Summary
Purpose
Intracranial electroencephalography (EEG) is performed as part of an epilepsy surgery evaluation when noninvasive tests are incongruent or the putative seizure-onset zone is near eloquent cortex. Determining the seizure-onset zone using intracranial EEG has been conventionally based on identification of specific ictal patterns with visual inspection. High-frequency oscillations (HFOs, >80 Hz) have been recognized recently as highly correlated with the epileptogenic zone. However, HFOs can be difficult to detect because of their low amplitude. Therefore, the prevalence of ictal HFOs and their role in localization of epileptogenic zone on intracranial EEG are unknown.
Methods
We identified 48 patients who underwent surgical treatment after the surgical evaluation with intracranial EEG, and 44 patients met criteria for this retrospective study. Results were not used in surgical decision making. Intracranial EEG recordings were collected with a sampling rate of 2,000 Hz. Recordings were first inspected visually to determine ictal onset and then analyzed further with time-frequency analysis. Forty-one (93%) of 44 patients had ictal HFOs determined with time-frequency analysis of intracranial EEG.
Key Findings
Twenty-two (54%) of the 41 patients with ictal HFOs had complete resection of HFO regions, regardless of frequency bands. Complete resection of HFOs (n = 22) resulted in a seizure-free outcome in 18 (82%) of 22 patients, significantly higher than the seizure-free outcome with incomplete HFO resection (4/19, 21%).
Significance
Our study shows that ictal HFOs are commonly found with intracranial EEG in our population largely of children with cortical dysplasia, and have localizing value. The use of ictal HFOs may add more promising information compared to interictal HFOs because of the evidence of ictal propagation and followed by clinical aspect of seizures. Complete resection of HFOs is a favorable prognostic indicator for surgical outcome.
Keywords: High-frequency oscillations, Intracranial EEG, Time-frequency analysis, Surgical outcome, Nonlesional epilepsy
Twenty percent to 30% of all patients diagnosed with epilepsy will be medically intractable (Hauser & Hesdorfeer, 1990; Schuele & Luders, 2008). For patients with intractable focal epilepsy, the identification and resection of the epileptogenic zone, defined as “the minimum amount of cortex [that] must be resected, inactivated, or completely disconnected to produce seizure freedom” (Lüders et al., 2006), can be curative (Engel, 1996; Wiebe et al., 2001). In children with predominantly neocortical epilepsy, intracranial electroencephalography (ICEEG) is often used to further define the epileptogenic zone. With rapid digitization rates of ICEEG and high signal to noise ratio (SNR) observed in intracranial recordings compared to scalp EEG, it is possible to identify very fast oscillations after applying sufficient high-pass filtering (i.e., 80 Hz). These oscillations have been termed high-frequency oscillations (HFOs, >80 Hz) (Bragin et al., 1999a; Staba et al., 2004; Engel et al., 2009).
Differentiating between physiologic and epileptogenic HFOs is critical. Physiologic HFOs in the high gamma/low ripple range (70–150 Hz) have been observed in primary motor cortex (Huo et al., 2010, 2011): those in the ripple range (80–200 Hz) have been associated with memory consolidation (Buzsaki et al., 1992; Axmacher et al., 2008; Le Van Quyen et al., 2008), and much faster oscillations (>600 Hz) have been associated with somatosensory evoked responses (Curio et al., 1994; Hashimoto et al., 1996; Ozaki et al., 1998). Following Bragin et al., 1999a,b description of pathologic HFOs in the range of 200–600 Hz, there have been numerous reports regarding the association between frequency bandwidth and specific pathologies (Jacobs et al., 2008, 2009a,b; Kobayashi et al., 2009; Zijlmans et al., 2009; Jacobs et al., 2010; Zijlmans et al., 2011). It has been suggested that HFOs generated by the hippocampus tend to be more robustly identified and of faster frequency, including the fast ripple range, whereas neocortical structures tend to generate pathologic HFOs in the ripple range (Jacobs et al., 2008).
Recent studies have shown that resection of interictal HFO regions correlate with seizure freedom in children, suggesting that the majority of HFO discharges observed during ICEEG monitoring are epileptogenic (Jacobs et al., 2010; Wu et al., 2010). However, sometimes it is difficult to distinguish HFOs from artifact by visual inspection during the interictal state, especially after high-pass filtering (e.g., >80 Hz). The presence of HFOs during ictal onset has been increasingly recognized as well, but few studies report the usefulness of ictal HFO analysis compared to conventional visual analysis of ICEEG in the pediatric population (Bragin et al., 1999b; Akiyama et al., 2005, 2006; Jirsch et al., 2006; Le Van Quyen et al., 2006; Ochi et al., 2007; Staba et al., 2007; Urrestarazu et al., 2007; Jacobs et al., 2009a, 2010). We hypothesized that complete resection of cortex generating ictal HFOs is associated with a more favorable clinical outcome when compared to incomplete HFO resection.
Materials and Methods
Patient population
Patients were identified retrospectively and included in the study if they had ICEEG recording followed by surgical resection between September 2008 and December 2009. Information regarding HFO location was not available for surgical decision making. Exclusion criteria were the following: (1) patients who had ICEEG with no focal resection (hemispherectomy, but patients who first had a focal resection and then subsequently went to hemispherectomy were included) and (2) patients with <1 year postsurgical follow-up. The placement of intracranial electrodes was guided by standard presurgical evaluation, including prolonged scalp video-EEG monitoring and multimodality brain imaging studies as described previously (Seo et al., 2011). Resections were individually tailored based on the results of ICEEG and functional mapping. The patients' antiepileptic drugs were stopped or reduced during the ICEEG monitoring in order to record sufficient seizures. This study was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board.
Intracranial EEG (ICEEG) video monitoring data acquisition
All patients underwent surgery for the placement of intracranial grid and/or strip electrodes made with platinum disks in silastic and arranged in a grid pattern with center-to-center interelectrode distance of 1 cm (INTEGRA, Plainsboro, NJ, U.S.A.). Each electrode has an exposed surface diameter of <2.5 mm. The ICEEGs were recorded with a sampling rate of 2,000 Hz per channel (Stellate, Montreal, QC, Canada). The recordings were performed referentially with a scalp electrode as a reference, but subsequently referenced to two adjacent intracranial electrodes without active epileptiform discharges or nonphysiologic artifacts in order to diminish 60 Hz environmental artifacts and eliminate myogenic artifacts.
Selection of seizures and identification of ictal onset
All seizures included in the study were identified as the patients' habitual clinical seizures. If the patient had more than one seizure type recorded, two of each type were selected for analysis. In the case of more than two seizures recorded for a given type, the recording with the least amount of artifact was selected for analysis. All electrodes without prolonged artifact were retained. The ictal onsets were defined by initial visual inspection and had to be clearly distinguishable from the ongoing background rhythms as well as not explained by artifact or physiologic state changes (Gotman et al., 1993) using regular bandwidth filtering with high pass filter of 1 Hz and low pass filter of 100 Hz. Evolution of rhythmic discharges in frequency, time, and amplitude was necessary to identify the visualized onset, and distinguish from transient artifactual changes. Time-frequency analysis (TFA) for the identification of HFOs was started 5 min prior to the visualized ictal onset. TFA was performed in 1-s time windows over the course of the 5 min before the visualized ictal onset.
Data analysis
TFA was performed using the power spectrum with short-time Fast Fourier Transform (STFT) (Prism: Spectrum and Insight II; Persyst Development Corp, Prescott, AZ, U.S.A.). This analysis was performed on at least two seizures for each clinical seizure type. TFA was applied to all additional seizures that demonstrated a different EEG onset based on visual inspection, even if the clinical semiology was unchanged from previously analyzed examples. For each seizure, TFA was applied to the 5-min duration before the onset point defined by the initial visual inspection, with a one second time window. Once HFOs were detected, the window resolution was increased to 200 msec from that point until generalization; the overlap between windows was 50%. The ordinate scale in the time window was spectral power from 10 to 100 μV2. TFA windows for all electrodes were then visually reviewed simultaneously in an array for the detection of spectral power changes above the threshold. TFA was applied to each bandwidth: 80–150 Hz, 150–300 Hz, and 300–500 Hz separately.
In addition to TFA, each time window segment (200 msec) was analyzed with frequency domain analysis (FDA, [Prism: Spectrum and Insight II; Persyst Development Corp]) with averaged power within each separate frequency bandwidth. Then each time window segment for each separate frequency bandwidth was transformed to an FDA topography overlaid onto an image of the grids either from the intraoperative photographs of the brain with electrode placement or the three-dimensional (3D) reconstructed brain images using the patients' own coregistered magnetic resonance imaging (MRI) and computed tomography (CT) with grids (CURRY6; Compumedics Limited, Abbotsford, Victoria, Australia). The resection areas were confirmed with intraoperative postresection photographs.
Determination of SOZ
Seizure-onset zone (SOZ) electrodes were defined by TFA. Although rapid propagation of HFOs often occurred in the seizures analyzed, it was possible to distinguish a small number of electrodes with the first evident spectral power increase detected by the STFT power spectrum. Empirically, we found that up to five adjacent electrodes could show similar onset, followed by a well-defined group of electrodes with HFOs prior to diffuse spread. It was critical to distinguish relevant pathologic HFOs from HFOs related to artifact, so specific criteria were applied. SOZ inclusion criteria were the following: (1) presence of a group of five or fewer electrodes showing significant spectral power shift in the measured bands (80–500 Hz); (2) onset of ictal HFOs prior to clinical seizure; (3) spectrogram demonstrating a discrete localized “on” phenomenon (Fig. S2 for an example of this); and (4) electrodes with early HFO spread were included up to the point of diffuse spread. Specific exclusion criteria were the following: (1) absence of ictal HFOs on spectrogram before clinical onset and (2) spectrogram showing widespread scatter pattern with lack of discrete “on” phenomenon (consistent with high frequency artifact, see Fig. S3 for characteristics of an “artifactual” HFO). For the case of patients with more than one seizure type, electrodes showing ictal HFOs for each seizure type were added, without weighting, to define the ictal HFOs for the patient. Although these areas were usually tightly overlapping, in some cases the addition of different seizure types increased the total number of electrodes.
Furthermore, we aimed to determine the difference between electrodes identified using TFA versus conventional visual inspection of ICEEG. Using conventional bandpass filters as described earlier, the putative epileptogenic zone was identified by visual inspection for each seizure analyzed. Criteria for electrodes included in the epileptogenic zone were presence of rhythmic, evolving epileptiform discharges at visualized ictal onset, or in neighboring electrodes within 10 s of ictal onset but prior to diffuse spread. This was performed by a blinded reviewer (HG), a pediatric epileptologist who did not participate in the surgical evaluations or frequency analyses described.
Determination of completeness of HFO resection
After resection was completed, all patients had intraoperative photographs demonstrating extent of resection. This information was compared to coregistered preoperative MRI and 3D reconstructions of grids to identify whether the cortex associated with each electrode was resected. In 16 (36%) of 44 cases, a postoperative MRI was obtained for clinical purposes. In these 16 patients, a second method was used to validate the visual assessment of resection margin using intraoperative photographs: the resection areas were assessed by coregistration of postoperative MR images and CT with grid locations (CURRY6; Compumedics Limited, Abbotsford, Victoria, Australia). Complete HFO resection was defined as inclusion of all ICEEG electrodes with ictal HFOs, as defined by TFA. HFO resection ratio was defined as the ratio of the number of electrodes with ictal HFOs included in the resected area to the total number of electrodes with ictal HFOs.
Classification of surgical outcome
Surgical outcome was measured using the following four categories: seizure-free, >90% of seizure reduction (>90%), >50% seizure reduction (>50%), and no improvement. The clinical outcome at the most recent postsurgical follow-up clinic visit was used. For two patients who had a focal resection followed by second surgery with functional hemispherectomy, their first ICEEG recordings were analyzed and surgical outcome was scored based on postsurgical follow-up from the initial surgery. The seizure-free outcomes were compared between the two groups of patients with complete and incomplete resection of the ictal HFOs using Fisher's exact test.
Classification of MRI findings
Imaging studies were assessed by a neuroradiologist (ABR certified with added qualification in neuroradiology) with 15 years of experience in epilepsy imaging (JLL). Analysis of imaging was performed in a two-step process. Initially, the imaging studies were reviewed blinded to clinical history (other than intractable epilepsy), results of other tests, and resection location. The studies were then re-reviewed with knowledge of all additional clinical history, EEG results, additional imaging results, and resection location (by analysis of resection location on postoperative imaging studies). In two subjects, MRI findings were identified on second review that were not noted initially. In one case there was a small region of nonspecific subcortical white matter signal increase on fluid-attenuated inversion recovery (FLAIR) images (nonspecific classification), and in another case there were findings of hippocampal sclerosis (increased hippocampal signal and volume loss) that were overlooked (lesional classification).The MRI studies were classified as either (1) nonlesional: no finding of intracranial parenchymal abnormality or only nonspecific findings (volume loss or small regions of white matter increased signal, not related to subcortical regions or clear gyral malformation) or (2) lesional: abnormalities that included findings compatible with cortical dysplasia (at least two of the following: abnormal gyral pattern, cortical thickening, abnormal blurring of the cortical/white matter interface, increased T2 signal in cortex and/or white matter) as well as findings consistent with tuberous sclerosis, tumor, cavernoma, encephalomalacia, or other localized brain lesion.
Results
Patient characteristics and overall surgical outcome
We identified 48 patients (age range 9 months to 25 years, mean age 10 years, male-to-female ratio 29:19) who underwent ICEEG-video monitoring that ranged from 2 to 13 days. All but one patient was younger than 18 years of age. All 48 patients had epilepsy surgery. Three patients who had functional hemispherectomy as the initial surgery and one patient with insufficient follow-up were excluded. Therefore, a total of 44 patients were included. The postsurgical follow-up duration ranged between 12 and 26 months (mean 14 months) (Table 1). Overall seizure-free outcome was seen in 52% (23 patients). For the remaining patients, the distribution of the surgical outcomes was >90%, seven patients (16%); >50%, nine patients (20%); no improvement, five patients (11%). Seventy-seven percent (34/44) of our patient population were diagnosed as focal cortical dysplasia based on final pathology. Of the remaining 10 patients, 8 had tuberous sclerosis, 1 had subpial gliosis, and 1 had chronic inflammation. These patients were more likely to be seizure-free. Seven of 10 noncortical dysplasia cases were seizure-free (70% v. 39%, p = 0.025 Fisher's exact test).
Table 1.
Summary of the presence of HFOs, resections, and surgical outcome
| Surgical procedure |
Pathology |
|||||||
|---|---|---|---|---|---|---|---|---|
| No./total | % | Temporal | ExtraT. | Multilobar | FCD | TSC | Other | |
| Total No. patients identified | 48 | |||||||
| Total No. patients included in this study | 44 | |||||||
| Presence of ictal HFOs (*p = 0.001) | 41/44 | 93 | ||||||
| Complete HFOs resection | 22/41 | 54 | ||||||
| Surgical outcome | ||||||||
| Seizure-free | 18/22 | 82 | 6 | 5 | 7 | 12 | 5 | 1 (chronic inflammation) |
| >90% | 2/22 | 9 | 1 | 0 | 1 | 2 | 0 | 0 |
| >50% | 2/22 | 9 | 0 | 2 | 0 | 0 | 2 | 0 |
| No improvement | 0/22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incomplete HFO resection | 19/41 | 46 | ||||||
| Surgical outcome | ||||||||
| Seizure-free | 4/19 | 21 | 0 | 2 | 2 | 3 | 1 | 0 |
| >90% | 4/19 | 21 | 0 | 3 | 1 | 3 | 0 | 1 (subpial gliosis) |
| >50% | 6/19 | 32 | 2 | 2 | 2 | 6 | 0 | 0 |
| No improvement | 5/19 | 26 | 1 | 1 | 3 | 5 | 0 | 0 |
| No HFOs presence | 3/44 | 7 | ||||||
| Surgical outcome | ||||||||
| Seizure-free | 1/3 | 0 | 1 | 0 | 3 | 0 | 0 | |
| >90% | 1/3 | 1 | 0 | 0 | 0 | 0 | 0 | |
| >50% | 1/3 | 1 | 0 | 0 | 0 | 0 | 0 | |
HFOs, high-frequency oscillations; SOZ, seizure-onset zone; ExtraT., extratemporal; FCD, focal cortical dysplasia; TSC, tuberous sclerosis complex; >90%, >90% seizure reduction; >50%, >50% seizure reduction.
p-value: Fisher's exact test – two sided p-value.
Completeness of HFO resection and surgical outcome
Overall, the majority of patients (41/44, 93%) had ictal HFOs. In those with ictal HFOs, between 1 and 21 electrodes were identified in each patient, with a median of 4. Complete HFO resection, defined as cortical resection of all electrodes showing ictal HFOs, occurred in 22/41 (54%) (Table 1). The distribution of surgical outcomes was the following: seizure-free, 18 patients (82%); >90%, 2 (9%); >50%, 2 (9%); no improvement, 0 (0%). Of the 19 patients who had incomplete HFO resection (19/41, 46%), only 4 (21%) were seizure-free. The distribution of surgical outcomes was the following: seizure-free, four patients (21%); >90%, 4 (21%); >50%, 6 (32%); no improvement, 5 (26%). Complete resection of ictal HFOs resulted in a significantly better rate of seizure freedom (82%) than incomplete resection (21%, p < 0.0001). In incomplete HFO resection cases, there were several factors limiting surgical margins that may have led to incomplete resection: (1) Ictal HFOs were in motor cortex in four patients (seizure-free, 1; >90%, 1; >50%, 2); (2) ictal HFOs were in functional language area defined by electrical cortical stimulation in two patients (>50%, 1; no improvement, 1); (3) ictal HFOs included hemisphere contralateral to subdural grid placement and eventual surgical side, detected by interhemispheric electrodes, observed in one patient (seizure-free); (4) interictal diffuse random high frequencies made it difficult to distinguish ictal HFOs in one patient (no improvement); (5) HFOs spread to more >30% of entire electrodes within <20 msec in three patients (seizure-free, 1; >90%, 1; no improvement, 1). For the other eight patients with incomplete HFO resection, HFOs defined by TFA were not included in the surgical plan, since the epileptogenic zone defined by conventional visual inspection did not identify those electrodes (seizure-free, 1; >90%, 2; >50%, 3; no improvement, 2). Failure to resect HFOs in any of the three bandwidths was equally associated with poor surgical outcome. No statistical difference was noted when comparing outcome with resection of ictal HFOs in different bandwidths (Fisher's exact test, p = 0.538).
Complete resection of the putative epileptogenic zone, as defined by conventional visual inspection rather than TFA, was observed in 26 patients. Fifteen (58%) of 26 were seizure-free, compared to 82% seizure-free with complete HFO resection, showing a trend toward favorable prognosis with HFO resection over resection of conventionally defined epileptogenic zone. This did not reach statistical significance (p = 0.118) (Table 2).
Table 2.
Comparison of surgical outcome between complete resection of HFO defined by TFA and complete resection of epileptogenic zone defined by conventional visual inspection
| Complete HFO resection | % | Complete resection of SOZ defined by visual inspection | % | |
|---|---|---|---|---|
| Total number of patients | 22 | 26 | ||
| Seizure-free | 18 | 82 | 15 | 58 |
| Not seizure-free | 4 | 18 | 11 | 42 |
p = 0.118, Fisher's exact test.
Comparison between different pathologic diagnoses was limited by the small sample size of noncortical dysplasia cases. Eight of 10 noncortical dysplasia patients had complete HFO resection, which showed a trend toward statistical significance (80% v. 42%, p = 0.069, Fisher's exact test). This trend likely contributes to the superior seizure-free outcome in this group, since complete resection of HFOs correlated with outcome overall.
Confirmation of resection margin based on postoperative MRI versus intraoperative photographs
In the 16 patients with available postresection MRIs, resection margins, confirmed by fusion of postresection MRI and preresection 3D grid reconstruction, did not differ substantially from the ones confirmed by pre- and postresection intraoperative photographs. In 13 (82%) of 16, the resection margins were identical using the two methods (Fig. S1 shows this similarity). In two patients, the resections were slightly larger on MRI compared to intraoperative photographs. In one patient the resection appeared smaller on MRI. In these three slightly incongruent cases, the electrodes differentially included/excluded by the two methods did not change the categorization of complete or incomplete HFO resection between methods. The HFO resection ratio in these patients was not altered, since they met complete HFO resection criteria (100%) using both methods.
Nonlesional MR image cases (normal MRI) versus lesional cases
Fifteen patients had normal or nonspecific MRI findings; six patients (40%) became seizure-free (Table 3) postoperatively. Six (40%) of the 15 nonlesional patients had complete HFO resection; five of these six patients (83%) became seizure-free. Figures 1, 2 and S1 shows the pattern of onset and spread of HFOs and the correlation of STFT findings and surgical merging in one of the patients who had a normal MRI. In contrast, 9 of the 15 nonlesional patients had incomplete HFO resection. Only one patient (1 of 9, 11%) categorized as nonlesional with incomplete HFO resection became seizure-free. This patient had an HFO resection ratio >0.3 but <0.5. Twenty-six patients (63%) were considered lesional. Of these, 18 (69%) of 26 had complete HFO resection. Fifteen (83%) of these 18 were seizure-free (Fig. S2 shows the spreading pattern of HFO, STFT analysis, and correlation between surgical margin and lesion in a patient who had lesional MRI).
Table 3.
Comparison of the surgical outcome with HFO resection ratio between nonlesional versus lesional cases among 41 patients who had HFOs at the seizure onset
| Nonlesional | Lesional | |
|---|---|---|
| HFO resection ratio | ||
| 1 (%) | 6 (40) | 18 (69) |
| Seizure-free | 5 | 15 |
| Not seizure-free | 1 | 3 |
| ≥0.8 (%) | 2 (13) | 3 (12) |
| Seizure-free | 0 | 1 |
| Not seizure-free | 2 | 2 |
| ≥0.5 (%) | 1 (6) | 3 (12) |
| Seizure-free | 0 | 1 |
| Not seizure-free | 1 | 2 |
| ≥0.3 (%) | 3 (20) | 2 (7) |
| Seizure-free | 1 | 0 |
| Not seizure-free | 2 | 2 |
| <0.3 (%) | 3 (20) | 0 (0) |
| Seizure-free | 0 | 0 |
| Not seizure-free | 3 | 0 |
| Average of total number of implanted electrodes | 85 | 74 |
| Total | 15 | 26 |
HFO resection ratio = (number of electrodes with HFOs in resected area)/(total number electrodes with HFOs presence).
Figure 1.
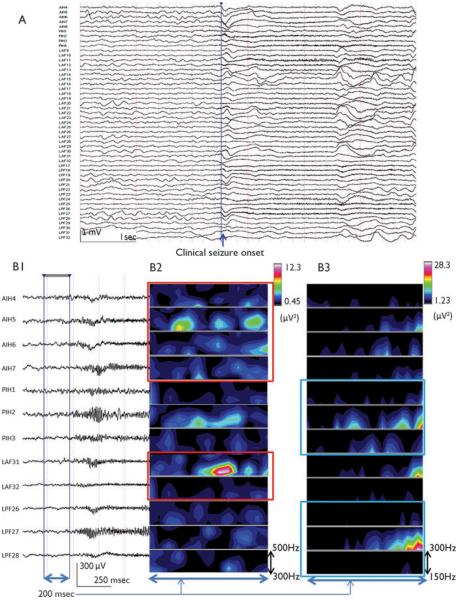
Nonlesional MRI (normal MRI) case. Ictal onset was followed by immediate spread to symptomatogenic zone in a 13-year-old left-handed girl with seizures since 8 years of age (A: showing partial number of electrodes; 49 of total 96 electrode placement, B1: expanded view of ICEEG at line in A, high-pass filtered at 40Hz; which approximately 250 msec before the clinical seizure onset, reveals the initial appearance of ictal HFO). After applying TFA through 1-min time window TFA analysis for each bandwidth (80–150, 150–300, 300–500 Hz) in 5-min segmentation before the initial seizure onset, HFOs were found (B2: 300–500 Hz, B3: 150–300 Hz) approximately 250 msec preceding clinical seizure onset defined by both video and muscle artifact seen in the electrocardiography electrode.
Epilepsia © ILAE
Figure 2.
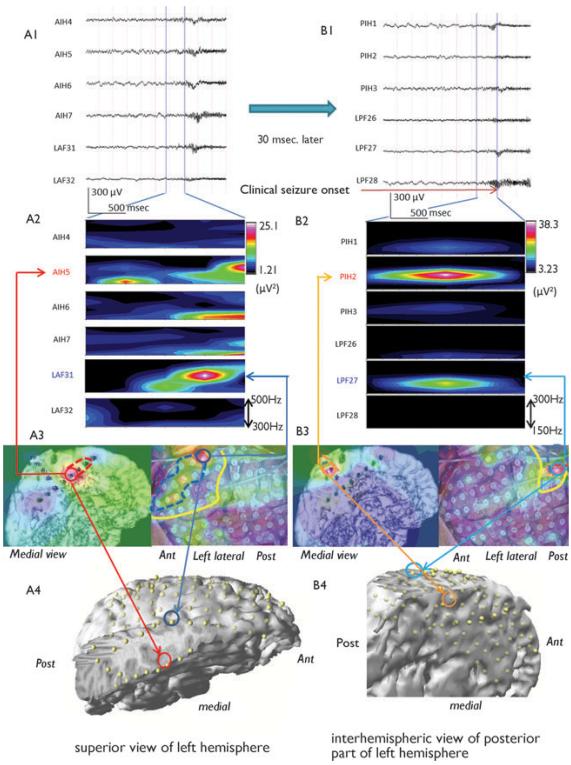
Ictal ICEEG recording showing ictal HFOs (A1) and symptomatogenic onset (high pass, 40 Hz) (B1). Time frequency analysis (TFA) using short-time Fast Fourier Transform (STFT) revealed ictal HFOs (300–500 Hz) localized in anterior mesial frontal (AIH5) and superior-posterior (PIH2) with secondary spread to lateral direction (LAF31, LPF27) (A2, B2). Frequency domain analysis superimposed on the picture of 3D reconstructed interhemispheric strip and intraoperative photo with grid placement showing the propagation of HFOs (A3, B3). 3D reconstructed left hemisphere image showing the intracranial electrodes by coregistration of MRI and post–grid placement CT images (A4, B4). The red (A4) and orange (B4) circle indicate AIH5 and PHI2 electrodes, respectively, as the first electrodes of SOZ, and dark blue (A4) and light blue (B4) indicate LAF31 and LPF27, respectively, as the first electrodes of secondary spreading location. The each area defined by dotted line on A3 and B3 indicate the area of the SOZ and secondary spread area. The yellow lines in A3 and B3 indicate the resection margin. Post, posterior; Ant, anterior.
Epilepsia © ILAE
Discussion
Planning a resection margin in pediatric epilepsy surgery is challenging. When an MRI-visible lesion exists, this can serve as a “target” or starting point for resection. In fact, in our surgical population the number of electrodes implanted was higher in the group of nonlesional cases (85 electrodes) compared to the lesional cases (72 electrodes) (Table 3). The most common pathologic cause found in pediatric cases is focal cortical dysplasia (FCD) (Wyllie et al., 1998); in this condition, the epileptogenic zone usually encompasses a larger area than is visible on MRI (Hemb et al., 2010; Kim et al., 2010). Even in other pathologies with well-circumscribed lesions on MRI, lesionectomy is not always sufficient for seizure freedom (Bollo et al., 2008). Therefore a successful surgical outcome depends on both MRI and other modalities to define the boundaries of the epileptogenic network that should be resected (Krsek et al., 2009).
With the ability to combine temporal resolution with excellent spatial resolution by direct electrode coverage over the cortex, ICEEG remains the gold standard for defining the epileptogenic zone in intractable neocortical epilepsy. However, conventional visual analysis of ICEEG has significant limitations of its own, such as the following: (1) surface findings of grid electrodes may not well represent deeper cortical sources in the bottom of a sulcus; (2) depth electrode recording is available for the deeper source, but often impractical and only feasible in a small region of interest; (3) in the case of an epileptogenic focus with a tangential orientation, the source spreads quickly and may be undetectable above the noise because its amplitude (power) is low and widespread (Jacobs et al., 2009a); and (4) the grid electrodes may be misplaced outside the true SOZ.
HFO analysis is emerging as an important tool for improving the accuracy of the information ICEEG provides. In our study, we found that ictal HFOs were commonly present. Our report found that 93% of patients had ictal HFOs during their habitual seizure onset. This is consistent with observations of prior studies demonstrating HFOs detectable in the SOZ prior to clinical seizure onset. In a study of 79 seizures in nine patients, 78 of 79 showed ictal HFOs (Ochi et al., 2007). Other work describing ictal patterns in ICEEG found the most common ictal onset pattern was a low voltage fast activity in 15 of 28 patients (Wetjen et al., 2009). This study was performed using a lower sampling rate (250 Hz) with low pass filter of 100 Hz. This low voltage, high-frequency ictal activity is usually identified by conventional settings in the high beta and low gamma bandwidths. However, by increasing the low pass filter it is usually possible to detect rhythmic oscillations faster than 80–100 Hz, fulfilling the current definition of HFOs (Bragin et al., 1999b). The upper limit of significant brain activity defined as HFOs is currently being explored, but may be as high as 1,000–2,500 Hz (Usui et al., 2010). In our experience, we have found that this pattern of low voltage fast activity detectable with conventional visual inspection is the “tip of the iceberg” of high-frequency activity. When a higher sampling rate and TFA is performed, HFOs may be evident during ictal onset or even several seconds before. In addition, classically evolving spike and wave detectable by visual inspection is often preceded by HFOs, particularly in neocortical epilepsy (Worrell et al., 2004; Khosravani et al., 2009).
This study represents the largest to date examining the relationship between ictal HFO resection and surgical outcome. Seizure outcome was significantly improved in cases with complete removal of HFOs (82% vs. 21%). In the 19 cases with incomplete resection, 11 cases could be attributed to either avoidance of adjacent eloquent cortex (6 cases) or difficulty identifying the HFO source as a clearly defined resectable region associated with ictal onset (5 cases). For instance, HFOs may be present diffusely or contralaterally near ictal onset, or occur frequently interictally, which possibly might instead represent physiologic HFOs. Moreover, interictal HFOs have been reported as pathologic and also as physiologic phenomena. It is not yet clear how to definitively distinguish between these two important HFO types, especially for the purpose of surgery (Rampp & Stefan, 2006; Nagasawa et al., 2011). However, the promise of ictal HFOs is the ability to distinguish pathologic from physiologic phenomenon by the temporal and spatial relationship to the clinical ictal event.
In our study, the remaining eight patients with incomplete HFO resection may have benefited from improved seizure outcome with likely little additional risk if the resection margin had included all HFO-generating cortices. The fact that four patients (21%) with incomplete HFO resection became seizure-free suggests ictal HFOs are an imperfect biomarker of the epileptogenic zone. Although not 100% specific, they represent at the least a complementary tool for assessing the epileptogenic zone. Recent reports showed a correlation between seizure-free outcome and percentage of HFO-generating cortex included in the resection (Ochi et al., 2007; Modur et al., 2011). Modur et al. (2011) even suggested that defining the epileptogenic zone with HFO analysis may allow for smaller resections than those typically recommended based on conventional visual analysis in neocortical epilepsy, as long as HFO resection is possible. In our population, we found a similar trend: percentage of HFOs resected correlated well with surgical outcome, and appeared more important than presence or absence of an MRI lesion in determining outcome (Table 3).
Our surgical outcome based on conventional visual inspection criteria alone yielded a 58% seizure-free rate, comparable to previously published seizure-free rates for pediatric epilepsy surgery series (Wyllie et al., 1998; Bauman et al., 2008; Goyal & Robinson, 2008). Resection of HFOs yielded a higher seizure-free outcome (82%), although not quite approaching statistical significance. It is possible that this difference may be confounded by limitations of study design, including the difference in how ictal onset was identified in the two methods.
The question remains as to whether HFOs can be a biomarker for pathologic etiology. In this regard, we cannot draw any conclusions from our study, since the majority of our patients had a pathologic diagnosis of focal cortical dysplasia (77%, 34/44). It is not clear whether ictal HFOs are more prevalent with certain epileptogenic pathologies or seizure types. Recently a few studies concluded that there was no particular relationship between HFO rates or any other feature of epileptic discharges and type of underlying pathology (Jacobs et al., 2010). HFOs have been found in both hippocampus and neocortex of patients with different types of seizures and underlying pathologies (Jacobs et al., 2009a; Kim et al., 2010). Moreover, much higher frequency range (>500 Hz) has been found at seizure onset in patients with mesial temporal lobe epilepsy (Worrell et al., 2008; Kobayashi et al., 2010a).
In order to use ictal HFO analysis as part of the decision-making process for resection planning, a few important questions need to be answered. First, how do we reliably differentiate a physiologic signal in many eloquent cortical areas from the pathologic ones? This may be determined in the near future (Engel et al., 2009; Frei et al., 2010); however, it remains a fundamental issue. Secondly, does absence of ictal HFOs predict surgical failure and should surgery be discouraged? Third, is the ictal HFO more specific than the interictal HFO? Fourth, HFO analysis of ICEEG is subject to the same undersampling bias inherent to ICEEG after grid placement. Electrode coverage is limited based on surgical risk and does not typically cover deep sources. Because of potential surgical risks, how important is it to assess deep sulcal ictal onsets?
Our study has some significant limitations. First, in more than half of the patients, postresection MRI was not obtained; therefore, we relied on the intraoperative postresection photo, which may have not given an accurate view, especially in the case of medial frontal or medial temporal resection. However, we did not find significant differences in the result regardless of whether a postoperative photograph or postoperative MRI coregistration confirmation was used. Second, the reference electrode was chosen from the electrophysiologically quiet region per conventional visual analysis, but may have been potentially contaminated with HFOs, including both pathologic and physiologic HFOs, which may not be detectable visually with conventional filter settings. Third, surgical resection margin was not only based on ICEEG, but also on the MRI, positron emission tomography (PET), ictal single photon emission computed tomography (SPECT) abnormality, magnetoencephalography (MEG), and even on the postresection electrocorticography. Fourth, although it is common practice at our institution to perform broad multilobar grid coverage in cases of presumed focal cortical dysplasia (FCD), slightly fewer electrodes were placed in lesional cases (Table 3). More restrictive sampling for cases in which lobar localization is more assured based on the presurgical evaluation may contribute to undersampling of HFO-containing regions. Because surgical outcome in lesional epilepsy is generally good, this may have created a selection bias in our sample.
Recent studies have demonstrated that it is feasible to detect HFOs in epileptic patients noninvasively using advanced neurophysiology techniques (Xiang et al., 2010) and even with scalp EEG recordings (Kobayashi et al., 2010b; Andrade-Valenca et al., 2011; Kobayashi et al., 2011; von Ellenrieder et al., 2011). In addition, automated detection of interictal HFOs has been introduced as a possible surrogate marker of the epileptogenic zone (Gardner et al., 2007; Schevon et al., 2009; Brázdil et al., 2010; Crépon et al., 2010; Akiyama et al., 2011; Zijlmans et al., 2011). These advances may better standardize the study of HFOs and expand the study of HFOs to patients without intractable epilepsy, including generalized epilepsy syndromes such as childhood absence epilepsy.
Ictal HFOs are commonly found in ICEEG in pediatric patients with intractable epilepsy. Complete resection of ictal HFOs is a favorable prognostic indicator for surgical outcome. Our data suggest that supplementing conventional visual inspection with ictal HFO analysis may improve surgical outcome. Owing to the distribution of etiologies in the current study, these findings are largely applicable to children with cortical dysplasia. Future prospective studies using automated detection methods and involving a larger number of surgical candidates are necessary before incorporating HFO analysis into the standard clinical decision making process.
Supplementary Material
Acknowledgments
We are very grateful to all of Epilepsy Surgery Program team at Cincinnati Children's Hospital Medical Center for the contribution to this study, and we are especially grateful to Dr. Jeffrey Tenney for his review and grammatical suggestions for the final manuscript.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, Weiss SK, Rutka JT, Carter Snead O., 3rd Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Otsubo H, Ochi A, Galicia EZ, Weiss SK, Donner EJ, Rutka JT, Snead OC., 3rd Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC, III, Rutka JT. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–1811. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- Andrade-Valenca L, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Bauman JA, Feoli E, Romanelli P, Doyle WK, Devinsky O, Weiner HL. Multistage epilepsy surgery: safety, efficacy, and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2008;62(Suppl. 2):489–505. doi: 10.1227/01.neu.0000316252.47028.2d. [DOI] [PubMed] [Google Scholar]
- Bollo RJ, Kalhorn SP, Carlson C, Haegeli V, Devinsky O, Weiner HL. Epilepsy surgery and tuberous sclerosis complex: special considerations. Neurosurg Focus. 2008;25:E13. doi: 10.3171/FOC/2008/25/9/E13. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid - treated rats with chronic seizures. Epilepsia. 1999b;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Halámek J, Jurák P, Daniel P, Kuba R, Chrastina J, Novák Z, Rektor I. Interictal high-frequency oscillations indicate seizure onset zone in patients with focal cortical dysplasia. Epilepsy Res. 2010;90:28–32. doi: 10.1016/j.eplepsyres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Crépon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Harer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Surgery for seizures. N Engl J Med. 1996;334:647–652. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Frei MG, Zaveri HP, Arthurs S, Bergey GK, Jouny CC, Lehnertz K, Gotman J, Osorio I, Netoff TI, Freeman WJ, Jefferys J, Worrell G, Quyen Mle V, Schiff SJ, Mormann F. Controversies in epilepsy: debates held during the Fourth International Workshop on Seizure Prediction. Epilepsy Behav. 2010;19:4–16. doi: 10.1016/j.yebeh.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Levtova V, Farine B. Graphic representation of the EEG during epileptic seizures. Electroencephalogr Clin Neurophysiol. 1993;87:206–214. doi: 10.1016/0013-4694(93)90020-v. [DOI] [PubMed] [Google Scholar]
- Goyal M, Robinson S. Expanding the role of surgery in intractable extratemporal pediatric epilepsy. Neurosurg Focus. 2008;25:E15. doi: 10.3171/FOC/2008/25/9/E15. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Mashiko T, Imada T. Somatic evoked high-frequency magnetic oscillations reflect activity of inhibitory interneurons in the human somatosensory cortex. Electroencephalogr Clin Neurophysiol. 1996;100:189–203. doi: 10.1016/0168-5597(95)00244-8. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorfeer DC. Incidence and prevalence. In: Hauser WA, Hesdorffer DC, editors. Epilepsy: frequency, causes, and consequences. Demos; NewYork: 1990. pp. 1–51. [Google Scholar]
- Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon N, Vinters HV, Mathern GW. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology. 2010;74:1768–1775. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Xiang J, Wang Y, Kirtman EG, Kotecha R, Fujiwara H, Hemasilpin N, Rose DF, Degrauw T. Gamma oscillations in the primary motor cortex studied with MEG. Brain Dev. 2010;32:619–624. doi: 10.1016/j.braindev.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Huo X, Wang Y, Kotecha R, Kirtman EG, Fujiwara H, Hemasilpin N, Degrauw T, Rose DF, Xiang J. High gamma oscillations of sensori-motor cortex during unilateral movement in the developing brain: a MEG study. Brain Topogr. 2011;23:375–384. doi: 10.1007/s10548-010-0151-0. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009a;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009b;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Mehrotra N, Rigby M, Hader WJ, Pinnegar CR, Pillay N, Wiebe S, Federico P. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- Kim DW, Kim HK, Lee SK, Chu K, Chung CK. Extent of neocortical resection and surgical outcome of epilepsy: intracranial EEG analysis. Epilepsia. 2010;51:1010–1017. doi: 10.1111/j.1528-1167.2010.02567.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Jacobs J, Gotman J. Detection of changes of high-frequency activity by statistical time-frequency analysis in epileptic spikes. Clin Neurophysiol. 2009;120:1070–1077. doi: 10.1016/j.clinph.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Agari T, Oka M, Yoshinaga H, Date I, Ohtsuka Y, Gotman J. Detection of seizure-associated high-frequency oscillations above 500 Hz. Epilepsy Res. 2010a;88:139–144. doi: 10.1016/j.eplepsyres.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010b;51:2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia. 2011;52:1812–1819. doi: 10.1111/j.1528-1167.2011.03169.x. [DOI] [PubMed] [Google Scholar]
- Krsek P, Maton B, Jayakar P, Dean P, Korman B, Rey G, Dunoyer C, Pacheco-Jacome E, Morrison G, Ragheb J, Vinters HV, Resnick T, Duchowny M. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72:217–223. doi: 10.1212/01.wnl.0000334365.22854.d3. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends Neurosci. 2006;29:419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippo-campal formation of humans. J Neurosci. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. 2006;8(Suppl. 2):S1–S9. [PubMed] [Google Scholar]
- Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011;52:1792–1801. doi: 10.1111/j.1528-1167.2011.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Juhasz C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2011;33:569–583. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Suzuki C, Yaegashi Y, Baba M, Matsunaga M, Hashimoto I. High frequency oscillations in early cortical somatosensory evoked potentials. Electroencephalogr. Clin Neurophysiol. 1998;108:536–542. doi: 10.1016/s0168-5597(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Rampp S, Stefan H. Fast activity as a surrogate marker of epileptic network function? Clin Neurophysiol. 2006;117:2111–2117. doi: 10.1016/j.clinph.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan A, Schroeder C, Goodman R, McKhann G, Jr, Emerson R. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuele SU, Luders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet neurol. 2008;7:514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- Seo JH, Holland K, Rose D, Rozhkov L, Fujiwara H, Byars A, Arthur T, DeGrauw T, Leach JL, Gelfand MJ, Miles L, Mangano FT, Horn P, Lee KH. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology. 2011;76:41–48. doi: 10.1212/WNL.0b013e318204a380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Usui N, Terada K, Baba K, Matsuda K, Nakamura F, Usui K, Tottori T, Umeoka S, Fujitani S, Mihara T, Inoue Y. Very high frequency oscillations (over 1000 Hz) in human epilepsy. Clin Neurophysiol. 2010;121:1825–1831. doi: 10.1016/j.clinph.2010.04.018. [DOI] [PubMed] [Google Scholar]
- von Ellenrieder N, Andrade-Valença LP, Dubeau F, Gotman J. Automatic detection of fast oscillations (40–200 Hz) in scalp EEG recordings. Clin Neurophysiol. 2011;123:670–680. doi: 10.1016/j.clinph.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetjen NM, Marsh WR, Meyer FB, Cascino GD, So E, Britton JW, Stead SM, Worrell GA. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu SQ, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740–748. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- Xiang J, Wang Y, Chen Y, Liu Y, Kotecha R, Huo X, Rose DF, Fujiwara H, Hemasilpin N, Lee K, Mangano FT, Jones B, DeGrauw T. Noninvasive localization of epileptogenic zones with ictal high-frequency neuromagnetic signals. J Neurosurg Pediatr. 2010;5:113–122. doi: 10.3171/2009.8.PEDS09345. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Res. 2009;85:287–292. doi: 10.1016/j.eplepsyres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122:664–671. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.