Abstract
Metabolism is involved directly or indirectly in all processes conducted in living cells. The brain, popularly viewed as a neuronal–glial complex, gets most of its energy from the oxygen-dependent metabolism of glucose, and the mitochondrial pyruvate dehydrogenase complex (PDC) plays a key regulatory role during the oxidation of glucose. Pyruvate dehydrogenase kinase (also called PDC kinase or PDK) is a kinase that regulates glucose metabolism by switching off PDC. Four isoforms of PDKs with tissue specific activities have been identified. The metabolisms of neurons and glial cells, especially, those of astroglial cells, are interrelated, and these cells function in an integrated fashion. The energetic coupling between neuronal and astroglial cells is essential to meet the energy requirements of the brain in an efficient way. Accumulating evidence suggests that alterations in the PDKs and/or neuron-astroglia metabolic interactions are associated with the development of several neurological disorders. Here, the authors review the results of recent research efforts that have shed light on the functions of PDKs in the nervous system, particularly on neuron-glia metabolic interactions and neuro-metabolic disorders.
Keywords: Aerobic glycolysis, neuro-metabolic disorders, neuronal-glial interaction, oxidative phosphorylation, pyruvate dehydrogenase complex, pyruvate dehydrogenase kinase.
1. INTRODUCTION
Pyruvate dehydrogenase complex (PDC) having a predominant role in the regulation of mammalian metabolism represents the point-of-no-return regarding the utilization of carbohydrate, plays a leading role in maintaining glucose homoeostasis and is immensely involved in the metabolic pathway required for energy production and movement [1-3]. PDC is entirely nuclear encoded and consists of multiple copies of three structurally distinct, but functionally interdependent, enzymes (E1 through E3) [4]. Mammalian PDC is a huge complex with several components: pyruvate dehydrogenase (E1), dihidrolipoyl transactylase (E2), E3 binding domain (E3BP) and dihydrolipoyl dehydrogenase (E3) [4-8]. Pyruvate dehydrogenase (E1) is the first component enzyme of PDC.
Pyruvate is the end product of glycolysis in the cytosol. Glycolysis, a series of enzymatically catalyzed reactions occurring within cells, by which glucose and other sugars are broken down to yield lactic acid or pyruvic acid, releasing energy in the form of ATP, is well differentiated into two types on the basis of oxygen consumption during the reaction. The conversion of glucose to lactate in the presence of oxygen has been termed aerobic glycolysis, whereas the conversion of glucose to lactate in the absence of oxygen has been termed anaerobic glycolysis. Recently, it was hypothesized that, in the brain, both aerobic and anaerobic glycolysis terminate with the formation of lactate from pyruvate by lactate dehydrogenase (LDH). If this hypothesis is correct, the lactate must be the mitochondrial substrate for oxidative energy metabolism via its oxidation to pyruvate, possibly via mitochondrial LDH. A new finding supports the hypothesis that lactate, at least in an in vitro setting, is indeed the principal end product of neuronal aerobic glycolysis [9].
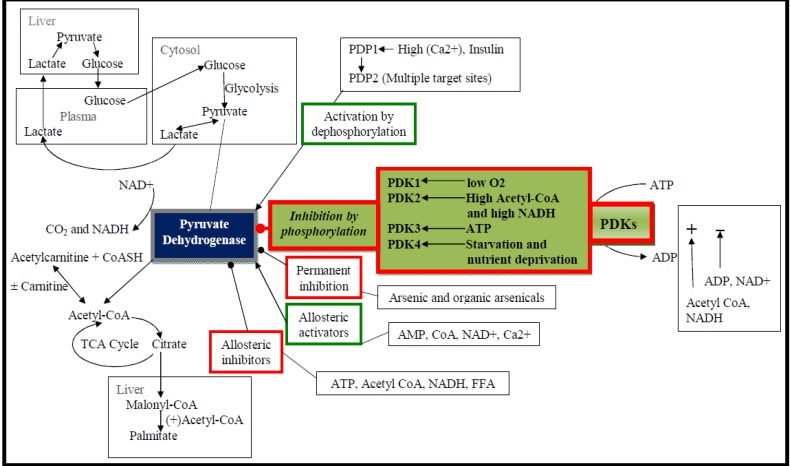
Under aerobic conditions, pyruvate enters the mitochondria, where it is transformed to acetyl-coenzyme A (acetyl-CoA), producing dihydronicotinamide adenine dinucleotide (NADH) and carbon dioxide. Acetyl-CoA subsequently enters the Krebs (citric acid) cycle, and thus, provides energy (adenosine triphosphate or ATP) to the cell [10]. This reaction is catalyzed by the enzyme-coenzyme complex PDC. PDC is located in the mitochondrial matrix space, and is responsible for irreversibly converting pyruvate into acetyl CoA, the primary fuel of the citric acid cycle (CAC). Reactions of the CAC and fatty acid oxidation are performed in the mitochondrial matrix. The mitochondrial PDC reaction connects glycolysis to oxidative metabolism to provide oxidative fuel for the generation of ATP. The PDC reaction also provides acetyl-CoA for fatty acids synthesis in fat synthesizing tissues when carbohydrate intake is excessive. PDC activity is under the control of pyruvate dehydrogenase kinases (also pyruvate dehydrogenase complex kinases, PDC kinases, or PDKs) 1 to 4, which phosphorylate the E1 subunit of PDC and suppress the catalysis of pyruvate to acetyl-CoA [11]. The activity of PDK is regulated by the concentrations of the metabolic products of pyruvate (NADH and acetyl-CoA). The PDH/PDK system acts as a key regulator of mitochondrial activity and plays an important role in the switching of the metabolism from oxidative phosphorylation to aerobic glycolysis that accompanies malignant transformation.
PDK isozymes together with the related branched chain dehydrogenase kinase comprise a novel family of serine kinases unrelated to cytoplasmic Ser/Thr/Tyr kinases [12-17]. PDKs are involved in the regulation of glucose oxidation, which could ultimately affect whole body glucose metabolism [18-20]. They consist of two subunits, that is, an α subunit with kinase activity, and a β, which is a regulatory subunit. PDK is a kinase enzyme that acts to inactivate PDC by phosphorylating it using ATP. PDK is involved in the regulation of the PDC activity (Fig. 1), which catalyzes the oxidative decarboxylation of pyruvate to acetyl CoA [10]. Although PDC is regulated by several mechanisms, including allosteric inhibition by acetyl CoA and NADH, covalent modification of PDC is extremely important for the long-term regulation of metabolic processes. PDKs phosphorylate PDC, whereas pyruvate dehydrogenase phosphatases (PDPs) catalyze the reverse reaction. PDC is dephosphorylated and active in the well-fed state to facilitate the oxidation of carbohydrates, but PDC is phosphorylated and inactive in the starved state to conserve substrates required for gluconeogenesis [21]. When carbohydrate stores are reduced in mammals, PDC activity is down-regulated to limit the consumption of glucose via oxidative phosphorylation in tissues that can use fatty acids or ketone bodies, such as, heart and skeletal muscle. The important exception is neuronal tissue, which processes glucose almost exclusively for ATP production.
Fig. (1).
The foremost tasks of PDK and PDP in the regulation of PDC and related metabolic processes. PDC is a large multi-enzyme complex located within the matrix compartment of mitochondria, and links glycolysis to the tricarboxylic acid cycle by catalyzing the irreversible oxidative decarboxylation of pyruvate, which leads to the generation of CO2, NADH, and acetyl-CoA. PDC activity is regulated by reversible phosphorylation. PDK inactivates PDC by phosphorylation. Conversely, PDP activates PDC by dephosphorylation. The regulation of PDC is linked to the metabolisms of glucose and fatty acid. PDC, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase; NADH, nicotinamide adenine dinucleotide hydride; FFA, free fatty acids; PDP, pyruvate dehydrogenase phosphatase; PEPCK; phosphoenolpyruvate carboxykinase; TCA, tricarboxylic acid cycle.
Hitherto, four tissue-specific isoforms (PDK 1–4) of PDK have been identified in the mammalian mitochondria [22]. PDK1 has been found in the heart [23, 24], pancreatic islets [25], and skeletal muscles [26]. PDK2 is ubiquitously expressed [27], whereas PDK3 has only been detected in the testes, kidneys, and brain [27], and PDK4 in the heart, skeletal muscles, liver, kidneys, brain, and pancreatic islets [23, 25, 26, 28]. PDKs phosphorylate three serine sites on PDC E1α, and whereas PDKs can phosphorylate sites 1 and 2, PDK1 uniquely phosphorylates site 3 [23, 25-27, 29, 30]. In rodents, there are two isoenzymic forms of PDK, which share up to 70% amino acid identity [31], and are designated PDK1 and PDK2. PDK4 was recently shown to have higher kinase activity that the other three PDK isoforms, regardless of the presence of L2 or the E2p/E3BP core [32]. Both isoenzymes, obtained as recombinant proteins, were found to be able of catalyze the phosphorylation and inactivation of PDC [31, 33]. Each PDK isoform has different specific activities and different sensitivities to pyruvate and ADP, which allows for individual responses to changing metabolic demands by targeting specific isoforms for upregulation [19, 34]. The existence of multiple isoenzymes of PDK in mammalian tissues suggests that their functionalities differ in particular tissues. Furthermore, the fraction of active PDC is reduced by the activities of dedicated PDK isoforms [35].
According to a recent report on protein expression in rat brain, PDK isoenzyme mRNAs are highly expressed in the cerebral cortex, hippocampus, amygdala, thalamus and other brain regions that could play important roles in regulating PDHE1α1 phosphorylation/dephosphorylation cycles and its activity in these regions under physiological conditions. In addition, PDK2 is the most abundant isoenzyme in the rat brain under physiological conditions, with PDK4 rarely expressed. The factors accounting for the regional heterogeneity of the isoforms and balance between phosphorylation and phosphatase activities remain unknown [36].
2. PDKs GOVERN THE ACTIVITY OF PDC
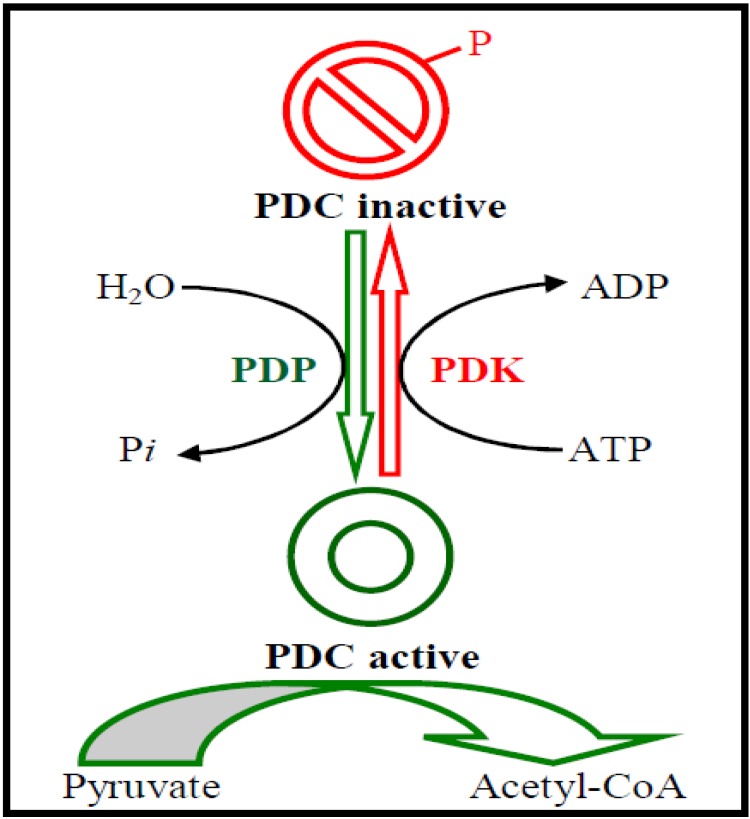
PDK participates in the regulation of PDC in which PDH is the first component. PDK can phosphorylate a serine residue on PDH at three possible sites. Phosphorylation at site 1 nearly completely deactivates the enzyme while phosphorylation at sites 2 and 3 makes only a small contribution to the complex inactivation. Therefore, phosphorylation at site 1 is responsible for PDH deactivation. PDK inhibition by pyruvate facilitates PDH activation, favoring glucose oxidation and malonyl-CoA formation: the latter suppresses long-chain fatty acid (LCFA) oxidation. PDK activation by high mitochondrial acetyl-CoA/CoA and NADH/NAD(+) concentration ratios that reflect high rates of LCFA oxidation causes blockade of glucose oxidation [18, 37-39]. On the other hand, PDP removes the phosphate groups on PDH and reactivates the enzyme (Fig. 2). To satisfy the discrete tissue-specific roles that PDC must fulfill to manage fuel consumption and storage, a set of dedicated regulatory enzymes provide highly adaptable control of the fraction of active PDC (PDCa) [11, 20, 40-48]. Four PDK isozymes [12, 15, 44, 47] govern the activity of PDC [35], and short-term and long-term mechanisms act to alter the activities and levels of PDK to manage the amount required for the storage of fuels [11, 20, 40-47]. It is reported that PDK is the effector that ultimately conveys the inhibitory signal from phosphorylated c-Jun-N-terminal kinase (pJNK) to PDH. In other words, it may be inferred that increased phosphorylated JNK association to mitochondria may up-regulate PDK activity, thus causing increased phosphorylation (and inhibition) of PDC [49]. The mechanism on how JNK may be modulating PDK remains unclear. Other signaling pathways may also contribute to increased PDK2 expression.
Fig. (2).
Regulation of PDC by phosphorylation-dephosphorylation. PDC catalyzes the oxidative decarboxylation of pyruvate, to form acetyl-CoA. PDKs catalyze the phosphorylations of serine residues of E1 of PDC, inhibiting the complex, whereas PDPs reverse this inhibition via dephosphorylation. PDC, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase; PDP, pyruvate dehydrogenase phosphatase; ADP, adenosine diphosphate; ATP, adenosine triphosphate.
3. ACTIVATION AND INHIBITION OF PDKs
PDKs are activated by ATP, NADH and acetyl-CoA. They are inhibited by ADP, NAD+, CoA-SH and pyruvate [19]. PDK activation involves interaction with the E2 subunits of PDC to sense changes in the oxidation state and acetylation of lipoamide caused by NADH and acetyl-CoA. During starvation, PDK levels increase in most tissues, including the skeletal muscles, via increased gene transcription [21, 50, 51]. Under the same conditions, the amount of PDP decreases, which prevents muscles and other tissues from catabolizing glucose and gluconeogenesis precursors. The metabolism then shifts toward fat utilization [39]. Muscle protein and the supply of gluconeogenesis precursors is minimized sparing available glucose for use by the brain. Furthermore, PDC levels are emerging as important biomarkers in acute injury and neurodegeneration. In the mouse model of AD, mitochondrial bioenergetic deficits, including a decrease in PDC level and activity, were found to precede the pathological manifestation of the disease [52].
The tightly-regulated balance existing between PDK and PDP isoenzyme expression is disrupted following traumatic brain injury (TBI), which may, in turn, impair or shutdown PDC-regulated glucose metabolism. Brain PDK2 and PDP1 isoenzymes could play a critical role in the tight control of the PDHE1α1 phosphorylation/dephosphorylation cycle and activity under normal conditions. The rapid and divergent changes between PDK and PDP isoenzyme expression could render PDHE1α1 hyperphosphorylated and inactivated in the post-injury period, thus blocking PDC-regulated glucose metabolism and ATP production in TBI [36].
Glycolytic inhibitors are currently the subject of intense research in order to explore their therapeutic potential [53]. Currently, there is considerable interest in the development of specific PDK inhibitors as potential treatments for cancer, diabetes, and brain disorders. Dichloroacetate (DCA), a small-molecule inhibitor of mitochondrial PDK, activates PDH by inhibition of PDK in a dose-dependent fashion in vitro [54] resulting in increased delivery of pyruvate into the mitochondria. DCA down-regulates glycolysis in vitro and in vivo, and has substantial therapeutic benefit in many types of cancers [55, 56] deserving further investigation to test its therapeutic potential in brain disorders.
Regulation of PDK2 and PDK4 expression by insulin is physiologically important. PDK2 and PDK4 are both increased in conditions in which insulin levels are low (e.g., starvation and diabetes). It is observed that glucocorticoids, free fatty acids (FFAs), and insulin play important roles in setting the level of PDK expression. This level in turn determines the phosphorylation state and therefore the activation state of PDC. Thus, the inactivation of PDC that occurs in most of the major tissues of the body during starvation and diabetes is likely explained by the effects that a decrease in insulin and an increase in glucocorticoids and FFAs have on PDK4 expression under these conditions [51].
4. ROLE OF PDKs IN NEURON-GLIA METABOLIC COUPLING
Glial cells play a major role in brain metabolism, since they control the chemical composition of the fluid that surrounds neurons, including the levels of ions and nutrients. The structural conditions required and expressional patterns of different isomers of glucose transporter and monocarboxylate transporter have established the molecular foundation of the metabolic coupling between glia and neurons [57, 58]. The responses of the two main types of brain cells (neurons and astrocytes) to energy deprivation is a complex function of their capacity to produce ATP and the activities of various pathways involved in ion homeostasis [59]. Astrocyte processes are wrapped around synaptic contacts whereas their end-feet surround intraparenchymal capillaries and provide a cellular zone interposed between the bloodstream and other elements of the brain parenchyma. This latter structural feature has long been suggested to indicate that astrocytes participate in the transit of substances from the blood to other brain cells. The metabolisms of neurons and glial cells are interrelated, and these cells function in an integrated fashion. Several important enzymes, such as pyruvate carboxylase and glutamine synthetase, are only found in astrocytes [60, 61], and PDC plays a key role in the regulation of glucose oxidation. In order for glucose to be oxidized to CO2, the pyruvate formed during glycolysis must enter the TCA cycle, and this is accomplished via PDC in the mitochondria, as it controls the rate of pyruvate entry into the TCA cycle as acetyl coenzyme A (acetyl- CoA). PDH is inactivated by phosphorylation at its decarboxylase moiety by a tightly bound Mg2+/ATP-dependent protein kinase, and activated by dephosphorylation by a loosely bound Mg2+- and Ca2+-dependent phosphatase.
The control of PDHα phosphorylation is accomplished by a set of 4 different PDKs (PDK1-4) and 2 different PDPs (PDP1 and 2), which are all differentially expressed in mammalian tissues [27]. The regulation of PDC at the protein expression or activity levels contributes to the differential metabolic phenotype of neurons and astrocytes and to the directional shuttling of monocarboxylates between these cell types [62]. It has been demonstrated that all subunits of PDC are expressed in cultured astrocytes and neurons, but astrocytes express significantly higher immunoreactivities for all subunits than neurons [62]. These higher expressions of PDK2 and PDK4 in astrocytes are consistent with the higher PDHα phosphorylation status, lower PDC activity, and higher lactate production displayed by cultured astrocytes.
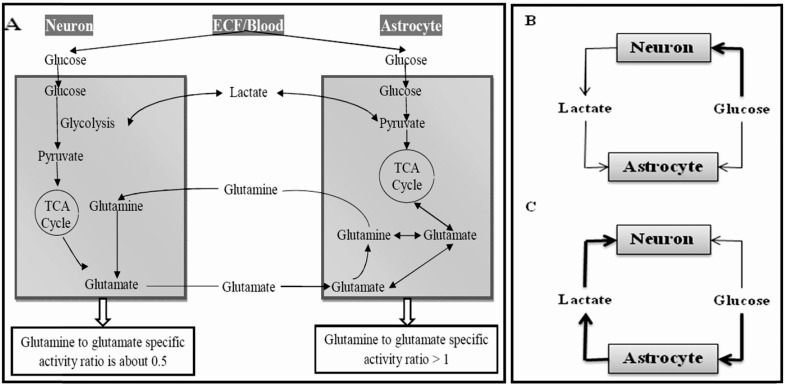
Astrocytes are central players in neurometabolic coupling and undergo plastic adaptations in parallel with adaptive mechanisms that characterize synaptic plasticity [63-65]. The basic mechanism involves glutamate-stimulated aerobic glycolysis, that is, the sodium-coupled reuptake of glutamate by astrocytes and the ensuing activation of Na-K-ATPase, which triggers glucose uptake and processing via glycolysis, and results in the release of lactate from astrocytes. More often than not, neurons, astrocytes and blood vessels function in a complex network, and this metabolic linkage as well as astrocytic support are obligatory for neuronal functioning. The interstitial compartment comprises the space between endothelial cells and astrocytic end feet (the basal lamina) and also the space between neuronal and astrocytic processes. These two regions are well connected, so that no significant concentration gradients are expected between these compartments for abundant molecules like glucose and lactate [66-68]. Fig. (3A) schematically represents a consensual model for glucose and lactate fluxes in the mammalian neuropil. After entering neurons and astrocytes, glucose is phosphorylated by hexokinase, a reaction that in the brain is irreversible due to lack of significant glucose-6-phosphatase activity [69, 70]. There are at present two extreme views of metabolic coupling in the brain, which accommodate the higher rate of oxygen consumption and ATP synthesis in neurons, but differ sharply in the identity of the lactate source during activity, and therefore in the direction of lactate flux. The default or conventional model of neurometabolic coupling states that neurons capture most of the glucose flux and release some lactate, which is taken up by astrocytes (Fig. 3B). This model advocates that astrocytes are more aerobic than neurons, i.e. their oxygen to glucose index (OGI) is higher. The alternative model is termed the “astrocyte-to-neuron-lactate shuttle hypothesis (ANLSH)” [71], which utters that astrocytes take most of the glucose and export it in the form of lactate, which is then taken up and oxidized by neurons (Fig. 3C). In this model, the more aerobic cell is the neuron. Several scholarly reviews that ponder the evidence for and against the two models of neurometabolic coupling have been published in recent years [68, 72-83]. There are also mathematical models, whose conclusions obtained under different assumptions have supported one model or the other [76, 84-88]. For example, some studies imply that neurons with basal activation show no net import of pyruvate or lactate [74], while Mangia and colleagues claim just the opposite of ANLSH, that is, neurons shuttle the lactate into astrocytes, and the only way this would work in reverse (i.e. astrocyte-to-neuron) is when the astrocytic glucose transport capacity is increased 12-fold [84]. According to a recent study by Genc et al., although the ANLSH is energetically more favorable for the neuron in terms of ATP produced, both under hypoxic and normoxic conditions, it is not the case of significant advantage for the astrocyte in the long term. Considering the fact that astrocytes are more resilient to hypoxia, they believe that rather than a "classical-or-ANLSH" choice for the cells, neurons and astrocytes can switch between one model or the other, depending on the energy requirements of the neuron, so as to maintain the survival of the neuron under hypoxic or glucose-and-oxygen-deprived conditions [89]. However, further investigations will surely prove useful in analyzing these models in more detail as well as in understanding such an energy demand-dependent switching [90].
Fig. (3).
Metabolic coupling between astrocytes and neurons. (A) Neurons and astrocytes are surrounded by the ECF, which contains glucose and lactate. The glucose pool feeds the cells and is replenished by blood-derived glucose. Lactate flows from and towards the cells, and is cleared into blood at a very low rate. (B) The conventional model of coupling proposes the major uptake of glucose by neurons, net production of lactate by neurons, and consumption of lactate by astrocytes. (C) The lactate shuttle model or ANLSH proposes the major uptake of glucose by astrocytes, net production of lactate by astrocytes, and consumption of lactate by neurons (In B and C, the thickness of the arrows represents the respective fluxes). TCA, tricarboxylic acid cycle; ECF, extracellular fluid; ANLSH, astrocyte-to-neuron-lactate shuttle hypothesis.
5. ALZHEIMER'S DISEASE AND PDKs
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. Dysfunction in glucose metabolism, bioenergetics, and mitochondrial function are consistent antecedents to the development of Alzheimer pathology [52, 91-93]. A decline in brain glucose metabolism and mitochondrial function can appear decades prior to the onset of histopathological and/or clinical features and thus may serve as a biomarker of AD risk as well as a therapeutic target [94-97]. Oxidative stress and synaptic damage have been implicated in AD pathogenesis [98-103]. It is well documented that oxidative damage to mitochondrial membranes and proteins impairs mitochondrial oxidative phosphorylation efficiency and results in increased electron leak, increased H2O2 levels and higher oxidative stress [104, 105]. Key enzymes involved in mitochondrial bioenergetics, such as PDH and α-ketoglutarate dehydrogenase (αKGDH), are often the targets of oxidative modifications. This leads to decease the enzyme activity, decrease the efficiency of the mitochondrial electron transport, and increase the production of free radicals [106, 107]. In parallel with the decline in glucose metabolism in AD, there is a generalized shift away from glucose-derived energy production, which is associated with a decrease in the expression of glycolytic enzymes coupled to a decrease in the activity of the PDC [108]. Alterations in the brain metabolic profile in AD are further evidenced by concomitant activation of compensatory pathways that promote the usage of alternative substrates, such as ketone bodies, to compensate for the decline in glucose-driven ATP generation. Potentiation of mitochondrial bioenergetics and enhancement of brain glucose metabolism are expected to prevent the decline in brain glucose metabolism, promote healthy aging, and therefore prevent AD. Interestingly, PDH is an important candidate within this category whose activity can be upregulated by inhibiting the PDK. Brain lipid peroxidation and decreased brain glucose utilization are characteristics of this neurodegenerative disease [109]. Acrolein, a byproduct of lipid peroxidation that accumulates within the brain in the course of AD, decreases the PDC activity. Specifically, acrolein binds lipoic acid, a component of both PDC and αKGDH [110]. Inactivation of PDC by acrolein or other mechanisms may be at least partially responsible for mitochondrial dysfunction and impaired cerebral energy metabolism associated with AD [110-112].
6. BRAIN AGING AND PDKs
Whole body glucose metabolism is suppressed by the aging process. It has been reported that energy metabolism appears to decline in the brain of aging animals and this decline may be more pronounced in certain brain regions. A study focused on the presence of different level of mRNAs for PDK isoenzymes in rat brain regions has inferred that the level of PDK1 mRNA is relatively high in the cerebellum and cerebral cortex compared with the medulla oblongata and hippocampus. Aging decreases PDK1 and PDK2 mRNAs levels in the cerebellum and increases PDK2 mRNA levels in the hippocampus and cerebral cortex, whereas PDK4 mRNA expression is unaffected by aging. These results provide evidence of differences in the regional abundances of the mRNAs of PDK isoenzymes in the rat brain and that their levels are affected by aging [113].
The decrease in the neurological activities during normal brain aging has been found to be related to mitochondrial dysfunction [114]. Interest in the association between mitochondrial dysfunction and the pathobiology of aging and age-related disorders was kindled when the free radical theory of aging was posited some six decades ago. Aging and neurodegeneration are common outcomes of mitochondrial dysfunction, and are associated with a decrease in PDH activity caused by phosphorylation of its E (1α) subunit. The phosphorylation of PDH is likely to be mediated by PDK, the protein levels, and the activity, which increases with age. The age-dependent decrease and increase in ATP production and lactate accumulation, respectively, in brain tissue appear to represent a shift from aerobic glycolysis (mitochondrial PDH-dependent) to anaerobic glycolysis (cytosolic LDH-dependent). The mechanistic implications of this shift are primarily based on the inactivation of the E1α subunit of mitochondrial matrix PDH with the consequent diminished metabolism of acetyl-CoA.
Neurons in general do not survive in vitro in the presence of glucose, but can survive in the presence of low-molecular-weight agents, such as pyruvate, which are supplied by glial cells. It appears that neurons utilize relatively little glucose in vivo, and that glial cells may supply substance(s) other than glucose, for example pyruvate, as the primary source of energy [115]. Reactions catalyzed by PDC functionally link glycolysis in the cytoplasm to oxidative phosphorylation (OXPHOS) in mitochondria [116]. PDC is central to the mitochondrial fuel metabolism, and thus, to organismal health and survival. Deficiencies of PDC, due to normal aging or the impositions of congenital or acquired diseases, display strikingly similar pathologies. Therefore, PDC and its regulatory kinases, PDKs, may be potential therapeutic targets for the treatment of multiple age-related disorders of nervous system [117].
7. GLIOBLASTOMA AND PDKs
Malignant gliomas or malignant glial neoplasms represent the most common type of primary brain tumor and constitute a spectrum of clinicopathologic entities from low- to high-grade malignancies. Glioblastoma, having highly expressed PDK2 [118], is the most frequent and malignant of gliomas [119]. Metabolic modulation may be a viable therapeutic approach in the treatment of glioblastoma [118]. A unique metabolic signature routinely observed in cancer cells is an energy-dependence-shift from normal oxidative phosphorylation to aerobic glycolysis. In cancer cells, however, pyruvate is converted to lactate regardless of the presence of oxygen. This may be in part due to the upregulation of the PDK activity and/or inhibition of PDH in cancer cells. High rates of aerobic glycolysis can promote the malignant transformation and survival of cancer cells [120]. Recent studies have shown that an anti-apoptotic mechanism in cancer cells is mediated by aerobic glycolysis, also known as the Warburg effect. One of the major regulators of aerobic glycolysis is PDK. Enhanced glycolysis and increased lactate production is a common property of invasive cancers, which may result in the suppression of apoptosis [55, 121].
The hypoxic tumor microenvironment elevates the levels of hypoxia inducible factor (HIF). HIF is able to attenuate mitochondrial respiration through the induction of PDK1, which in part accounts for the Warburg effect that describes the propensity for cancers to avidly take up glucose and convert it to lactate with the concurrent decrease in mitochondrial respiration [122]. Similarly, normoxic overexpression of HIF1α in most, if not all, human cancers stimulates glucose uptake, glycolysis, and PDK, which inhibits PDC [123-125]. Consequently, OXPHOS is suppressed and pyruvate accumulates, which stabilizes HIF1α, creating a positive feedback loop between HIF1α and glycolysis [120, 126], which can be reversed by the knockdown of PDK [127]. DCA inhibition of PDK frees up the mitochondrial gate-keeping enzyme PDH which is then able to convert pyruvate to acetyl-CoA and initiate normal oxidative phosphorylation via the Krebs cycle [55]. These findings suggest that the PDK/PDH axis may contribute to glioma cell metabolism and tumor growth.
8. BRAIN INJURY AND PDKs
While oxidative damage to cerebral energy metabolism is a critical contributor to delayed and necrotic neuronal death, oxidative stress is also a powerful initiator of apoptosis, which also contributes significantly to ischemic neural cell death. Mitochondria normally generate reactive oxygen species and contribute significantly to the elevated net production of these destructive agents during reperfusion. The mitochondria are both targets and sources of oxidative stress. This dual relationship is particularly evident in experimental paradigms modeling ischemic brain injury. Mitochondria play central roles in acute brain injury. Following TBI, the degree of mitochondrial injury or dysfunction can be an important determinant of cell survival or death [128]. Alterations in mitochondrial respiratory capacity have been demonstrated following TBI in adult animals and humans [129-131]. Dysregulated glucose metabolism and energy failure is a metabolic characteristic and an indicator of poor prognosis for patients with severe TBI [129, 132-137]. Recent studies suggest an important role for PDH in the altered brain energy metabolism following TBI [138-141]. Brain PDK and PDP isoenzymes appear to be very sensitive to the effects of traumatic injury: controlled cortical impact-induced TBI (CCI-TBI) and craniotomy induced a marked increase in PDK protein expression and a reduction in PDP protein expression, which would favor increased PDHE1α1 phosphorylation and inhibition with subsequent uncoupling of PDH-regulated glucose metabolism. Furthermore, all of the four PDK isoenzymes appear to be involved in altered PDHE1α1 phosphorylation and activity at different stages after TBI due to their distinct spatiotemporal induction patterns. Divergent changes in PDK and PDP isoenzyme expression were consistently observed at various times following CCI-TBI [36]. How the post-injury cortical spreading depression (CSD) is, however, correlated with PDK/PDP expression in TBI remains unanswered.
9. CONCLUSIONS AND FUTURE PERSPECTIVES
Efficient cerebral activity requires an adequate energy supply for each of the cellular mechanisms involved. The concept of metabolic coupling between neurons and glia, particularly astrocytes, in the context of maintaining energy metabolism homeostasis in the brain has been thought about for some time. Furthermore, astrocytic and neuronal metabolisms are closely coupled to fulfill the anabolic and energy needs associated with brain activation. Glial cells play a major role in brain metabolism, by controlling the chemical composition of the fluid that surrounds neurons. Structural relationships between astrocytes and other elements of nervous tissue are of particular relevance in this discussion of brain energy metabolism at the cellular level. Enhanced understanding of neuron–astrocyte metabolic interactions offers a potential means for developing novel therapeutic strategies for many neurological disorders associated with metabolic deficits. The dedicated PDK/PDP system responds to metabolite and hormone signals that vary the PDC activity in response to changes in nutritional states. PDKs are involved in the regulation of glucose oxidation, which could ultimately affect whole body glucose metabolism. The metabolisms of neurons and glial cells are interlinked, and these cells function in an integrated fashion. Furthermore, the regulation of PDC protein expression or activity contributes to the metabolic phenotypes of neurons and astrocytes and to the directional shuttling of monocarboxylates between these cells. Up to now, the astrocyte–neuron lactate shuttle hypothesis still promises to help unravel important cellular and molecular aspects of neurometabolic coupling. Astrocytes are central players in this coupling as they take up glucose from blood vessels for neurons. On the other hand, PDC activity is tightly regulated by the phosphorylation by four isoforms of PDKs. There is mounting evidence of crosstalk between astroglial and neuronal cells in terms of maintaining the energy requirements for neurological activities.
Metabolic disorders based on the PDH/PDK system cause defects in the synthesis, metabolism, transportation, and storage of biochemical compounds, which in turn may lead to tissue intoxication or energy deficiency in the brain resulting in life threatening neuro-metabolic disorders in addition to dysfunction of several other vital organs. The precise role played by PDKs in the nervous system, especially in glial-neuron interactions remains unclear, and much remains to be determined regarding how PDKs participate in signaling pathways and the metabolic control of glia-neuron interactions. Further investigations are required to verify the role played by PDKs in the nervous system, especially in glia-neuron interactions, and to investigate the behaviors of PDKs and their corresponding microRNAs. We are convinced that such studies will lead to a major breakthrough in neuroscience research and establish new dimensions for translational research into disorders of the nervous system.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation (NRF) grants funded by the Ministry of Education, Science and Technology (MEST) of Korean government (2011-0028240). This study was also supported by a grant of the Korean Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111345).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Dunford EC, Herbst EA, Jeoung NH, Gittings W, Inglis JG, Vandenboom R, LeBlanc PJ, Harris RA, Peters SJ. PDH activation during in vitro muscle contractions in PDH kinase 2 knockout mice: effect of PDH kinase 1 compensation. Am J Physiol Regul Integr Comp Physiol. 2011;300(6 ):R1487–1493. doi: 10.1152/ajpregu.00498.2010. [DOI] [PubMed] [Google Scholar]
- 2.Denton RM, Halestrap AP. Regulation of pyruvate metabolism in mammalian tissues. Essays Biochem. 1979;15:37–77. [PubMed] [Google Scholar]
- 3.Peters SJ, St Amand TA, Howlett RA, Heigenhauser GJ, Spriet LL. Human skeletal muscle pyruvate dehydrogenase kinase activity increases after a low-carbohydrate diet. Am J Physiol. 1998;275(6 Pt 1 ):E980–986. doi: 10.1152/ajpendo.1998.275.6.E980. [DOI] [PubMed] [Google Scholar]
- 4.Smolle M, Prior AE, Brown AE, Cooper A, Byron O, Lindsay JG. A new level of architectural complexity in the human pyruvate dehydrogenase complex. J Biol Chem. 2006;281(28 ):19772–19780. doi: 10.1074/jbc.M601140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanderson SJ, Miller C, Lindsay JG. Stoichiometry organisation and catalytic function of protein X of the pyruvate dehydrogenase complex from bovine heart. Eur J Biochem. 1996;236(1 ):68–77. doi: 10.1111/j.1432-1033.1996.00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265(16 ):8971–8974. [PubMed] [Google Scholar]
- 7.Maeng CY, Yazdi MA, Niu XD, Lee HY, Reed LJ. Expression purification and characterization of the dihydrolipoamide dehydrogenase-binding protein of the pyruvate dehydrogenase complex from Saccharomyces cerevisiae. Biochemistry. 1994;33(46 ):13801–13807. doi: 10.1021/bi00250a034. [DOI] [PubMed] [Google Scholar]
- 8.Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272(32 ):19746–19751. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- 9.Schurr A, Payne RS. Lactate not pyruvate is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience. 2007;147(3 ):613–619. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–259. doi: 10.1016/s0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 11.Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv. Enzyme Regul. 2001;41:269–288. doi: 10.1016/s0065-2571(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 12.Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270(48 ):28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- 13.Machius M, Chuang JL, Wynn RM, Tomchick DR, Chuang DT. Structure of rat BCKD kinase: nucleotide-induced domain communication in a mitochondrial protein kinase. Proc Natl Acad Sci U S A. 2001;98(20 ):11218–11223. doi: 10.1073/pnas.201220098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steussy CN, Popov KM, Bowker-Kinley MM, Sloan RB, Jr., Harris RA, Hamilton JA. Structure of pyruvate dehydrogenase kinase. Novel folding pattern for a serine protein kinase. J Biol Chem. 2001;276(40 ):37443–37450. doi: 10.1074/jbc.M104285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowles J, Scherer SW, Xi T, Majer M, Nickle DC, Rommens JM, Popov KM, Harris RA, Riebow NL, Xia J, Tsui LC, Bogardus C, Prochazka M. Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J Biol Chem. 1996;271(37 ):22376–22382. doi: 10.1074/jbc.271.37.22376. [DOI] [PubMed] [Google Scholar]
- 16.Bowker-Kinley M, Popov KM. Evidence that pyruvate dehydrogenase kinase belongs to the ATPase/kinase superfamily. Biochem J. 1999;344 (Pt 1 ):47–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn RM, Chuang JL, Cote CD, Chuang DT. Tetrameric assembly and conservation in the ATP-binding domain of rat branched-chain alpha-ketoacid dehydrogenase kinase. J Biol Chem. 2000;275(39 ):30512–30519. doi: 10.1074/jbc.M005075200. [DOI] [PubMed] [Google Scholar]
- 18.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112(3 ):139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 19.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5 ):E855–862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 20.Sugden MC, Bulmer K, Holness MJ. Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem Soc Trans. 2001;29(Pt 2 ):272–278. doi: 10.1042/0300-5127:0290272. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Wu P, Popov KM, Harris RA. Starvation and diabetes reduce the amount of pyruvate dehydrogenase phosphatase in rat heart and kidney. Diabetes. 2003;52(6 ):1371–1376. doi: 10.2337/diabetes.52.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popov KM, Hawes JW, Harris RA. Mitochondrial alpha-ketoacid dehydrogenase kinases: a new family of protein kinases. Adv Second Messenger Phosphoprotein Res. 1997;31:105–111. [PubMed] [Google Scholar]
- 23.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998;329(Pt 1 ):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di RM, Feng QT, Chang Z, Luan Q, Zhang YY, Huang J, Li XL, Yang ZZ. PDK1 plays a critical role in regulating cardiac function in mice and human. Chin Med J (Engl) 2010;123(17 ):2358–2363. [PubMed] [Google Scholar]
- 25.Sugden MC, Bulmer K, Augustine D, Holness MJ. Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-alpha: implications for glucose-stimulated insulin secretion. Diabetes. 2001;50(12 ):2729–2736. doi: 10.2337/diabetes.50.12.2729. [DOI] [PubMed] [Google Scholar]
- 26.Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D. Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J Appl Physiol. 2004;96(6 ):2082–2087. doi: 10.1152/japplphysiol.01318.2003. [DOI] [PubMed] [Google Scholar]
- 27.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1 ):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381(1 ):1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- 29.Teague WM, Pettit FH, Yeaman SJ, Reed LJ. Function of phosphorylation sites on pyruvate dehydrogenase. Biochem Biophys Res Commun. 1979;87(1 ):244–252. doi: 10.1016/0006-291x(79)91672-3. [DOI] [PubMed] [Google Scholar]
- 30.Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem. 2001;276(40 ):37223–37229. doi: 10.1074/jbc.M103069200. [DOI] [PubMed] [Google Scholar]
- 31.Popov KM, Kedishvili NY, Zhao Y, Gudi R, Harris RA. Molecular cloning of the p45 subunit of pyruvate dehydrogenase kinase. J Biol Chem. 1994;269(47 ):29720–29724. [PubMed] [Google Scholar]
- 32.Wynn RM, Kato M, Chuang JL, Tso SC, Li J, Chuang DT. Pyruvate dehydrogenase kinase-4 structures reveal a metastable open conformation fostering robust core-free basal activity. J Biol Chem. 2008;283(37 ):25305–25315. doi: 10.1074/jbc.M802249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popov KM, Kedishvili NY, Zhao Y, Shimomura Y, Crabb DW, Harris RA. Primary structure of pyruvate dehydrogenase kinase establishes a new family of eukaryotic protein kinases. J Biol Chem. 1993;268(35 ):26602–26606. [PubMed] [Google Scholar]
- 34.LeBlanc PJ, Peters SJ, Tunstall RJ, Cameron-Smith D, Heigenhauser GJ. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J Physiol. 2004;557(Pt 2 ):559–570. doi: 10.1113/jphysiol.2003.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem . 1998;273(28 ):17680–17688. doi: 10.1074/jbc.273.28.17680. [DOI] [PubMed] [Google Scholar]
- 36.Xing G, Ren M, O'Neill JT, Verma A, Watson WD. Controlled cortical impact injury and craniotomy result in divergent alterations of pyruvate metabolizing enzymes in rat brain. Exp Neurol. 2012;234(1 ):31–38. doi: 10.1016/j.expneurol.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31(Pt 6 ):1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 38.Tokmakov AA. Comparative homology modeling of pyruvate dehydrogenase kinase isozymes from Xenopus tropicalis reveals structural basis for their subfunctionalization. J Mol Model. 2011. [DOI] [PubMed]
- 39.Laurencikiene J, Stenson BM, Arvidsson Nordstrom E, Agustsson T, Langin D, Isaksson B, Permert J, Ryden M, Arner P. Evidence for an important role of CIDEA in human cancer cachexia. Cancer Res. 2008;68(22 ):9247–9254. doi: 10.1158/0008-5472.CAN-08-1343. [DOI] [PubMed] [Google Scholar]
- 40.Randle PJ. Fuel selection in animals. Biochem Soc Trans. 1986;14(5 ):799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- 41.Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4(14 ):3224–3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- 42.Sugden MC, Orfali KA, Fryer LG, Holness MJ, Priestman DA. Molecular mechanisms underlying the long-term impact of dietary fat to increase cardiac pyruvate dehydrogenase kinase: regulation by insulin cyclic AMP and pyruvate. J Mol Cell Cardiol. 1997;29(7 ):1867–1875. doi: 10.1006/jmcc.1997.0425. [DOI] [PubMed] [Google Scholar]
- 43.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14(4 ):263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2 ):217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 45.Priestman DA, Mistry SC, Halsall A, Randle PJ. Role of protein synthesis and of fatty acid metabolism in the longer-term regulation of pyruvate dehydrogenase kinase. Biochem J. 1994;300(Pt 3 ):659–664. doi: 10.1042/bj3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denton RM, McCormack JG, Rutter GA, Burnett P, Edgell NJ, Moule SK, Diggle TA. The hormonal regulation of pyruvate dehydrogenase complex. Adv Enzyme Regul. 1996;36:183–198. doi: 10.1016/0065-2571(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 47.Roche TE, Baker JC, Yan X, Hiromasa Y, Gong X, Peng T, Dong J, Turkan A, Kasten SA. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog. Nucleic Acid Res Mol Biol. 2001;70:33–75. doi: 10.1016/s0079-6603(01)70013-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Iwasaki Y, Zhao LF, Nishiyama M, Taguchi T, Tsugita M, Kambayashi M, Hashimoto K, Terada Y. Hormonal regulation of glycolytic enzyme gene and pyruvate dehydrogenase kinase/phosphatase gene transcription. Endocr J. 2009;56(8 ):1019–1030. doi: 10.1507/endocrj.k09e-178. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Lam PY, Han D, Cadenas E. Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 2009;583(7 ):1132–1140. doi: 10.1016/j.febslet.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holness MJ, Smith ND, Bulmer K, Hopkins T, Gibbons GF, Sugden MC. Evaluation of the role of peroxisome-proliferator-activated receptor alpha in the regulation of cardiac pyruvate dehydrogenase kinase 4 protein expression in response to starvation, high-fat feeding and hyperthyroidism. Biochem J. 2002;364(Pt 3 ):687–694. doi: 10.1042/BJ20011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands glucocorticoids and insulin. Diabetes. 2002;51(2 ):276–283. doi: 10.2337/diabetes.51.2.276. [DOI] [PubMed] [Google Scholar]
- 52.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(34 ):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34 ):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 54.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38(11 ):1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1 ):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE. 2007;2007(381 ):pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- 57.Balmaceda-Aguilera C, Cortes-Campos C, Cifuentes M, Peruzzo B, Mack L, Tapia JC, Oyarce K, Garcia MA, Nualart F. Glucose Transporter 1 and Monocarboxylate Transporters 1, 2, and 4 Localization within the Glial Cells of Shark Blood-Brain-Barriers. PLoS One. 2012;7(2 ):e32409. doi: 10.1371/journal.pone.0032409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumoto K, Akazawa S, Ishibashi M, Trocino RA, Matsuo H, Yamasaki H, Yamaguchi Y, Nagamatsu S, Nagataki S. Abundant expression of GLUT1 and GLUT3 in rat embryo during the early organogenesis period. Biochem. Biophys Res Commun. 1995;209(1 ):95–102. doi: 10.1006/bbrc.1995.1475. [DOI] [PubMed] [Google Scholar]
- 59.Silver IA, Deas J, Erecinska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience. 1997;78(2 ):589–601. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- 60.Yu AC, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41(5 ):1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195(4284 ):1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 62.Halim ND, McFate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia. 2010;58(10 ):1168–1176. doi: 10.1002/glia.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283(5401 ):496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 64.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exper Biol. 2006;209(Pt 12 ):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 65.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neuroscience. 2006;9(2 ):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 66.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3 ):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barros LF, Bittner CX, Loaiza A, Porras OH. A quantitative overview of glucose dynamics in the gliovascular unit. Glia. 2007;55(12 ):1222–1237. doi: 10.1002/glia.20375. [DOI] [PubMed] [Google Scholar]
- 68.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cerebral Blood Flow Metabol. 2007;27(11 ):1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dienel GA, Nelson T, Cruz NF, Jay T, Crane AM, Sokoloff L. Over-estimation of glucose-6-phosphatase activity in brain in vivo Apparent difference in rates of [2-3H]glucose and [U-14C]glucose utilization is due to contamination of precursor pool with 14C-labeled products and incomplete recovery of 14C-labeled metabolites. J Biol Chem. 1988;263(36 ):19697–19708. [PubMed] [Google Scholar]
- 70.Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623(2 ):208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- 71.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Pro Natl Acad Sci U S A. 1994;91(22 ):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mangia S, Giove F, Tkac I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Ugurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cerebral Blood Flow Metabol. 2009;29(3 ):441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hertz L, Dienel GA. Energy metabolism in the brain. Int Rev Neurobiol. 2002;51:1–102. doi: 10.1016/s0074-7742(02)51003-5. [DOI] [PubMed] [Google Scholar]
- 74.Gjedde A, Marrett S, Vafaee M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cerebral Blood Flow Metabol. 2002;22(1 ):1–14. doi: 10.1097/00004647-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(Pt 1 ):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Occhipinti R, Somersalo E, Calvetti D. Astrocytes as the glucose shunt for glutamatergic neurons at high activity: an in silico study. J Neurophysiol. 2009;101(5 ):2528–2538. doi: 10.1152/jn.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chih CP, Lipton P, Roberts EL Jr. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24(10 ):573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- 78.Bonvento G, Herard AS, Voutsinos-Porche B. The astrocyte--neuron lactate shuttle: a debated but still valuable hypothesis for brain imaging. J. Cerebral Blood Flow Metabol. 2005;25(10 ):1394–1399. doi: 10.1038/sj.jcbfm.9600127. [DOI] [PubMed] [Google Scholar]
- 79.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cerebral Blood Flow Metabol. 2005;25(1 ):2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 80.Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Internat. 2004;45(2-3 ):321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12 ):1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 82.Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia. 2007;55(12 ):1238–1250. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- 83.Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111(2 ):522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109 (Suppl 1 ):55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A. 2005;102(45 ):16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cerebral Blood Flow Metabol. 2006;26(7 ):865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 87.Aubert A, Costalat R. Compartmentalization of brain energy metabolism between glia and neurons: insights from mathematical modeling. Glia. 2007;55(12 ):1272–1279. doi: 10.1002/glia.20360. [DOI] [PubMed] [Google Scholar]
- 88.Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ. Response to 'comment on recent modeling studies of astrocyte-neuron metabolic interactions': much ado about nothing. J Cerebral Blood Flow Metabol. 2011;31(6 ):1346–1353. doi: 10.1038/jcbfm.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Genc S, Kurnaz IA, Ozilgen M. Astrocyte-neuron lactate shuttle may boost more ATP supply to the neuron under hypoxic conditions--in silico study supported by in vitro expression data. BMC Syst Biol. 2011;5:162. doi: 10.1186/1752-0509-5-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smallbone K, Simeonidis E, Swainston N, Mendes P. Towards a genome-scale kinetic model of cellular metabolism. BMC Syst Biol. 2010;4:6. doi: 10.1186/1752-0509-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10 ):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer's disease highlights common regional vulnerability across AD mouse models. Brain Res. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibson GE, Shi Q. A mitocentric view of Alzheimer's disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl2 ):S591–607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6 ):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2009;36(5 ):811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101(1 ):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5 ):676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9 ):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 99.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26(35 ):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19(14 ):2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 101.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8 ):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 102.DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5(4 ):417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 103.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594 ):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 104.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4 ):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 105.Reddy PH, Beal MF. Amyloid beta mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14(2 ):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park LC, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, Gibson GE. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J Neurochem. 1999;72(5 ):1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. [DOI] [PubMed] [Google Scholar]
- 107.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24(36 ):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blass JP. he mitochondrial spiral. An adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 109.Pratico D, Yao Y, Rokach J, Mayo M, Silverberg GG, McGuire D. Reduction of brain lipid peroxidation by CSF drainage in Alzheimer's disease patients. J Alzheimers Dis 385-389. 2004;6(4):385–389. doi: 10.3233/jad-2004-6405. discussion 443-389. [DOI] [PubMed] [Google Scholar]
- 110.Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox Res. 2003;5(7 ):515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- 111.Sun L, Luo C, Long J, Wei D, Liu J. Acrolein is a mitochondrial toxin: effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion. 2006;6(3 ):136–142. doi: 10.1016/j.mito.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Lovell MA, Xie C, Markesbery WR. Acrolein a product of lipid peroxidation inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radical Biol Med. 2000;29(8 ):714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 113.Nakai N, Obayashi M, Nagasaki M, Sato Y, Fujitsuka N, Yoshimura A, Miyazaki Y, Sugiyama S, Shimomura Y. The abundance of mRNAs for pyruvate dehydrogenase kinase isoenzymes in brain regions of young and aged rats. Life Sci. 2000;68(5 ):497–503. doi: 10.1016/s0024-3205(00)00947-4. [DOI] [PubMed] [Google Scholar]
- 114.Navarro A, Sanchez Del Pino MJ, Gomez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol. 2002;282(4 ):R985–992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 115.Katoh-Semba R, Keino H, Kashiwamata S. A possible contribution by glial cells to neuronal energy production: enzyme-histochemical studies in the developing rat cerebellum. Cell Tissue Res. 1988;252(1 ):133–139. doi: 10.1007/BF00213834. [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Bai L, Li J, Sun C, Zhao J, Cui C, Han K, Liu Y, Zhuo X, Wang T, Liu P, Fan F, Guan Y, Ma A. Proteomic analysis of mitochondria reveals a metabolic switch from fatty acid oxidation to glycolysis in the failing heart. Science in China Series C Life Sci. 2009;52(11 ):1003–1010. doi: 10.1007/s11427-009-0140-2. [DOI] [PubMed] [Google Scholar]
- 117.Stacpoole PW. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell. 2012. [DOI] [PubMed]
- 118.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2(31 ):31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 119.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5 ):1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26 ):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 121.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99(7 ):989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc. 2007. pp. 35–53. [DOI] [PubMed]
- 123.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3 ):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27(21 ):7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3 ):187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 126.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51 ):41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 127.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, Jeoung NH, Harris RA, Verma A. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283(33 ):22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36(4 ):347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- 129.Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93(5 ):815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- 130.Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14(1 ):23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 131.Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol Res. 1997;19(3 ):334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- 132.Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25(6 ):763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prins ML, Lee SM, Fujima LS, Hovda DA. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J Neurochem. 2004;90(3 ):666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- 134.Lifshitz J, Friberg H, Neumar RW, Raghupathi R, Welsh FA, Janmey P, Saatman KE, Wieloch T, Grady MS, McIntosh TK. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J Cereb Blood Flow Metab. 2003;23(2 ):219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- 135.Hillered L, Enblad P. Nonischemic energy metabolic crisis in acute brain injury. Crit Care Med. 2008;36(10 ):2952–2953. doi: 10.1097/CCM.0b013e3181872178. [DOI] [PubMed] [Google Scholar]
- 136.Hattori N, Huang SC, Wu HM, Yeh E, Glenn TC, Vespa PM, McArthur D, Phelps ME, Hovda DA, Bergsneider M. Correlation of regional metabolic rates of glucose with glasgow coma scale after traumatic brain injury. J Nucl Med. 2003;44(11 ):1709–1716. [PubMed] [Google Scholar]
- 137.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561(1 ):106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 138.Roche TE, Hiromasa Y. Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes heart ischemia and cancer. Cell Mol Life Sci. 2007;64(7-8 ):830–849. doi: 10.1007/s00018-007-6380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xing G, Ren M, Watson WD, O'Neill JT, Verma A. Traumatic brain injury-induced expression and phosphorylation of pyruvate dehydrogenase: a mechanism of dysregulated glucose metabolism. Neurosci Lett. 2009;454(1 ):38–42. doi: 10.1016/j.neulet.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 140.Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma. 2007;24(5 ):772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- 141.Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101(5 ):1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]