Abstract
We study the influence of fluid flow on the ability to trap optically a 20 nm polystyrene particle from a stationary microfluidic environment and then hold it against flow. Increased laser power is required to hold nanoparticles as the flow rate is increased, with an empirical linear dependence of 1 μl/(min×mW). This is promising for the delivery of additional nanoparticles to interact with a trapped nanoparticle; for example, to study protein-protein interactions, and for the ability to move the trapped particle in solution from one location to another.
The ability of optical trapping to immobilize and manipulate nanoparticles has opened up new possibilities for manipulation at the nanometer scale1. For example, this trapping can be used in the characterization and placement of colloidal quantum dots2,3. It is also of interest for the study of nanoscale biological interactions; for example, those involving protein-protein interactions4, antigen–antibody binding5,6, and the manipulation of individual virus particles7.
Two challenges hinder the progress of this technique. First, the gradient force for trapping scales as the cube of a spherical particle's radius, so smaller particles require much higher intensities to trap8. This can lead to situations where the trapping intensity is larger than the damage threshold of the particle. For example, to trap smaller than 20 nm silica particles required greater than 1.5 W of green laser power, and for 85 nm polystyrene particles, the damage was so quick at the required intensity that trapping could not be observed8. Second, reduction in the particle size decreases viscous drag, so that the escape from the trap due to Brownian motion is faster, on average. In spite of these challenges, some biological nanoparticles have been trapped by conventional methods such as the tobacco mosaic virus and bacteria9, but these are usually highly elongated, and therefore more polarizable than simple spheres of the same volume. Optical tweezers have also been used to trap dielectric nano-spheres8,10,11, carbon nanotubes12, semiconductor nanowires13, DNA strains14, and metal nanoparticles15,16. Also, excellent reviews on the fundamentals of plasmonic trapping are available17,18,19,20.
One approach for trapping of these small nanoparticles with conventional optical tweezers is tethering a micrometer-sized bead to them21,22,23,24. Tethering, however, introduces steric issues that hinder studying of processes such as protein-protein interaction and processes which require binding to a surface25.
To achieve large trap stiffness and highly localized field intensities, many have used nanophotonic and plasmonic optical traps11,26,27,28,29,30,31,32,33,34,35. These traps, however, are still perturbative in the sense that the traditional gradient force calculation can be used to quantify the trapping, and therefore, they require either large local intensities to trap small objects36,37,38, or highly polarizable particles.
Trapping with apertures in metal films has been used to overcome the problem of required high intensities. In 2009, trapping 50 nm polystyrene particles with 1 mW of power was demonstrated using an aperture in a metal film39. It was confirmed by comprehensive numerical calculations that this aperture trapping method does not follow the traditional third power scaling with particle size. The particle to be trapped strongly modifies the local electromagnetic environment and thereby facilitates the trapping – for this reason, the approach was referred to as self-induced back-action (SIBA) optical trapping.
Shaped apertures can lead to further improved trapping performance of even smaller particles. For example, we have used a double-hole aperture to trap 12 nm particles40. Rectangular apertures have been used to trap particles as small as 22 nm24, with a propensity for trapping of multiple particles simultaneously. Later, we used the double-hole aperture to trap and unfold single proteins41, which is a promising step for studying the interactions of biological particles at the single particle level (e.g., protein binding).
Those past works, however, did not demonstrate the ability to trap against flow, which is essential for introducing additional nanoparticles, for example in the study of binding. Trapping against flow is also essential to applications where we wish to move the trapped particle through a fluid, for example, if we wish to relocate a quantum dot trapped at the end of an aperture fiber42,43. For these applications, trapping against flow needs to be demonstrated.
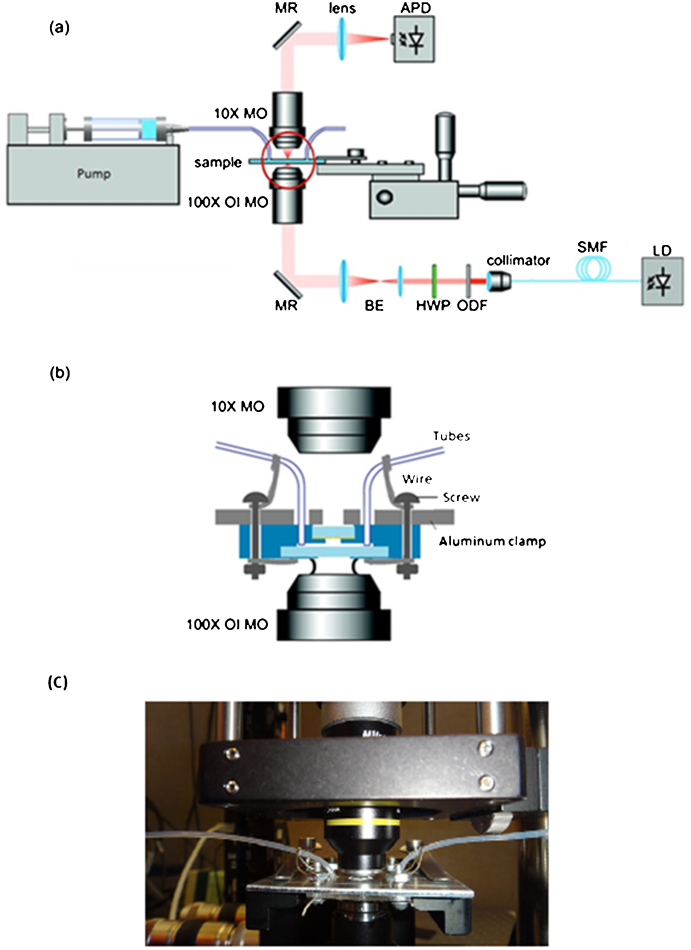
Figure 1a) shows the modified trapping setup with microfluidic delivery. The setup is based on the inverted microscope configuration, modified from the Thorlabs optical tweezer kit (OTKB) that has been used in our past experiments40,41; however, it includes a microfluidic assembly designed to fit in the trap. This setup is using a 820 nm continuous laser (Sacher Lasertechnik Group, Model TEC 120) that has better detection efficiency for the photodetector and helps with trapping smaller objects (due to the favorable wavelength-dependent scaling).
Figure 1.
(a) Schematic drawing of the nanoscale double-hole optical trap. (b) An enlargement of the red circle in part (a), showing details of the composition of the sample in the microfluidic chip, the setup of the oil immersion microscope objective, and the condenser microscope objective. (c) Photograph of the working setup. Abbreviations used: LD = laser diode; SMF = single-mode fiber; ODF = optical density filter; HWP = half-wave plate; BE = beam expander; MR = mirror; MO = microscope objective; OI MO = oil immersion microscope objective; APD = avalanche photodetector.
Figure 1b) shows the microfluidic assembly. The fabrication process is given in the supplementary information (Fig. S1). Details of the fabrication are also given in the supplementary information. An image of completed chip is shown in Figure 1c). The resultant channel dimensions were 65 μm by 800 μm. The solution was flown into the channel using a Fusion Syringe Pump (Model Fusion200) which controls the flow rate.
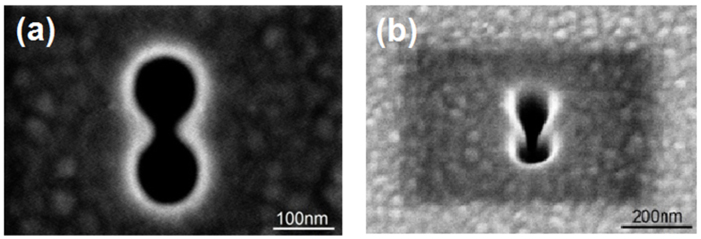
Figure 2 shows scanning electron microscope (SEM) images of a double nanohole, (a) normal to the surface and (b) at 35° from vertical, which were taken by a Hitachi S-4800 FESEM. The separation of the two sharp tips is measured to be 25 nm that according to our last works is the best separation tip for trapping of 20 nm particle (within fabrication tolerances)40. The double nanohole was milled in a commercially available 100 nm thick Au film on glass substrate with a 2 nm Ti adhesive layer (EMF Corp.) by a focussed ion beam system (Hitachi FB-2100 FIB).
Figure 2. SEM image of the double nano-hole in Au film, taken (a) normal to the surface and (b) at 35°.
Results
In this work, we create a microfluidic environment that is integrated into our nanoaperture trapping setup, and we characterize the ability to trap nanoparticles against fluid flow for varying flow rates. We find that stable trapping can be achieved for 20 nm polystyrene particles (Thermo Scientific, Cat No. 3020A, Normal diameter 20 nm) for flow rates exceeding 10 μl/min at powers below 10 mW (corresponding to 0.5 μm/s flow velocities at the trapping surface).
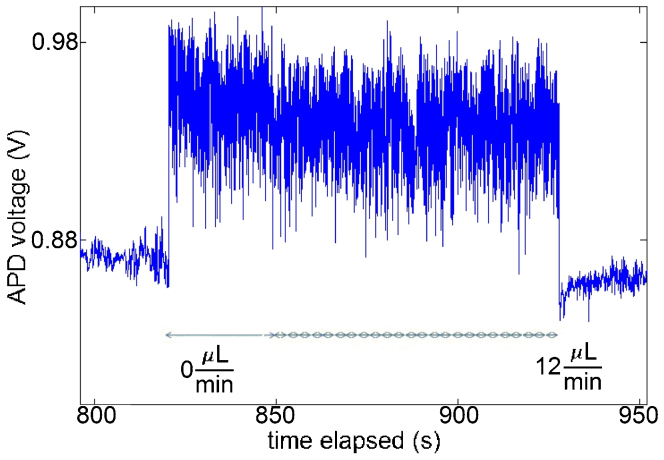
Figure 3 shows the time evolution of the transmitted optical power through a double nanohole for 10 mW of incident power, using 20 nm polystyrene spheres, while the solution flow-rate was increased in 0.5 μL/min increments after trapping (shown by the step at 825 seconds). This figure shows that the trapped nanoparticle is released at a flow rate 12 μL/min. The measurements were repeated on different days with freshly made nanosphere suspensions each time and the data was repeatable for all types of holes and all nanospheres used. Moreover, this event was not observed when pure water without nanospheres was used. We have seen no evidence of heating effects in our experiments. Previously, we trapped the protein BSA with similar laser intensities41; and since that protein denatures irreversibly at 50°C and we did not see such denaturing, we believe that the heating is minimal.
Figure 3. Optical transmission through a double-hole with 25 nm tip separation and with 10 mW of incident power.
This figure shows trapping and releasing of a 20 nm polystyrene nanosphere with increasing flow after trapping was achieved. Double-arrows show 0.5 μL/min increments in the flow rate.
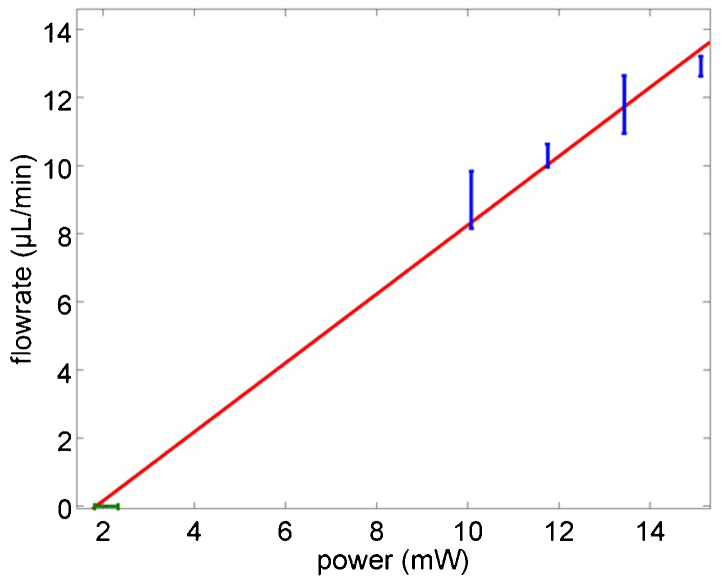
Figure 4 shows the dependence of the incident optical power and the flow-rate for which the trapped particle was released. The vertical error bars are standard deviations of the flow-rates for multiple trap and release events (greater than four events for each power). As an additional experiment, shown by the green data point in Figure 4, we first trapped a particle without flow and then reduced the power slowly until the particle was released. The horizontal error bar is the standard deviation of the power at which a trapped particle with high power has been released by decreasing the incident optical power without flow. With lower powers, the time to trap increases, as expected from the Arrhenius behaviour40. For example, at 10 mW it took several hours to trap, and at 13.5 mW it takes 10 minutes. The straight line is a linear fit of all the data which has a slope of 1 μl/(min×mW). So far, we have not seen any departure from the linear behavior, but this is limited by the laser intensity achievable in our present setup.
Figure 4. Critical flow rate as a function incident power, studied using 20 nm polystyrene spheres and a double-nanohole with 25 nm tip separation.
The error bars are the standard deviation of the data and the straight line is a linear fit to the data.
Discussion
To explain the linear relation between the power and flow rate at which the particle is released, we consider the interplay between the trapping force and Stokes' drag. The trapping force scales linearly with power. This is true for perturbative gradient force, but more generally true from Maxwell stress tensor (MST) analysis39. The Stokes' drag force scales linearly with flow rate. Therefore, it is expected that the critical flow rate where flow overcomes the trapping force will scale linearly with power as well, as found in the experiments.
The finite power is required to hold the particle in a stationary environment. This is because of the Brownian motion of the particle. We can estimate the optical trapping force based on the Stokes' drag force required to release the particle. For a flow rate of 10 μl/min, the fluid velocity at the centre of the channel is 3.2 mm/s. Considering a laminar flow, for a high aspect ratio rectangular microfluidic channel, the velocity close to the edge of the channel can be approximated as: v = 6Q z/(h2 w), where Q is the flow rate, z is the distance from the edge of the channel near the center, h is the height of the channel and w is the width44. Using this equation, we estimate that the velocity at the center of the particle (10 nm from the edge) is 5.9 mm/s, which in turn gives a Stokes' drag force on the 20 nm particle of 2 fN, including corrections for the adjacent boundary.
For applications where the purpose is interaction of the trapped particles with other nanoparticles, for example, to study protein-protein interactions, this flow capability is useful. Under the present conditions, we have shown that it is possible to deliver a secondary particle to the trapping site in about thirty seconds, with a flow rate of 1.7 μl/min, which for a 20 nm trapped particle allows for stable trapping for powers of 3.5 mW and larger. Even though the flow rate is lower at the surface, the additional particles can be delivered predominantly in the middle of the channel and then subsequently diffuse to the surface.
In summary, we have created a microfluidic environment integrated in our optical trapping setup to study trapping of nanoparticles against fluid flow. It was demonstrated that the flow-rate in which the trapped particle is released depends linearly on the incident optical power. In the future, we hope to extend these studies to particle-particle interactions and optical trapping at the ends of fiber probes. In particular, we have begun to integrate double nanohole structures at the end of an optical fiber, coated with gold. This approach is similar to our past work42, except that a double nanohole will be used.
Author Contributions
A.Z.O. and J.B. performed the nanofabrication and microfluidic integration. The experiments were conceived and designed by A.Z.O. and R.G. The experiments were carried out by A.Z.O. A.Z.O. and R.G. co-wrote the manuscript.
Supplementary Material
Flow-dependent optical trapping of 20 nm polystyrene nanospheres using a double-nanohole in a gold film
Acknowledgments
The authors acknowledge funding from the NSERC Discovery Grant program (Canada).
References
- Ashkin A., Dziedzic J. M. & Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature 330, 769–771 (1987). [DOI] [PubMed] [Google Scholar]
- Curto A. G. et al. Unidirectional Emission of a Quantum Dot Coupled to a Nanoantenna. Science 329, 930–933 (2010). [DOI] [PubMed] [Google Scholar]
- Pattantyus-Abraham A. G. et al. Site-Selective Optical Coupling of PbSe Nanocrystals to Si-Based Photonic Crystal Microcavities. Nano Letters 9, 2849–2854 (2009). [DOI] [PubMed] [Google Scholar]
- Anker J. N. et al. Biosensing with plasmonic nanosensors. Nat Mater 7, 442–453 (2008). [DOI] [PubMed] [Google Scholar]
- Ferreira J. et al. Attomolar Protein Detection Using in-Hole Surface Plasmon Resonance. Journal of the American Chemical Society 131, 436–437 (2008). [DOI] [PubMed] [Google Scholar]
- Romanuik S. F. et al. Sensing of antibodies secreted by microfluidically trapped cells via extraordinary optical transmission through nanohole arrays. IEEE Sensors 11 (2011). [Google Scholar]
- Seisenberger G. et al. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 294, 1929 (2001). [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Bjorkholm J. E. & Chu S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 11, 288 (1986). [DOI] [PubMed] [Google Scholar]
- Ashkin A. & Dziedzic J. M. Optical Trapping and Manipulation of Viruses and Bacteria. Science 235, 1517 (1987). [DOI] [PubMed] [Google Scholar]
- Dholakia K., Reece P. & Gu M. Optical micromanipulation. Chem. Soc. Rev. 37, 42 (2008). [DOI] [PubMed] [Google Scholar]
- Kwak E. S. et al. Optical trapping with integrated near-Field apertures. J. Phys. Chem. B 108, 13607 (2004). [Google Scholar]
- Maragò O. M. et al. Femtonewton Force Sensing with Optically Trapped Nanotubes. Nano Lett. 8, 3211 (2008). [DOI] [PubMed] [Google Scholar]
- Reece P. J. et al. Characterisation of semiconductor nanowires based on optical tweezers. Nano Lett. 11, 2375 (2011). [DOI] [PubMed] [Google Scholar]
- Yang A. H. J. et al. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 457, 71 (2009). [DOI] [PubMed] [Google Scholar]
- Pelton M. et al. Optical trapping and alignment of single gold nanorods by using plasmon resonances. Opt. Lett. 31, 2075 (2006). [DOI] [PubMed] [Google Scholar]
- Selhuber-Unkel C., Zins I., Schubert O., Sönnichsen C. & Oddershede L. B. Quantitative optical trapping of single gold nanorods. Nano Lett. 8, 2998 (2008). [DOI] [PubMed] [Google Scholar]
- Juan M. L., Righini M. & Quidant R. Plasmon nano-optical tweezers. Nature Photonics 5, 349–356 (2011). [Google Scholar]
- Erickson D., Serey X., Chen Y.-F. & Mandal S. Nanomanipulation using near field photonics. Lab on a Chip 11, 995–1009 (2011). [DOI] [PubMed] [Google Scholar]
- Miao X., Wilson B. K., Pun S. H. & Lin L. Y. Optical manipulation of micron/submicron sized particles and biomolecules through plasmonics. Opt. Express 16, 13517–13525 (2008). [DOI] [PubMed] [Google Scholar]
- Kim J. Joining plasmonics with microfluidics: from convenience to inevitability. Lab on a Chip 12, 3611–3623 (2012). [DOI] [PubMed] [Google Scholar]
- Wang M. D., Yin H., Landick R., Gelles J. & Block S. M. Stretching DNA with optical tweezers. Biophys. J. 72, 1335 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbondanzieri E. A., Greenleaf W. J., Shaevitz J. W., Landick R. & Block S. M. Direct observation of base-pair stepping by RNA polymerase. Nature 438, 460 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M. & Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113 (1994). [DOI] [PubMed] [Google Scholar]
- Chen C. et al. Enhanced Optical Trapping and Arrangement of Nano-Objects in a Plasmonic Nanocavity. Nano Lett. 12, 125–132 (2012). [DOI] [PubMed] [Google Scholar]
- Ashok P. C. & Dholakia K. Optical trapping for analytical biotechnology. Current Opinion in Biotechnology 23, 16–21 (2012). [DOI] [PubMed] [Google Scholar]
- Huang L. & Martin O. J. F. Reversal of the optical force in a plasmonic trap. Opt. Lett. 33, 3001–3003 (2008). [DOI] [PubMed] [Google Scholar]
- Nieto-Vesperinas M., Chaumet P. C. & Rahmani A. Near-field photonic forces. Phil. Trans. Math. Phys. Eng. Sci. 362, 719–737 (2004). [DOI] [PubMed] [Google Scholar]
- Okamoto K. & Kawata S. Radiation force exerted on subwavelength particles near a nanoaperture. Phys. Rev. Lett. 83, 4534–4537 (1999). [Google Scholar]
- Novotny L., Bian R. X. & Xie X. S. Theory of nanometric optical tweezers. Phys. Rev. Lett. 79, 645–648 (1997). [Google Scholar]
- Yang A. H. J. et al. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 457, 71–75 (2009). [DOI] [PubMed] [Google Scholar]
- Righini M. et al. Nano-optical trapping of Rayleigh particles and Escherichia coli bacteria with resonant optical antennas. Nano Lett. 9, 3387–3391 (2009). [DOI] [PubMed] [Google Scholar]
- Ohlinger A., Nedev S., Lutich A. A. & Feldmann J. Optothermal Escape of Plasmonically Coupled Silver Nanoparticles from a Three-Dimensional Optical Trap. Nano Letters 11, 1770–1774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner J. S., Baffou G., McCloskey D. & Quidant R. Plasmon-Assisted Optofluidics. ACS Nano 5, 5457–5462 (2011). [DOI] [PubMed] [Google Scholar]
- Righini M., Zelenina A. S., Girard C. & Quidant R. Parallel and selective trapping in a patterned plasmonic landscape. Nat Phys 3, 477–480 (2007). [Google Scholar]
- Righini M., Volpe G., Girard C., Petrov D. & Quidant R. Surface Plasmon Optical Tweezers: Tunable Optical Manipulation in the Femtonewton Range. Physical Review Letters 100, 186804 (2008). [DOI] [PubMed] [Google Scholar]
- Yang A. H. J., Lerdsuchatawanich T. & Erickson D. Forces and Transport Velocities for a Particle in a Slotted Waveguide. Nano Lett. 9, 1182 (2009). [DOI] [PubMed] [Google Scholar]
- Hu J., Lin S., Kimerling L. C. & Crozier K. Optical trapping of dielectric nanoparticles in resonant cavities. Phys. Rev. A 82, 053819 (2010). [Google Scholar]
- Barth M. & Benson O. Manipulation of dielectric particles using photonic crystal cavities. Appl. Phys. Lett. 89, 253114 (2006). [Google Scholar]
- Juan M. L., Gordon R., Pang Y., Eftekhari F. & Quidant R. Self-induced back-action optical trapping of dielectric nanoparticles. Nat. Phys. 5, 915 (2009). [Google Scholar]
- Pang Y. & Gordon R. Optical Trapping of 12 nm Dielectric Spheres Using Double-Nanoholes in a Gold Film. Nano Letters 11, 3763 (2011). [DOI] [PubMed] [Google Scholar]
- Pang Y. & Gordon R. Optical Trapping of a Single Protein. Nano Letters 12, 402–406 (2012). [DOI] [PubMed] [Google Scholar]
- Neumann L. et al. Extraordinary optical transmission brightens near-field fiber probe. Nano Lett. 11, 355 (2011). [DOI] [PubMed] [Google Scholar]
- Weber-Bargioni A. et al. Hyperspectral nanoscale imaging on dielectric substrates with coaxial optical antenna scan probes. Nano Lett. 11, 1201 (2011). [DOI] [PubMed] [Google Scholar]
- Bruus H. Theoretical Microfluidics Technical University Denmark, 3rd Edition (2004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow-dependent optical trapping of 20 nm polystyrene nanospheres using a double-nanohole in a gold film