correlation with the pattern of KLF2 and CD62L expression. That this is more than a correlation was first suggested by elegant experiments that examined the impact of restoring Foxo1 transcriptional activity in PTEN null T cells by expressing a mutant of Foxo1 that could not be phosphorylated by PKB (Fabre et al., 2008). These experiments identified KLF2, CD62 and S1P1 as Foxo1 regulated genes. More recently is has been shown that Foxo1 null T cells fail to express KLF2 and fail to express CD62L (Kerdiles et al., 2009). This then explains how PI3K/PKB signaling pathways control expression of KLF2 and its target genes: in quiescent T cells, active Foxos drive expression of KLF2 whereas is response to PI(3,4,5)P3 accumulation there is PKB phosphorylation of Foxos that caused them to be exported from the nucleus hence terminating expression of KLF2 and its gene targets (Fig 2).
Figure 2. PKB/Foxo dependent regulation of KLF2 and its target genes.
Naive T cells contain low levels of PI(3,4,5)P3 and active PKB. In these cells Foxo transcription factors are actively inducing the expression of targets genes such as KLF2. KLF2 in turn induces the expression of an array of genes that determine T cell trafficking, CD62L, CCR7 and S1P1. In contrast, in effector T cells, activated PI3-Kinase maintains elevated levels of PI(3,4,5)P3 and resultant activated PKB. PKB phosphorylates Foxo transcription factors on multiple residues maintaining them within the cytosol, thus repressing KLF2 expression and that of its target genes.
What is the link between Foxo1 and mTOR in T cells that explains why expression of KLF2, CD62L and CCR7 are controlled by mTOR (Sinclair et al., 2008)? There is as yet no answer to this question only to state that rapamycin treatment of effector CTL, that causes them to re-express KLF2, CD62L and CCR7, has no discernable effect on PKB activity or Foxo phosphorylation. The molecular details of mTOR control of KLF2 expression in effector T cells thus remains to be determined. However, it should be emphasized that mTOR regulation of CD62L and CCR7 expression may be important for the immunosuppressive actions of the mTORC1 inhibitor rapamycin which is widely used clinically as an immunosuppressant. It was originally thought that rapamycin suppressed immune responses because of the role for mTOR as a nutrient sensor that controls protein synthesis and proliferation of T cells. However, it is now clear that rapamycin treatment restore expression of CD62L and CCR7 on effector CTL and re directs their trafficking from peripheral tissue to lymphnodes and spleen (Sinclair et al., 2008). The ability of rapamycin to redirect activated cytotoxic T cells to secondary lymphoid tissue could result in the containment of these cells within secondary lymphoid organs and hence prevent immune destruction of target cells in peripheral tissues. The regulation of lymphocyte trafficking might thus contribute to the clinical efficacy of rapamycin in various immunosuppressive protocols.
References
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–1238. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]
- Bayascas JR, Wullschleger S, Sakamoto K, Garcia-Martinez JM, Clacher C, Komander D, van Aalten DM, Boini KM, Lang F, Lipina C, Logie L, Sutherland C, Chudek JA, van Diepen JA, Voshol PJ, Lucocq JM, Alessi DR. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler JL, Walsh PT, Porrett PM, Choi Y, Turka LA. Cutting edge: T cell requirement for CD28 costimulation is due to negative regulation of TCR signals by PTEN. J Immunol. 2006;177:4262–4266. doi: 10.4049/jimmunol.177.7.4262. [DOI] [PubMed] [Google Scholar]
- Burgering B. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- Cantrell DA. Regulation and function of serine kinase networks in lymphocytes. Curr Opin Immunol. 2003;15:294–298. doi: 10.1016/s0952-7915(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Chao CC, Jensen R, Dailey MO. Mechanisms of L-selectin regulation by activated T cells. J Immunol. 1997;159:1686–1694. [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- Fabre S, Lang V, Harriague J, Jobart A, Unterman TG, Trautmann A, Bismuth G. Stable activation of phosphatidylinositol 3-kinase in the T cell immunological synapse stimulates Akt signaling to FoxO1 nuclear exclusion and cell growth control. J Immunol. 2005;174:4161–4171. doi: 10.4049/jimmunol.174.7.4161. [DOI] [PubMed] [Google Scholar]
- Fan H, Derynck R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. Embo J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Fox C, Hammerman P, Thompson C. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth K, Riley J, Harris M, Parry R, Rathmell J, Plas D, Elstrom R, June C, Thompson C. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;6:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Galkina E, Florey O, Zarbock A, Smith BR, Preece G, Lawrence MB, Haskard DO, Ager A. T lymphocyte rolling and recruitment into peripheral lymph nodes is regulated by a saturable density of L-selectin (CD62L) Eur J Immunol. 2007;37:1243–1253. doi: 10.1002/eji.200636481. [DOI] [PubMed] [Google Scholar]
- Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigenactivated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- Greiner E, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol Chem. 1994;269:31484–31490. [PubMed] [Google Scholar]
- Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KB, Trapman J, Krimpenfort P, Spits H. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbeek TJ, Spits H. T-cell lymphomas in T-cell-specific Ptendeficient mice originate in the thymus. Leukemia. 2007 doi: 10.1038/sj.leu.2405056. [DOI] [PubMed] [Google Scholar]
- Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: Natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem. 2007 doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- Huppa J, Gleimer M, Sumen C, Davis M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nature Immunology. 2003;8:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- Jarmin S, David R, Ma L, Chai J, Dewchand H, Takesono A, Ridley A, Okkenhaug K, Marelli-Berg F. T cell receptor-induced phosphoinositide-3-kinase p110delta activity is required for T cell localization to antigenic tissue in mice. J Clin Invest. 2008;118:1154–1164. doi: 10.1172/JCI33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci U S A. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono T, Venturi GM, Steeber DA, Tedder TF. Leukocyte rolling velocities and migration are optimized by cooperative L-selectin and intercellular adhesion molecule-1 functions. J Immunol. 2002;169:4542–4550. doi: 10.4049/jimmunol.169.8.4542. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositidedependent kinase 1. Embo J. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver N, Jacobs S, Wieman H, Wofford J, Coloff J, Rathmell J. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B, Cantley L. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1262–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, Horie K, Gaitanaris GA, Fidanza V, Ludwig T, Wiest DL, Gounari F, Tsichlis PN. Unequal contribution of Akt isoforms in the double-negative to doublepositive thymocyte transition. J Immunol. 2007;178:5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, Arthur JS, Alessi DR. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. Embo J. 2004;23:2071–2082. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J, von Andrian U. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Emery JL, Vanhaesebroeck B. Phosphoinositide 3-kinase in T cell activation and survival. Biochem Soc Trans. 2004;32:332–335. doi: 10.1042/bst0320332. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7 doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- Reif K, Nobes C, Thomas G, Hall A, Cantrell D. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Richards H, Longhi M, Wright K, Gallimore A, A A. CD62L (L-selectin) down-regulation does not affect memory T cell distribution but failure to shed compromises anti-viral immunity. J Immunol. 2008;180:198–206. doi: 10.4049/jimmunol.180.1.198. [DOI] [PubMed] [Google Scholar]
- Saibil S, Jones R, Deenick E, Liadis N, Elford A, Vainberg M, Baerg H, Woodget t.J., Gerondakis S, Ohashi P. CD4+ and CD8+ T cell survival is regulated differentially by protein kinase Ctheta, c-Rel, and protein kinase B. J. Immunol. 2007;178:2932–2939. doi: 10.4049/jimmunol.178.5.2932. [DOI] [PubMed] [Google Scholar]
- Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008 doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Tu L, Poe JC, Kadono T, Venturi GM, Bullard DC, Tedder TF, Steeber DA. A functional role for circulating mouse L-selectin in regulating leukocyte/endothelial cell interactions in vivo. J Immunol. 2002;169:2034–2043. doi: 10.4049/jimmunol.169.4.2034. [DOI] [PubMed] [Google Scholar]
- Unsoeld H, Voehringer D, Krautwald S, Pircher H. Constitutive expression of CCR7 directs effector CD8 T cells into the splenic white pulp and impairs functional activity. J Immunol. 2005;173:3013–3019. doi: 10.4049/jimmunol.173.5.3013. [DOI] [PubMed] [Google Scholar]
- Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, Bock CB, Miller AS, Albrecht RM, Kubes P, Steeber DA, Tedder TF. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19:713–724. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Ward S, Marelli-Berg F. Mechanisms of chemokine and antigendependent T-lymphocyte navigation. Biochem J. 2009;418:13–27. doi: 10.1042/BJ20081969. [DOI] [PubMed] [Google Scholar]
- Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cron P, Thompson V, Good V, Hess D, Hemmings B, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]