Abstract
Background
Recent findings suggest that chronic kidney disease (CKD) may be associated with increased risk of venous thromboembolism (VTE). Given the high prevalence of mild-to-moderate CKD in the general population, in depth analysis of this association is warranted.
Methods and Results
We pooled individual participant data from five community-based cohorts from Europe (HUNT2, PREVEND and Tromsø study) and United States (ARIC and CHS study) to assess the association of estimated glomerular filtration rate (eGFR), albuminuria and CKD with objectively verified VTE. To estimate adjusted hazard ratios (HRs) for VTE, categorical and continuous spline models were fit using Cox regression with shared-frailty or random-effect meta-analysis. A total of 1,178 VTE events occurred over 599,453 person-years follow-up. Relative to eGFR 100 mL/min/1.73m2, HRs for VTE were 1.29 (95%CI, 1.04-1.59) for eGFR 75, 1.31 (1.00-1.71) for 60, 1.82 (1.27-2.60) for 45 and 1.95 (1.26-3.01) for 30 mL/min/1.73m2. Compared with albumin-creatinine ratio (ACR) of 5.0 mg/g, the HRs for VTE were 1.34 (1.04-1.72) for 30 mg/g, 1.60 (1.08-2.36) for 300 mg/g and 1.92 (1.19-3.09) for 1000 mg/g. There was no interaction between clinical categories of eGFR and ACR (P=0.20). The adjusted HR for CKD defined as eGFR <60 mL/min/1.73m2 or albuminuria ≥30 mg/g (vs. no CKD) was 1.54 (95%CI, 1.15-2.06). Associations were consistent in subgroups according to age, gender, and comorbidities as well as for unprovoked versus provoked VTE.
Conclusions
Both eGFR and ACR are independently associated with increased risk of VTE in the general population, even across the normal eGFR and ACR ranges.
Keywords: chronic kidney disease, deep vein thrombosis, epidemiology, pulmonary embolism, thromboembolism
The overall incidence rate of venous thromboembolism (VTE) in developed countries is approximately 1.5 per 1,000 person-years, varying from <0.05 in children to nearly 10.0 per 1,000 person-years in the elderly.1-4 The 28-day case-fatality rate after a first VTE is as high as 11%.4 As none of the known VTE risk factors are present in up to 50% of VTE cases,3 identifying novel risk factors for VTE is the focus of intensive research.
Nephrotic syndrome and overt proteinuria are well-known risk factors for VTE.5, 6 Mild to moderate chronic kidney disease (CKD) is associated with a procoagulant profile,7-12 and might therefore also be related to VTE risk. Two recent studies suggested that CKD may be associated with increased VTE risk, with some conflicting results.13-15 Of the two key CKD defining kidney measures (i.e. glomerular filtration rate [GFR] and albuminuria), in the Atherosclerosis Risks in Communities (ARIC) study, a significant association was found only between reduced GFR and VTE incidence,14 whereas in the Prevention of Renal and Vascular End-stage Disease (PREVEND) study an association was observed only for elevated albuminuria.13 Possible explanations for these inconsistent findings might be limited statistical power of the individual studies and differences in study population characteristics or the selection of covariates.
Given the high prevalence of CKD (10 to 16%) in the general adult population,16-19 in-depth analysis of the association of CKD with VTE incidence is warranted. Hence, we conducted an individual-level meta-analysis of five prospective general population-based cohorts with information on GFR, albuminuria and incident VTE. This report explores the separate and combined associations of GFR and albuminuria with the risk of VTE.
Methods
Study selection criteria
To select eligible studies we utilized criteria similar to those of the CKD Prognosis Consortium;20, 21 eligible studies had to be community-based cohort studies with both baseline eGFR and urine albumin measurements. A PubMed search was performed on March 2 2010, using the following combination of terms: (eGFR OR GFR OR “glomerular filtration rate” OR “kidney function” OR “renal function” OR “microalbuminuria” OR albuminuria OR “albumin to creatinine ratio” OR ACR OR “urinary albumin concentration” OR UAC) AND (“venous thrombosis” OR “venous thromboembolism” OR “pulmonary embolism” OR “deep vein thrombosis” OR DVT) AND (adult [MeSH]) AND (humans [MeSH]). Two investigators (BKM and RTG) performed the search independently. No language or publication period restrictions were applied. Subsequently we searched general population studies, with albumin-to-creatinine ratio (ACR) ascertainment that participated in the CKD Prognosis Consortium,21 in PubMed for availability of VTE outcomes. Finally, additional eligible cohorts were sought during scientific meetings and via personal contacts. The ethical review committee of the University Medical Center of Groningen approved the project to receive and analyze the data. Review committees of each participating cohort approved sharing of the de-identified individual-level data and the conducted analyses presented in this paper.
Baseline study variables
Glomerular filtration rate (GFR) was estimated using the CKD Epidemiology Collaboration (CKD-EPI) equation that takes into account serum creatinine, age, sex and race.22 In 3 studies serum creatinine was not standardized to isotope dilution mass spectrometry (IDMS), hence we reduced the creatinine levels by 5%, the calibration factor used to adjust non-standardized Modification of Diet in Renal Disease (MDRD) Study samples to IDMS.23 In a sensitivity analysis, GFR was estimated using the MDRD equation.24 Albuminuria was quantified by the ratio of urinary albumin to urinary creatinine excretion in a spot or 24 hour urine sample.25, 26 CKD was defined as eGFR <60 mL/min/1.73m2 and/or ACR ≥30 mg/g, according to prevailing guidelines.25 History of cardiovascular disease was defined as history of self-reported myocardial infarction (MI) or stroke at study baseline. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication. Diabetes mellitus was defined as fasting glucose concentration ≥7.0 mmol/L (≥126 mg/dL), non-fasting glucose concentration ≥11.1 mmol/L (≥200 mg/dL), or use of glucose lowering drugs or self-reported diabetes. Smoking was dichotomized to current smokers versus former or nonsmokers. Hypercholesterolemia was defined as total cholesterol concentration 5.0 mmol/L (193 mg/dL) or more in patients with a history of MI or stroke and as 6.0 mmol/L (232 mg/dL) or more in patients without history of MI and stroke. Body mass index (BMI) was calculated as measured body weight in kilograms divided by height in meters squared.
Venous thromboembolism
Only objectively verified symptomatic VTE were considered in all cohorts. Deep vein thrombosis was confirmed by compression ultrasound or venography, and pulmonary embolism by ventilation/perfusion lung scanning, angiography, spiral computed tomography or at autopsy. Major trauma, surgery, significant immobilization or active cancer in the proceeding 3 months were the main determinants for classifying VTE as provoked.4, 13, 27, 28 Three of the 5 cohorts included additional risk factors in the definitions of provoked VTE, such as the use of oral contraceptives or hormone therapy, pregnancy, long-distance travel, active infectious disease, acute myocardial infarction, paresis/paralysis of the leg, and heart failure.13, 27, 28 In the absence of the aforementioned risk factors, VTE was classified as unprovoked.
Statistical analysis
Individual participant data from the cohorts were pooled. Cox proportional hazards models with shared frailty (i.e., random effects) were used to estimate adjusted hazard ratios (HRs) of VTE associated with eGFR, albuminuria and their combination. Individual cohort was considered as the shared frailty variable to account for between-study differences. As sensitivity analysis, continuous eGFR and ACR associations with VTE were also modeled using stratified (i.e., fixed effects) Cox proportional hazards regression.
Based on previous literature on risk factors for VTE and correlates of eGFR and ACR; age, sex, BMI, history of cardiovascular disease, hypertension, diabetes, total cholesterol, and current smoking were included in models as potential confounders.13, 14, 29 Since only 2 of the 5 studies also enrolled black participants, ethnicity specific results were presented for those 2 studies rather than including race as a covariate in the main model. In addition all eGFR models were adjusted for log-ACR, and all ACR models were adjusted for eGFR splines. To assess the shape of the relationship of eGFR and ACR with risk of VTE, we modeled eGFR and ACR using linear splines with knots at 45, 60, 75, 90 and 105 mL/min/1.73m2 for eGFR, and 10, 30, 300 and 1000 mg/g (to convert to mg/mmol multiply by 0.113) for ACR, respectively. eGFR of 100 mL/min/1.73 m2 and ACR of 5 mg/g were selected as reference points. HRs of eGFR association with VTE were estimated per 1 mL/min/1.73m2 increment of eGFR from 15 to 120 mL/min/1.73m2. HRs for the association of ACR with VTE were estimated per 8% increments of ACR from 2.5 to 1000 mg/g. Adjustment for prevalent baseline traditional VTE risk factors (ie, major trauma, surgery, significant immobilization or active cancer) was not performed because these were not uniformly available; however, given the temporary nature of these risk factors baseline prevalence of these risk factors is unlikely to influence long-term VTE risk. To quantify short-term influence of these risk factors on the association of eGFR and albuminuria with VTE, we performed stratified analysis for provoked versus unprovoked VTE, where the definition of provoked VTE incorporated presence of the traditional VTE risk factors.
Joint effects of eGFR and ACR on VTE were investigated using cross-tabulation of eGFR and ACR categories. The interaction between eGFR and ACR was assessed by likelihood ratio tests between the models with eGFR and measured ACR with and without their product terms. This methodology was also used to assess interactions of CKD with sex, race, age, hypertension, diabetes, smoking, hypercholesterolemia, history of cardiovascular disease and BMI. Moreover, for dichotomous CKD VTE risk association, pooled estimates of the HRs and 95% CIs of individual studies were obtained from random-effect meta-analysis. Heterogeneity among studies was estimated by χ2 test and the I2 statistics.30 Potential sources of heterogeneity were explored by meta-regression analysis.
Since ACR was measured in only a subset of the HUNT2 cohort, we adjusted the eGFR-VTE associations for log-ACR values based on multiple imputation.31 We created 20 complete data sets using the linear regression with the bootstrap method of the Stata “ice” command to achieve maximum accuracy for the imputed log-ACR.31, 32 Subsequently the “micombine” command with Cox regression was used to obtain HRs and correct 95% CIs. Age, sex, hypertension, diabetes and history of cardiovascular disease, as well as VTE and log-transformed follow-up time were used to impute log-ACR values. To avoid potential bias due to multiple imputation, analyses of the ACR-VTE risk association was based on measured ACR values only. Statistical significance was considered as a 2-tailed P <0.05. All statistical analyses were performed using Stata software version 11.2 (StataCorp LP, College Station, Texas).
Results
Figure 1 shows the flow diagram of the identified studies. Investigators of one of the eligible studies could not provide data.33 Characteristics of the included studies are presented in Table 1. Overall, 95,154 participants (46.7% males, 96.6% Caucasians) were included with 599,453 person-years of follow-up. During follow-up, 1,178 VTE occurred, 45% were classified as unprovoked and 39% were pulmonary embolism alone or in combination with deep vein thrombosis. In all cohorts combined, 94,882 (99.7%) participants had measured eGFR data and 39,524 (41.5%) had ACR data (in HUNT2 only 15% of participants had ACR measured; n=9,737). All other variables presented in Table 1 had less than 0.8% missing values in the pooled dataset except current smoking (4.4% missing).
Figure 1.
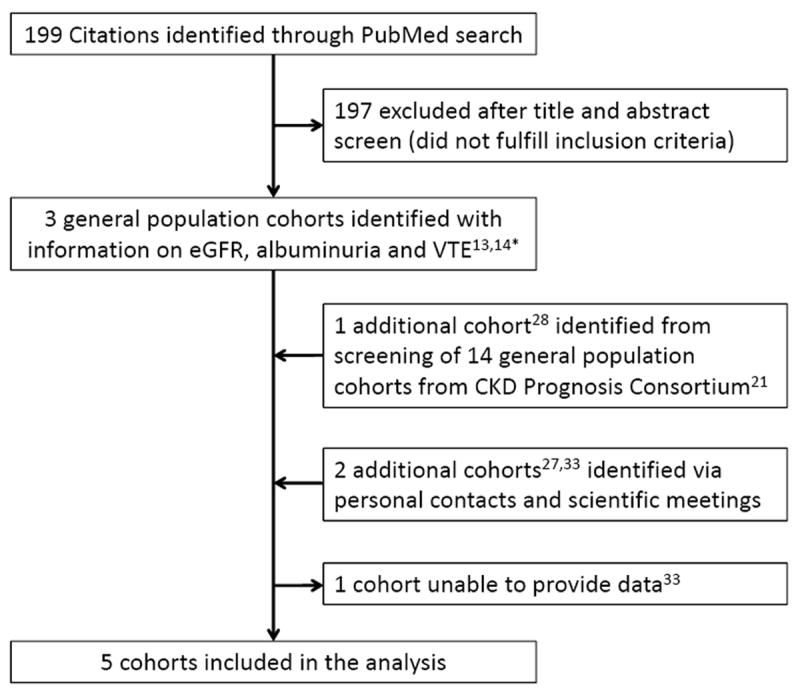
Flow diagram for selection of studies. * Reference 14 included analysis of 2 cohorts.
Table 1.
Study characteristics per cohort.
| Country of origin | ARIC* USA | CHS* USA | HUNT2 Norway | PREVEND Netherlands | Tromsø Norway |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Participants, n | 11,513 | 3,450 | 64,793 | 8,573 | 6,825 |
| Male, % | 44.1 | 39.7 | 46.9 | 50.0 | 49.2 |
| Mean age, years | 62.8 | 78.1 | 50.2 | 49.0 | 60.2 |
| Black, % | 22.5 | 16.6 | 0.0 | 1.0 | 0.0 |
| Hypertension, % | 47.7 | 72.5 | 45.1 | 34.1 | 50.2 |
| Diabetes, % | 16.8 | 22.2 | 3.0 | 4.4 | 3.4 |
| Hypercholesterolemia, % | 21.2 | 27.3 | 46.6 | 37.9 | 70.8 |
| Current smoking, % | 14.8 | 7.6 | 29.8 | 34.2 | 31.8 |
| History of MI or stroke, % | 10.1 | 16.5 | 4.9 | 4.5 | 8.5 |
| Mean body mass index, kg/m2 | 28.8 | 26.9 | 26.3† | 26.1 | 26.0 |
| Mean total cholesterol, mg/dL | 200 | 203 | 228 | 218 | 259 |
| Mean eGFR, mL/min/1.73m2 | 84.3 | 67.6 | 97.9 | 88.8 | 92.8 |
| Median ACR, mg/g | 3.7 | 10.2 | 7.7 | 7.0 | 5.4 |
| Mean follow-up, years | 8.0 | 4.5 | 5.2 | 9.3 | 10.8 |
| Venous thromboembolism, n | 260 | 61 | 509 | 122 | 226 |
MI denotes myocardial infarction and ACR, albumin creatinine ratio. eGFR was estimated by the CKD-EPI equation.22 To convert total cholesterol to mmol/L multiply by 0.0259. To convert ACR to mg/mmol multiply by 0.113.
Since albuminuria was measured at visit 4 in ARIC and the year7 CHS, we treated these visits as the baseline for ARIC and CHS.
In subject with measured ACR in HUNT2 study, mean BMI was 28 kg/m2 as depicted in supplementary Figure 4.
Estimates of adjusted HRs for VTE according to eGFR and ACR levels are presented in Figure 2. Risk of VTE started to be significantly increased at eGFR 88 mL/min/1.73m2. Relative to eGFR 100 mL/min/1.73m2, HRs for VTE were 1.29 (95%CI, 1.04-1.59) for eGFR 75, 1.31 (1.00-1.71) for 60, 1.82 (1.27-2.60) for 45 and 1.95 (1.26-3.01) for 30 mL/min/1.73m2. Similar findings were observed in analyses using the MDRD equation-based eGFR (Supplementary Appendix, Figure 1). The interpretation of results did not change in models comparing ACR as a covariate with and without use of imputed ACR from the HUNT2 study, indicating the validity of the multiple imputation (Supplementary Appendix, Figure 2). The association of ACR splines and VTE risk was largely linear on the log-log scale, with significantly increased risk observed at ACR 14 mg/g and higher. Compared with ACR of 5.0 mg/g, the HRs for VTE were 1.34 (1.04-1.72) for 30 mg/g, 1.60 (1.08-2.36) for 300 mg/g and 1.92 (1.19-3.09) for 1000 mg/g. (Figure 2B). Results of fixed-effect Cox proportional hazards models were identical to the random-effect models (Supplemental Figure 3).
Figure 2.
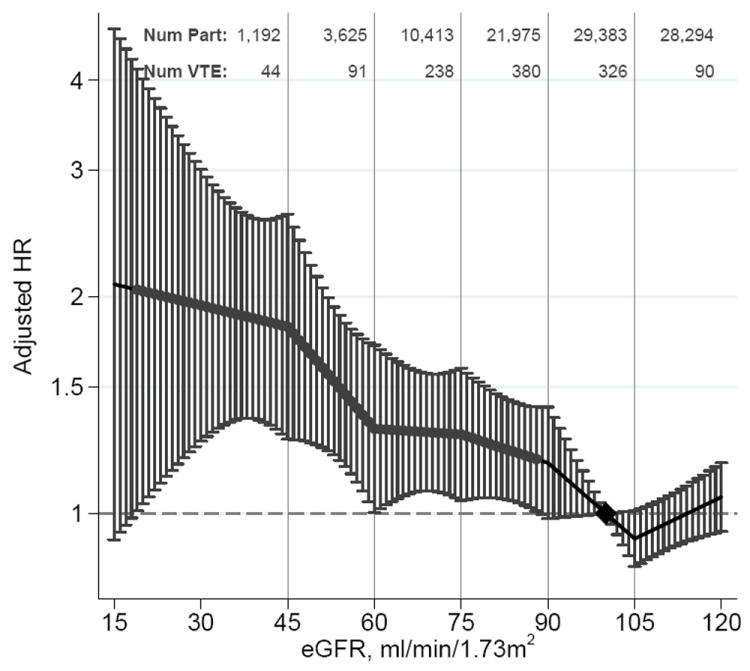
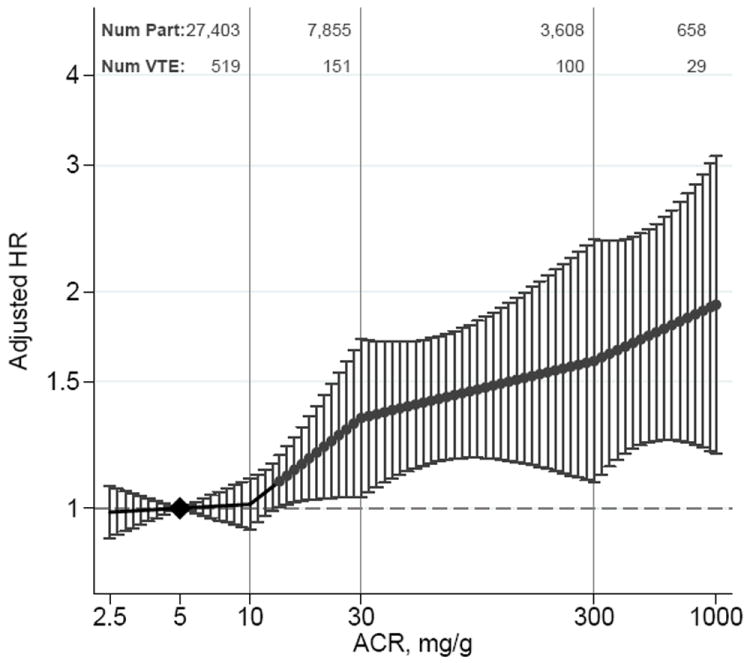
Pooled hazard ratios and 95% CIs for venous thromboembolism according to spline estimated glomerular filtration rate (eGFR) and albumin-to-creatinine ratio (ACR).
Hazard ratios and 95% CIs (error bars) according to eGFR (A) and ACR (B) adjusted for each other, age, sex, body mass index, history of cardiovascular disease, hypertension, diabetes, smoking, and total cholesterol. The reference (diamond) was eGFR 100 mL/min/1.73 m2 and ACR 5 mg/g (0.6 mg/mmol), respectively. Red dots represent statistically significance. Num Part and Num VTE denote the number of participants and number of VTE, respectively, in the range between the knots represented by the vertical gray lines. To convert ACR to mg/mmol multiply by 0.113.
Table 2 shows the adjusted HR of VTE in clinical categories of eGFR and ACR based on K/DOQI staging.25 The corresponding number of VTE and total number of participants according to these categories are presented in Supplemental Table 1. In general, the association of ACR with VTE risk was evident across most eGFR categories. The association between reduced eGFR and VTE risk was more obvious in those with normoalbuminuria (i.e., ACR<30mg/g). The risk increase was not clearly multiplicative with lower eGFR and higher ACR categories; tests for interaction of the separate categories (P>0.14) and overall (P=0.20) were not significant. The interaction of continuous eGFR with spline-terms and linear logACR was not significant (P=0.10).
Table 2.
Pooled estimates of adjusted hazard ratios (95%CIs) for venous thromboembolism according to clinical categories of eGFR and ACR.
| ACR | |||
|---|---|---|---|
|
| |||
| <30 mg/g (<3.3 mg/mmol) | 30-300 mg/g (3.4-33.8 mg/mmol) | >300 mg/g (≥33.9 mg/mmol) | |
| eGFR | |||
| ≥90 mL/min/1.73m2 | Reference | 1.66 (1.11-2.48) | 1.51 (0.48-4.73) |
| 60-89 mL/min/1.73m2 | 1.15 (0.96-1.38) | 1.47 (1.07-2.03) | 4.38 (2.64-7.26) |
| 45-59 mL/min/1.73m2 | 1.23 (0.87-1.74) | 1.37 (0.76-2.49) | 1.51 (0.48-4.77) |
| 30-44 mL/min/1.73m2 | 2.13 (1.26-3.62) | 2.11 (0.95-4.95) | 2.33 (0.74-7.34) |
eGFR denotes estimated glomerular filtration rate and ACR, albumin:creatinine ratio. From the HUNT2 study only subjects with measured ACR contributed to this analysis. eGFR was estimated by the CKD-EPI equation.22 Given the low numbers of individual with eGFR<30 (see supplemental Table 1), these individuals were excluded from this analysis.
When we compared individuals with CKD versus no-CKD, the pooled HR for overall VTE associated with CKD was 1.54 (95%CI, 1.15-2.06) (Figure 3). In figure 4, the impact of CKD on VTE incidence was consistent across the subgroups tested, except for a trend for BMI categories showing weaker association of CKD with VTE in subjects with BMI ≥25 versus <25 kg/m2 (P for interaction =0.07). Similarly, the significant heterogeneity observed for overall VTE among studies appeared to be due to differences in BMI across studies (β=-0.23, P=0.054) (Supplementary Appendix, Figure 4).
Figure 3.
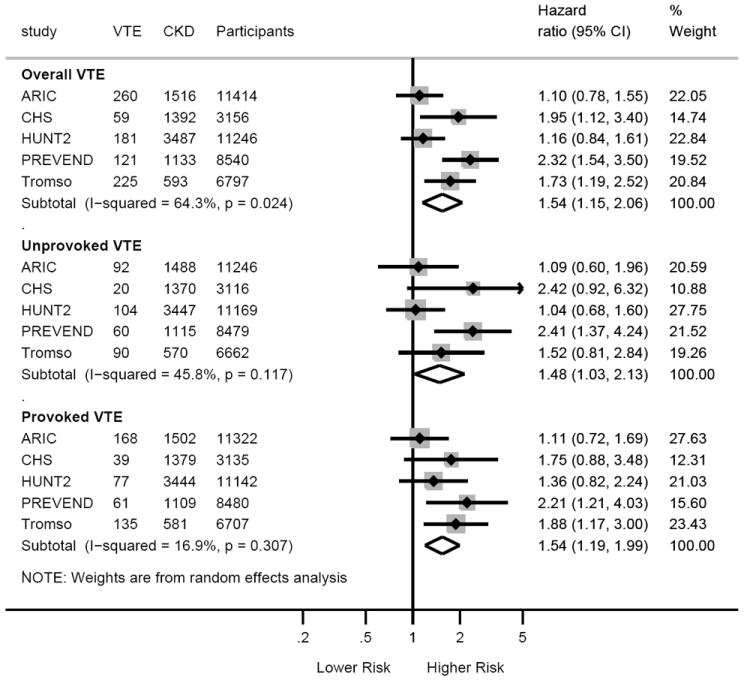
Overall and study-specific hazard ratios for overall, unprovoked and provoked venous thromboembolism in participants with CKD compared to those without CKD.
CKD was defined by eGFR of <60 mL/min/1.73m2 and/or ACR ≥30 mg/g.25 Hazard ratios are adjusted for age, sex, body mass index, history of cardiovascular disease, hypertension, diabetes, smoking, and total cholesterol. The slight differences in numbers with Table 1 are due to missing observations for either eGFR or ACR, and because for defining non-CKD both eGFR and ACR were required. For HUNT2 study only measured ACR was considered in this analysis.
Figure 4.
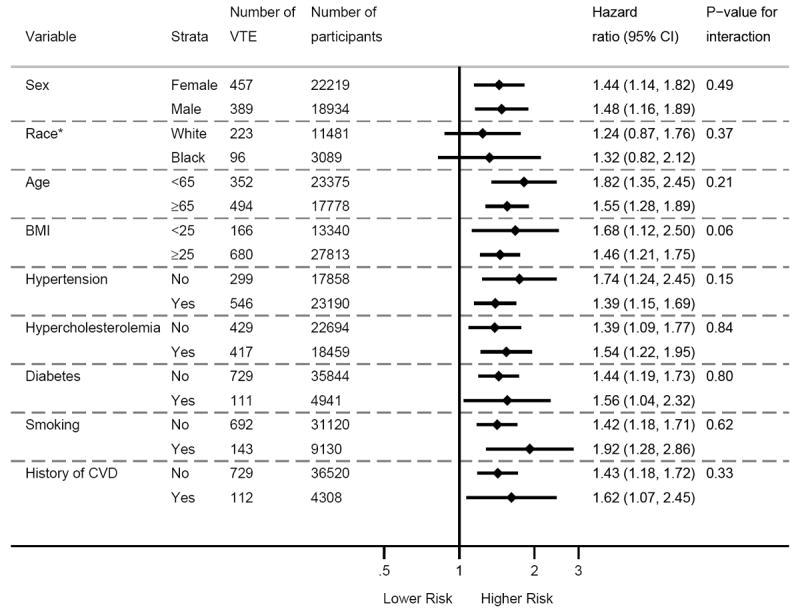
Association of CKD with VTE in subgroups according to traditional cardiovascular risk factors. CVD denotes cardiovascular disease. CKD was defined by eGFR of <60 mL/min/1.73m2 and/or ACR ≥30 mg/g 25. Hazard ratios are adjusted for other than the stratified risk factor itself, which included age, sex, body mass index, history of cardiovascular disease, hypertension, diabetes, smoking, and total cholesterol. *Race comparison was limited to ARIC and CHS studies, since the other studies enrolled only whites.
The HRs of VTE with CKD compared to no CKD were similar for unprovoked and provoked VTE (Figure 3). Analyses for continuous eGFR and ACR are presented in the supplemental Figure 5. Similarly, the HRs of pulmonary embolism and deep vein thrombosis with CKD were similar (Supplementary Appendix, Figure 6). Finally, of the covariates, only BMI and age showed significant strong association with VTE in all models of eGFR and ACR (data not shown).
Discussion
In this individual participant meta-analysis including 95,154 participants from prospective observational studies followed for an average of 6.3 years, both eGFR and elevated albuminuria were associated with increased risk of VTE independently of each other and traditional cardiovascular risk factors including BMI. For both eGFR and ACR, there was a dose-response relationship with increased risk of VTE starting in the non-CKD range of eGFR (i.e. ≥60 mL/min/1.73m2) and the normal range of ACR (i.e. <30 mg/g). There was no significant interaction between eGFR and ACR. CKD, defined by eGFR <60 mL/min/1.73m2 and/or ACR ≥30 mg/g, was similarly associated with both provoked and unprovoked VTE and with both pulmonary embolism and deep-vein thrombosis.
In this comprehensive analysis of large prospective general population-based cohorts, a clear association of eGFR and albuminuria with risk of VTE clarifies the previous inconsistent published findings of the ARIC and PREVEND studies. In addition to ARIC and PREVEND cohorts this analysis included previously unpublished data from three additional cohorts. The association of CKD with VTE was largely consistent in presence versus absence of various traditional cardiovascular risk factors, except a trend for relatively stronger association of CKD with VTE in subjects with BMI <25 kg/m2 as compared to BMI ≥25 kg/m2 (P=0.07). Difference in mean BMI among studies also explained most of the variability of the CKD-VTE risk association across studies. This finding is in line with several observational studies that reported an antagonistic interaction between BMI and CKD on mortality.34
The observation that risks do not fully multiply when both eGFR is low and ACR is high might be secondary to competing risk for mortality in low eGFR and high ACR categories as well as to limited power in the low eGFR and high albuminuria categories (supplemental Table 1). However, a significant interaction between eGFR and ACR categories in relation to mortality was not observed in a recent meta-analysis.21
CKD is associated with a broad range of diseases requiring hospitalization. This may have resulted in the association between CKD and provoked VTE. However, the association of eGFR and albuminuria with unprovoked VTE gives credence to a direct association of CKD with VTE. The high risk of VTE in individuals diagnosed with nephrotic-range proteinuria is assumed to be secondary to loss of anticoagulant proteins.6 The increased risk of VTE with mild to moderate CKD may be secondary to endothelial injury and/or the related changes in procoagulant proteins such increased levels of fibrinogen, factor VII, factor VIII, von Willebrand factor, and plasminogen activator inhibitor-1 or increased levels of D-dimers.7-12 An increased procoagulant state in CKD patients was also confirmed by functional coagulation assays such as prothrombin fragment 1+2, thrombin-antithrombin complex, plasmin-antiplasmin complex as well as in vitro thrombin generation assessed by calibrated automated thrombogram.7, 8, 10, 35 The well-known link of CKD with arterial cardiovascular disease and mortality36 is also assumed to be at least partially due to a hypercoagulable state.7, 8, 10
The high prevalence of CKD in the general population (10-16%) suggests that on the population level,16-19 CKD may explain a much larger proportion of VTE risk than most of the established rare hereditary VTE risk factors, such as antithrombin, protein C and protein S deficiencies.37 Assuming a CKD prevalence of 10% in the general population, the observed HR of 1.54 in our study corresponds to a population attributable risk of 5.1%, if the relationship is causal. In contrast to most established VTE risk factors, CKD, in particular albuminuria, is modifiable with medications (e.g., renin-angiotensin system inhibitors).38, 39 In fact, losartan use in patients with overt proteinuria >2.0 g/d ameliorates the hypercoagulable state in proteinuric patients.35 Taken together with our findings, studies evaluating the effect of albuminuria lowering drugs on the risk of VTE in patients with mild to moderate CKD are warranted. Further, because CKD is common, based on the current findings it would be useful to assess whether CKD might be associated with the risk of recurrent VTE.
We acknowledge that this study has limitations. First, measurement of creatinine, albuminuria and potential confounders were not standardized among all studies. For instance, some studies measured albumin and creatinine in fresh urine samples whereas other studies used frozen samples, and there was no centralized laboratory for all studies together. Care was taken, however, to use the same definitions for exposure variables and covariates across studies. Second, whereas we accounted for cardiovascular risk factors as potential confounders that are strongly associated with CKD and possibly associated with VTE, residual confounding may still remain. Although we were not able to account for hereditary thrombophilic defects, these are not known to be associated with mild to moderate CKD. In fact, one recent study reported a reno-protective effect of factor V Leiden.40 Third, event ascertainment across studies was comparable, but the definitions of unprovoked and provoked VTE were slightly different. However, we observed largely consistent findings for the association of CKD with overall, unprovoked and provoked VTE. Fourth, we are unable to account for anticoagulant medication use. However, given that CKD is associated with cardiovascular disease, ignoring anticoagulant medication use would have resulted in underestimated CKD-VTE risk association. Lastly, meta-regression analysis that explored the variation of HRs across studies was underpowered given the small number of studies in current analysis. Nevertheless, the association of BMI with the variation of HRs of the association of CKD with VTE risk reached borderline significance, suggesting that the heterogeneity across studies might be secondary to differences in mean BMI.
In conclusion, both eGFR and ACR are independently associated with increased risk of VTE in the general population, even in the non-CKD rage of eGFR and the normal range of ACR.
Supplementary Material
CLINICAL PERSPECTIVE.
Chronic kidney disease (CKD) is a major health problem, which affects 10 to 16% of the general adult population. Whereas associations of CKD with arterial thromboembolism and mortality are well known, the association of CKD with venous thromboembolism (VTE) is uncertain. In the present study, we assessed the association of CKD with venous thrombosis in 5 general population cohorts. The key CKD measures (i.e., decreased estimated glomerular filtration rate [eGFR] and elevated albumin-to-creatinine ratio [ACR]) were both associated with an increased risk of VTE, even for values in the normal ranges. Subjects with CKD (i.e., eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/g) had a 54% higher risk of VTE as compared to subjects without CKD. The associations were similar for unprovoked and provoked VTE, as well as for pulmonary embolism and deep-vein thrombosis. CKD measures showed largely similar associations with VTE across subgroups of traditional cardiovascular risk factors, such as hypertension, diabetes, age and gender. Given the effect size of the association, individual-level implications may be limited. Nevertheless, due to the high prevalence of CKD, population-level VTE burden due to CKD is estimated to be high, especially in populations with high CKD prevalence such as those with diabetes and hypertension. Future studies are warranted to assess whether CKD is also associated with recurrent VTE. If confirmed, these finding may have implications for the duration of anticoagulant treatment for first VTE.
Acknowledgments
The authors thank the staff and participants of the included studies for their important contributions.
Funding/Support: Mahmoodi’s fellowship at Johns Hopkins Bloomberg School of Public Health is supported by grants of Netherlands Organization for Scientific Research and the Dutch Kidney Foundation. Coresh, Matsushita and Sang are partly supported by the US National Kidney Foundation. No external support was required for current analysis. Sponsors for individual studies are as follows:
Atherosclerosis Risk in Communities Study (ARIC): The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Additional support was from the National Heart, Lung, and Blood Institute grant R01 HL59367.
Cardiovascular Health Study (CHS): The CHS was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Additional support was from the National Heart, Lung, and Blood Institute grant R01 HL59367.
The second Nord-Trøndelag Health Study (HUNT2): HUNT2 was funded by joint efforts of a large number of partners. Main contributions came from The Ministry of Health, through The National Institute of Public Health screening service (SHUS). The Nord-Trøndelag County Council, The Norwegian University of Science and Technology (NTNU) and The Norwegian Research Council also provided essential funding. The linked study of venous thrombosis (the TROL study) was a collaboration between NTNU, Trondheim, Norway and Leiden University Medical Center, Leiden, the Netherlands, respectively supported by the Research Council of Norway (grant nr. 148037) and the Netherlands Hearth Foundation (grant nr. 2000B185).
Prevention of REnal and Vascular ENd-stage Disease (PREVEND): The PREVEND study is supported by several grants from the Dutch Kidney Foundation, and grants from the Dutch Heart Foundation, the Dutch Government (NWO), the US National Institutes of Health (NIH) and the University Medical Center Groningen (UMCG).
Tromsø Study: The Tromsø Study is funded by the University of Tromsø and supported by grants from the Northern Norwegian Regional Health Authority.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 2.Nordstrom M, Lindblad B, Bergqvist D, Kjellstrom T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232:155–160. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 3.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: The Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, Navis G, van der Meer J. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation. 2008;117:224–230. doi: 10.1161/CIRCULATIONAHA.107.716951. [DOI] [PubMed] [Google Scholar]
- 6.Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: Pathophysiology and clinical management. Thromb Res. 2006;118:397–407. doi: 10.1016/j.thromres.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Dubin R, Cushman M, Folsom AR, Fried LF, Palmas W, Peralta CA, Wassel C, Shlipak MG. Kidney function and multiple hemostatic markers: Cross sectional associations in the Multi-Ethnic Study of Atherosclerosis. BMC Nephrol. 2011;12:3. doi: 10.1186/1471-2369-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Fried LF, Stehman-Breen C, Siscovick D, Newman AB. Chronic renal insufficiency and cardiovascular events in the elderly: Findings from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:81–90. doi: 10.1111/j.1076-7460.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- 9.Kario K, Matsuo T, Kobayashi H, Matsuo M, Sakata T, Miyata T, Shimada K. Factor vii hyperactivity and endothelial cell damage are found in elderly hypertensives only when concomitant with microalbuminuria. Arterioscler Thromb Vasc Biol. 1996;16:455–461. doi: 10.1161/01.atv.16.3.455. [DOI] [PubMed] [Google Scholar]
- 10.Gruden G, Cavallo-Perin P, Romagnoli R, Olivetti C, Frezet D, Pagano G. Prothrombin fragment 1 + 2 and antithrombin iii-thrombin complex in microalbuminuric type 2 diabetic patients. Diabet Med. 1994;11:485–488. doi: 10.1111/j.1464-5491.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 11.Gruden G, Cavallo-Perin P, Bazzan M, Stella S, Vuolo A, Pagano G. Pai-1 and factor vii activity are higher in iddm patients with microalbuminuria. Diabetes. 1994;43:426–429. doi: 10.2337/diab.43.3.426. [DOI] [PubMed] [Google Scholar]
- 12.Clausen P, Feldt-Rasmussen B, Jensen G, Jensen JS. Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: A 4-year prospective study. Clin Sci (Lond) 1999;97:37–43. [PubMed] [Google Scholar]
- 13.Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J. Microalbuminuria and risk of venous thromboembolism. JAMA. 2009;301:1790–1797. doi: 10.1001/jama.2009.565. [DOI] [PubMed] [Google Scholar]
- 14.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folsom AR, Lutsey PL, Astor BC, Wattanakit K, Heckbert SR, Cushman M. Chronic kidney disease and venous thromboembolism: A prospective study. Nephrol Dial Transplant. 2010;25:3296–3301. doi: 10.1093/ndt/gfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC. Prevalence of kidney damage in australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 18.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 19.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 20.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J. Lower estimated gfr and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 26.Crowe E, Halpin D, Stevens P. Early identification and management of chronic kidney disease: Summary of nice guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 27.Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromsø study. Haematologica. 2010;95:270–275. doi: 10.3324/haematol.2009.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quist-Paulsen P, Naess IA, Cannegieter SC, Romundstad PR, Christiansen SC, Rosendaal FR, Hammerstrom J. Arterial cardiovascular risk factors and venous thrombosis: Results from a population-based, prospective study (the HUNT 2) Haematologica. 2010;95:119–125. doi: 10.3324/haematol.2009.011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: A meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 30.Woodward M. Epidemiology: Study design and data analysis. Boca Raton: Chapman & Hall/CRC; 2005. [Google Scholar]
- 31.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 34.Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial. 2007;20:566–569. doi: 10.1111/j.1525-139X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoodi BK, Mulder AB, Waanders F, Spronk HM, Mulder R, Slagman MC, Vogt L, Navis G, Ten Cate H, Kluin-Nelemans HC, Laverman GD. The impact of antiproteinuric therapy on the prothrombotic state in patients with overt proteinuria. J Thromb Haemost. 2011;9:2416–2423. doi: 10.1111/j.1538-7836.2011.04525.x. [DOI] [PubMed] [Google Scholar]
- 36.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 37.Rosendaal FR. Risk factors for venous thrombotic disease. Thromb Haemost. 1999;82:610–619. [PubMed] [Google Scholar]
- 38.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 39.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Madhusudhan T, He T, Hummel B, Schmidt S, Vinnikov IA, Shahzad K, Kashif M, Muller-Krebs S, Schwenger V, Bierhaus A, Rudofsky G, Nawroth PP, Isermann B. Low but sustained coagulation activation ameliorates glucose-induced podocyte apoptosis: Protective effect of factor v leiden in diabetic nephropathy. Blood. 2011;117:5231–5242. doi: 10.1182/blood-2010-10-314773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


