Abstract
Objective
To evaluate the prevalence of insomnia in MS patients with comorbid depression, associations between psychological symptoms, MS symptoms and insomnia, and to test effects of a psychotherapy intervention on insomnia symptoms.
Methods
Participants with MS and depression were randomized to two 16-week protocol based psychotherapies: telephone administered cognitive behavioral therapy and telephone administered supportive emotion focused therapy. MS symptoms were measured at baseline. Depression, insomnia, anxiety were evaluated at pre-treatment, mid treatment (8 weeks), and post treatment (16 weeks).
Results
Data were available for 127 participants. Prevalence of insomnia ≥ 3 times per week was 78% at pre treatment and 43% at post treatment. Insomnia symptoms at baseline were associated with depression, MS related mood symptoms and anxiety. Middle of the night awakenings were associated with swallowing and speech problems. Improvements in insomnia were associated with improvement in depression and anxiety, but treatment type did not predict improvement. Participants with residual insomnia were more likely to have major depressive disorder, greater MS severity and elevated anxiety.
Conclusion
Rates of insomnia in patients with comorbid MS and depression are higher than those reported in the general MS population. The greatest improvements in insomnia were seen in those with the most improvement in depression and anxiety, particularly for sleep onset insomnia. Despite this improvement, a nearly half of participants continued to report insomnia. Results suggest additional treatment of insomnia is needed beyond the treatment of comorbid psychological disorders.
Keywords: multiple sclerosis, depression, insomnia
Multiple Sclerosis (MS) is a chronic autoimmune demeylinating disease of the central nervous system. In the variable course and progression of symptoms in this disease, patients may experience episodic, chronic, or progressive symptoms including fatigue, pain, spasticity, loss of use of their arms and legs, loss of vision, impaired bowel and bladder function, and sexual problems. Prevalence of poor self-reported sleep quality as well as sleep disorders, such as insomnia and restless legs syndrome, is higher in patients with MS compared with the general population.[1] This is attributable to multiple factors including pain and spasticity, medication side effects, as well as physical and emotional comorbidites such as depression. Several studies have reported more than half of patients with MS report poor sleep quality.[2–3] In addition to self-reported poor sleep quality, polysomnography demonstrates that patients with MS also have poorer objective sleep quality including more frequent arousals, greater wake after sleep onset, and poorer sleep efficiency.[4–5]
Poor sleep quality has been associated with greater disease severity, pain, and poorer mental and physical quality of life in patients with MS.[6] Insomnia is one of the most prevalent sleep complaints in MS. Prior studies have reported that over half of patients with MS have difficulty initiating and maintaining sleep, or early morning awakenings.[3, 7] This is significantly higher than the rates in the general population (10–15%).[8]
The relationship between MS and insomnia is complicated by the high prevalence of mood and anxiety disorders. Insomnia symptoms are commonly associated with depression and anxiety, and thus may cause or exacerbate sleep disturbance in patients with MS[9]. Prevalence of significant depressive symptoms in MS has been reported as 41.8% with 25% meeting criteria for major depressive disorder.[10–11] One study in patients with MS found that those classified as poor sleepers on a standardized measure of sleep quality were more likely to have clinically elevated anxiety and depression.[6] Anxiety, which is correlated with depression in MS patients, is also associated with sleep disturbance.[12–14] In longitudinal studies, there appears to be a different course in the relationship between depression, anxiety, and insomnia. Insomnia symptoms have been found to precede the onset of depression and recurrence of depressive disorders whereas insomnia symptoms occur concurrently or following anxiety.[15–16] Resent research suggests that treating comorbid insomnia may enhance treatment response to antidepressants.[17] Therefore, improvement in insomnia symptoms may contribute to quality of life and reduce risk for future depressive episodes. To date, no study has tested the results of treating depression on insomnia symptoms in patients with comorbid MS and depression.
This study presents secondary data analysis of a trial of telephone-administered psychotherapy for depression in MS.[18] The goals of this study were: 1) To determine the prevalence of insomnia in patients with MS and depression, 2) To evaluate the pre-treatment correlation between insomnia, anxiety and MS symptoms, 3) To determine the effects of treatment for depression on insomnia symptoms 4) To determine treatment-related factors associated with improvement in insomnia, including improvement in depression and anxiety symptoms as well as treatment type. The present study analyzed insomnia outcomes resultant from two validated, protocol based (manualized) treatments for depression: telephone-administered cognitive behavioral therapy (T-CBT), which is a structured approach to treat depression through increasing behavioral activation and enhancing cognitive strategies for coping and telephone administered Supportive Emotion Focused Therapy (T-SEFT), a supportive and relationship-based approach to treating depression. We hypothesized that insomnia symptoms would improve with both treatments but there would be a greater improvement with cognitive behavioral therapy due to the more structured behavioral aspects of this treatment.
Methods
Participants
Participants were recruited from the Kaiser Permanente Medical Group of Northern California (KP) and regional chapters of the Multiple Sclerosis Society. Eligibility criteria included 1) a diagnosis of MS confirmed by a neurologist, 2) functional limitation, which was defined as 3 out of 6 on Guy’s Neurological Disability Scale (GNDS) [19], 3) Significant depressive symptoms, defined as ≥16 on the Beck Depression Inventory-II (BDI-II)[20], and ≥14 on the Hamilton Depression Rating Scale (HDRS)[21], 4) the ability to speak and read English, 5) age ≥ 18 years. Exclusion criteria included 1) dementia, 2) currently psychotherapy attendance, 3) disability that would prevent participation in assessment or treatment (e.g. reading or writing limitation), 4) severe psychopathology, including psychosis, current substance abuse, or plan or intent to commit suicide, 5) current MS exacerbation, and 6) use of medications that affect mood other than antidepressants (e.g. steroidal anti-inflammatory agents). Use of antidepressant medications was not exclusionary.
Procedure
A full description of the eligibility assessment and follow-up procedures is described elsewhere.[18] Briefly, potential participants were identified through the KP patient database and subsequent to approval from their physicians, were sent a mailing asking them to return a pre-stamped postcard if they did not wish to be contacted further. Potential participants who did not return postcards were called 10 days later. During the phone calls, potential participants were provided with a brief description of the study and then asked to participate in a brief telephone screening to assess depressive symptoms and exclusionary criteria. Those who met initial screening criteria were invited to participate in a longer eligibility assessment involving a telephone interview and written questionnaires. Recruitment through the regional National Multiple Sclerosis Society was achieved through an announcement in chapter newsletters. Assessments were conducted at baseline, mid treatment (week 8), and post treatment (week 16) by trained interviewers who were blinded to treatment assignment. Written self-report materials were mailed with pre-stamped and addressed envelopes and participants were paid $10–$50 per assessment, based on the length of assessment.
Measures
Depression and Anxiety: Current DSM-IV diagnoses of Major Depressive Disorder (MDD) were assessed using the Structured Clinical Interview for the DSM-IV (SCID)[22] at baseline and post treatment. Reliability and validity for telephone administered SCID has been demonstrated (citation needed). In random reliability checks, evaluators for this study had 100% reliability on MDD diagnoses in this study. Evaluator-rated depression severity was assessed using a telephone administered version of the HDRS[21] at baseline, mid treatment, and post treatment. The telephone version of the HDRS used in this study was developed for the Medical Outcomes Study.[23] In order to avoid overlap with insomnia ratings, HDRS scores for this study were calculated without the 3 insomnia items. Self-reported depression severity was measured by the BDI-II.[20] The BDI-II was administered as self-report by mail at baseline, mid treatment and post treatment. BDI-II scores were also calculated minus the insomnia item. The Hospital Anxiety and Depression Scale (HADS), Anxiety Subscale was administered to assess self-reported anxiety symptoms.[24] This 7 items self-report measure assessed both physical and psychological anxiety symptoms including tension, worry, and restlessness. Items did not overlap with insomnia symptoms. Significant anxiety symptoms were defined at a HADS anxiety score ≥11.
Insomnia was measured by 3 items on the HDRS: early insomnia, middle insomnia, and late insomnia. Items were scored from 0 (not at all) to 2 (significant insomnia ≥ 3 days per week). In analyses of insomnia as a continuous variable, these items were summed to create an insomnia total score. Insomnia sub scores for early, middle, and late were also explored. In categorical analyses, presence of insomnia was defined as a score of 2 on at least one insomnia item. A score of 2 on early insomnia was defined as difficulty initiating sleep for ≥ 30 minutes 3 or more days per week for early insomnia. A score of 2 on middle insomnia was defined as middle of the night awakenings with wake after sleep onset ≥ 3 days per week for middle insomnia. A score of 2 on late insomnia was defined as early awakening and getting out of bed for ≥ 30 minutes on ≥ 3 days per week for late insomnia.
MS-related functional impairment was measured using The Guy’s Neurological Disability Scale which was telephone administered.[19] This structured interview assesses 11 basic areas of function (e.g. limb function, vision) and produces a total score that is highly related to objective measures of functional impairment based on neurological exam (r= .81).[19]
A brief screening of cognitive function was performed to determine eligibility criteria using a battery of telephone administered neuropsychological tests including Digit Span[25], The California Verbal Learning Test[26], The Controlled Oral Word Association Test-FAS version[27], and the Similarities test from the Wechsler Adult Intelligence Scale, third edition.[25] Participants who scored below the fifth percentile on any scale were determined to have dementia and excluded from the study.
Treatments and Clinicians
Participants were randomized to one of two 16-week telephone administered psychotherapies, T-CBT and T-SEFT. Sessions were 50 minutes in duration and occurred weekly. Randomization was stratified based on current MDD diagnosis and use of antidepressant medication. All treatments were administered by doctoral level psychologists. T-CBT was based on CBT for depression[28] with content added for MS and used a workbook as a guide through treatment.[29–30] The goal of treatment was to teach participants skills to manage cognitions and behaviors that contribute to depression and improve skills in managing stressful life events and interpersonal difficulties. T-SEFT was adapted from a manual from Greenberg and colleagues for process-experiential psychotherapy.[31] Goal of this treatment was increasing participants’ level of experiencing their internal world. Therapeutic tasks included maintaining attention on empathic attunement, developing the therapeutic bond, and facilitating direct expression of present emotional experience and current needs. Thus, T-SEFT controlled for all non-specific factors associated with T-CBT, including dosage, the therapeutic relationship, use of a psychotherapy treatment manual with a coherent theoretical justification and clearly described procedures, and individualized application of the treatment model.
Data analysis
Analyses were conducted in an intention-to-treat basis. Data were analyzed using SAS (v. 9.2 SAS Corporation, Cary, NC). Analyses compared baseline demographics between treatment groups using t-test for continuous data and χ2 for categorical variables. Bivariate correlations were used to evaluate associations between insomnia, depression, anxiety and MS symptoms. Finally, mixed modeling using Proc Mixed was used to test improvements in depression on the (HDRS and the BDI), anxiety, and treatment type as predictors of improvements in insomnia symptoms over time. Missing data in mixed models (n=1 pre-treatment and n=6 post treatment) was ignored under the assumption it was missing completely at random. Statistical significance was defined as p < .05 in two-tailed tests.
Results
Baseline Characteristics
Data were available for 127 participants (65 were randomized to the T-SEFT and 62 were randomized to the T-CBT groups). Of the 7 dropouts from the study, all but 2 participants (one from each group) agreed to continue with assessments. Baseline participant characteristics are listed in Table 1. Approximately half of the sample was on disability. Participants were predominantly middle aged, female, and White. There was a wide range of MS-related functional impairment reported on the GNDS but most scores were in the mild to moderate range (range=10–37, M= 23.37, SD= 6.25). The majority of participants reported having relapsing/remitting MS (n=112, 88%). The majority (n=89, 70%) met criteria for at baseline MDD and nearly half (n=60, 47%) reported clinically significant anxiety. There were no significant differences between treatment groups among baseline values.
Table 1.
Baseline Participant Characteristics
| T-CBT (n= 62) | T-SEFT (n=65) | |
|---|---|---|
| N (%) or M (SD) | N (%) or M (SD) | |
| Age | 48.6 (9.6) | 47.6 (10.1) |
| % Female | 51 (75.8) | 48 (78.5) |
| % White | 58 (93.5) | 56 (86.2) |
| Employment | ||
| %Employed | 17 (25.8) | 17 (26.2) |
| %Disability | 32 (51.6) | 32 (56.9) |
| GNDS Total Score | 23.9 (5.8) | 22.9 (6.7) |
| %Major Depressive Disorder | 46 (72.6) | 43 (67.7) |
| BDI | 26.7 (8.1) | 28.4 (7.9) |
| HDRS | 21.4 (3.9) | 21.7 (3.5) |
| HADS Anxiety Scale | 21.4 (3.9) | 10.6 (3.6) |
| % HADS Anxiety score ≥11 | 29 (47.7) | 31 (46.8) |
GNDS= Guy’s Neurological Disability Scale, BDI= Beck Depression Inventory, HDRS= Hamilton Depression Rating Scale, HADS= Hospital Anxiety and Depression Scale, Anxiety subscale only
Prevalence of Insomnia
Pre and post treatment prevalence of insomnia symptoms is listed in Table 2. At baseline, 78% (n=98) reported insomnia of any type ≥ 3 times per week. This declined to 43% (n= 53) at post treatment. At both time points early insomnia and middle insomnia were more common than late insomnia.
Table 2.
Insomnia prevalence greater than or equal to 3 times per week
| Insomnia Symptoms N (%)† | ||||
|---|---|---|---|---|
| Any Insomnia | Early | Middle | Late | |
| Baseline | 98 (77.8) | 60 (47.6) | 64 (50.8) | 29 (15.9) |
| Post treatment | 53 (43.4) | 36 (29.5) | 29 (23.8) | 11 (9.0) |
Percentages vary slightly between assessments due to missing data (n=1 at baseline and n=6 at post treatment)
Correlations between Insomnia, Depression, Anxiety, and MS symptoms
Correlations between insomnia, depression, anxiety and MS symptoms at baseline are listed in Table 3. The insomnia total score and middle insomnia was positively correlated with the HDRS score (p < .05 in both correlations). Anxiety was positively associated with total insomnia (p < .05) and early insomnia (p < .05). There were few correlations between MS symptoms and insomnia. Among MS symptoms and disability, total insomnia was positively correlated with MS related mood symptoms (p < .05) and middle insomnia was positively correlated with swallowing problems (p < .05) and speech problems (p < .05).
Table 3.
Correlations with insomnia symptoms at baseline (n= 126)
| Insomnia Symptoms | ||||
|---|---|---|---|---|
| Total Insomnia | Early | Middle | Late | |
| Depression*** | ||||
| HDRS | .23* | .06** | .28** | .09** |
| BDI | .07 | .07 | .01 | .05 |
| Anxiety | ||||
| HADS | .22* | .21* | .10 | .06 |
| MS-Symptoms | ||||
| GNDS Total Score | .13 | .05 | .09 | .11 |
| Cognitive | .07 | .08 | .08 | −.04 |
| Mood | .21* | .14 | .16 | .00 |
| Visual | −.02 | −.05 | .00 | .03 |
| Speech | .15 | .10 | .18* | −.00 |
| Swallowing | .10 | −.03 | .22* | .00 |
| Upper Limb | −.01 | −.10 | −.00 | .11 |
| Lower Limb | −.10 | −.15 | −.12 | .10 |
| Sexual | .06 | .14 | −.00 | −.05 |
| Bladder | .04 | −.03 | .03 | .09 |
| Bowel | .03 | .06 | −.05 | .03 |
| Fatigue | .05 | −.04 | .02 | .12 |
HDRS= Hamilton Depression Rating Scale, BDI= Beck Depression Inventory-II.
p<.05,
p<.01,
HDRS and BDI depression scores are calculated minus insomnia items
Predictors of insomnia improvement
First, two measures of depression (interview based and self-report) were tested in separate models as predictors of change in insomnia treatment. Models with depression predicting change in insomnia are listed in Tables 4 (HDRS) and 5 (BDI-II). Results were concordant for both depression measures for total insomnia and early insomnia. Higher depression at baseline and greater improvement in depressive symptoms over time were associated with a greater improvement in the total insomnia score (p<.01 for the HDRS and p<.05 for the BDI-II) and early insomnia (p<.01 for the HDRS and p<.05 for the BDI-II). There was a trend for a change in depression by time interaction in middle insomnia for the HDRS only (p=.06). Higher baseline HDRS scores were associated with greater improvements in middle insomnia (p<.0001) and higher baseline BDI-II scores were associated with greater improvement in late insomnia (p<.05).
Table 4.
Model Predicting Change in Insomnia
| Variable | Estimate | Standard Error |
p |
|---|---|---|---|
| Insomnia Total Score | |||
| Intercept | −.23 | .65 | .73 |
| Time | −.27 | .15 | .07 |
| Therapy group | −.15 | .27 | .58 |
| Baseline HDRS | .17 | .03 | <.0001 |
| Change in HDRS | .03 | .02 | .26 |
| Therapy group × time | .09 | .17 | .59 |
| Change in HDRS × time | .05 | 0.02 | <.01 |
| Early Insomnia | |||
| Intercept | −2.52 | .95 | <.01 |
| Time | −.03 | .19 | .86 |
| Therapy group | −.23 | .37 | .53 |
| Baseline HDRS | .14 | .05 | <.01 |
| Change in HDRS | .02 | .03 | .45 |
| Therapy group × time | .14 | .24 | .56 |
| Change in HDRS × time | .07 | .02 | <.001 |
| Middle Insomnia | |||
| Intercept | −4.43 | 1.07 | <.0001 |
| Time | −.37 | .22 | .09 |
| Therapy group | −.25 | .39 | .51 |
| Baseline HDRS | .24 | .06 | <.0001 |
| Change in HDRS | −.002 | .03 | .95 |
| Therapy group × time | −.13 | .29 | .65 |
| Change in HDRS × time | .04 | .02 | .05 |
| Late Insomnia | |||
| Intercept | −3.42 | 1.12 | <.01 |
| Time | −.15 | .42 | .71 |
| Therapy group | .30 | .51 | .56 |
| Baseline HDRS | .09 | .05 | .09 |
| Change in HDRS | .04 | .06 | .47 |
| Therapy group × time | .12 | .43 | .78 |
| Change in HDRS × time | .03 | .04 | .54 |
Models with anxiety predicting change in insomnia are listed in Table 5. Higher baseline anxiety and greater improvement in anxiety over time (HADS by time interaction) were associated greater improvement in the total insomnia score (p<.001, p<.05, respectively) and early insomnia (p<.05, and p<.001, respectively). Higher baseline insomnia was also associated with greater change in middle insomnia (p<.05) and change in anxiety over time was associated with greater improvement in late insomnia (p<.05).
Table 5.
| Variable | Estimate | Standard Error |
p |
|---|---|---|---|
| Insomnia Total core | |||
| Intercept | 1.89 | .44 | <.0001 |
| Time | −.33 | .14 | <.05 |
| Therapy group | −.12 | .28 | .68 |
| Baseline BDI | .04 | .02 | <.05 |
| Change in BDI | .01 | .01 | .68 |
| Therapy group × time | −.06 | .17 | .71 |
| Change in BDI × time | .02 | .01 | <.05 |
| Early Insomnia | |||
| Intercept | −1.25 | .57 | <.05 |
| Time | −.15 | .18 | .38 |
| Therapy group | −.24 | .36 | .51 |
| Baseline BDI | .05 | .02 | <.05 |
| Change in BDI | .01 | .02 | .59 |
| Therapy group × time | −.08 | .23 | .75 |
| Change in BDI × time | .02 | .01 | <.05 |
| Middle Insomnia | |||
| Intercept | −.80 | .54 | .14 |
| Time | −.49 | .21 | <.05 |
| Therapy group | −.09 | .35 | .79 |
| Baseline BDI | .03 | .02 | .08 |
| Change in BDI | .003 | .02 | .86 |
| Therapy group × time | −.27 | .26 | .31 |
| Change in BDI × time | .005 | .01 | .70 |
| Late Insomnia | |||
| Intercept | −3.31 | .77 | <.0001 |
| Time | −.13 | .40 | .75 |
| Therapy group | .48 | .50 | .34 |
| Baseline BDI | .05 | .02 | <.05 |
| Change in BDI | .004 | .03 | .90 |
| Therapy group × time | −.06 | .42 | .88 |
| Change in BDI × time | .02 | .02 | .44 |
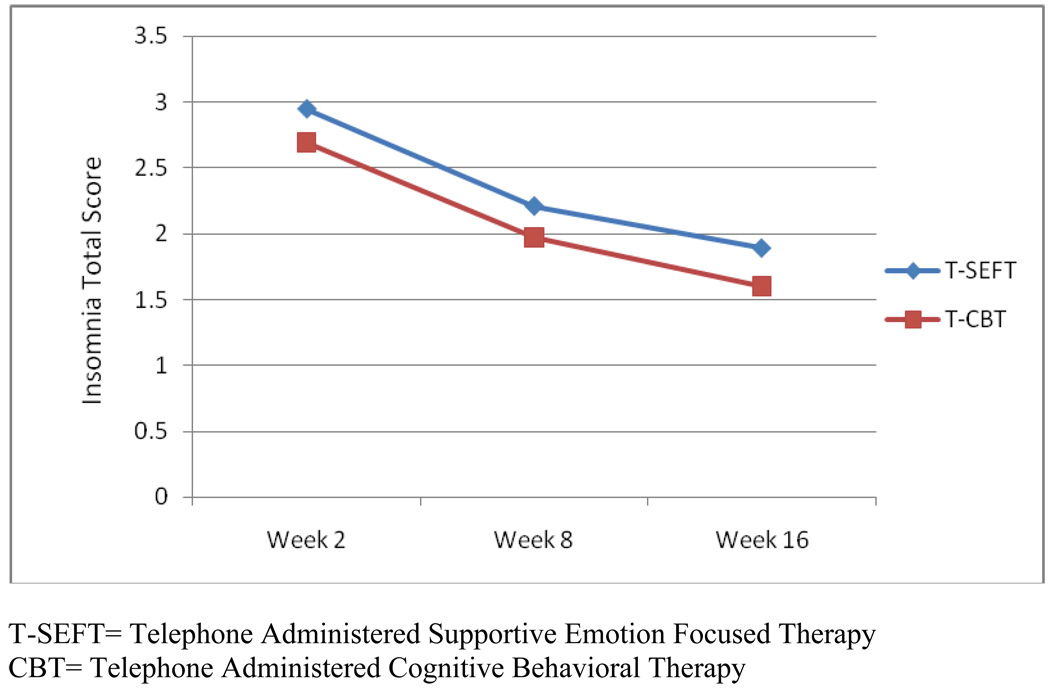
In all models, treatment type (T-CBT versus T-SEFT) and the treatment type by time interaction was not significant, indicating therapy group assignment was not associated with post treatment insomnia (Figure 1).
Figure 1.
Change in insomnia total score over time by psychotherapy group
Associations with insomnia at post treatment
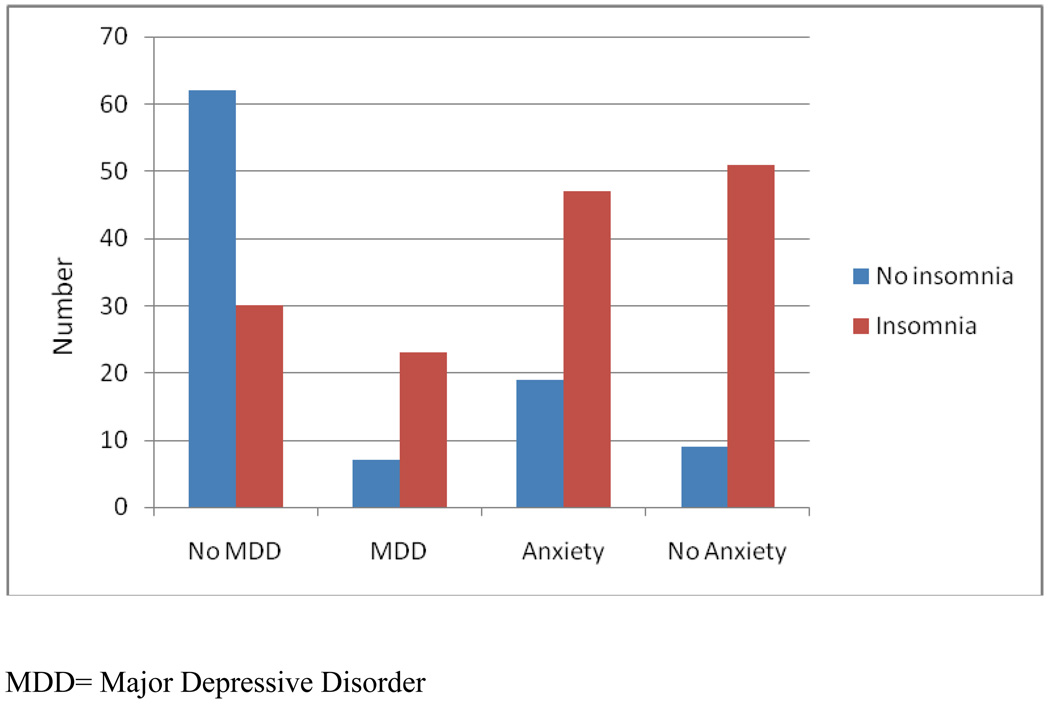
At the end of treatment, 27% (n=35) continued to meet criteria for MDD and 21% (n=26) had elevated anxiety symptoms. In univariate regression models, higher MS symptoms on the GNDS (B= .06, SE=.02, p<.01), higher depression on the HDRS (B= .13, SE=.02, p<.01) and higher anxiety scores (B= .10, SE=.03, p<.01) were associated with higher post treatment insomnia symptoms. When included in a multivariate model, with age and gender, only post treatment depression independently predicted post treatment insomnia (R2Δ = .17, p<.0001). Having insomnia at post treatment was associated with an increased risk of meeting criteria for MDD (OR= 1.8, p<.0001) and increased risk of elevated anxiety scores (OR= 1.16, p<.05). In addition, a significant number of participants without MDD or elevated anxiety symptoms continued to report insomnia post-treatment (n=30, 33% of participants without MDD, and n=35, 37% of participants without elevated anxiety Figure 2).
Figure 2.
Number of participants with insomnia at post treatment by depression and anxiety group.
Discussion
This study evaluated the prevalence of insomnia symptoms, association and response of these symptoms to psychotherapy for depression in patients with MS and depression. Results demonstrate that patients with comorbid MS and depression have levels of insomnia symptoms above and beyond that reported of the overall MS population, with over three quarters of the sample reporting clinically significant insomnia symptoms (difficulty initiating sleep, maintaining sleep, or early morning awakenings ≥ 3 days per week). In a study that used a diary based measure of sleep in a sample of patient with MS who were not selected on depression 42% reported difficulty initiating sleep, 53% reported extended awakenings, 58% reported waking and being unable to return to sleep ≥ 2 times per week,.[7] The current study used a higher threshold for defining insomnia yet found a greater prevalence.
We found that the severity of insomnia was correlated with interviewer rated depressive symptoms on the HDRS but not with self report depression on the BDI. This may reflect method variance on the measures as well as the high base rates of insomnia and depression, which contribute to restricted range. In addition, the items of the HDRS focus more on the somatic symptoms that are often related to anxiety, whereas the BDI focuses more on the cognitive symptoms of depression. Insomnia symptoms at baseline were also not associated with the majority of MS symptoms at baseline, including bowel and bladder symptoms, fatigue, and weakness in the limbs. Only speech and swallowing difficulties were correlated with middle of the night awakenings and mood symptoms were associated with the total insomnia score. The correlation between difficulty swallowing, speech problems and insomnia may suggest that those with the most severe MS symptoms, may also have more severe sleep disturbance due to greater overall disease severity, such as weakness in the upper airway.[32] Bulbar dysfunction in MS may contribute to both arousals during sleep as well as dysphagia.[33–35] We also found that patients with the most severe MS symptoms at baseline were more likely to have higher insomnia symptoms post-treatment. It is possible that insomnia symptoms due to depression would be likely to remit with improvement in depression whereas insomnia associated with MS severity would not improve with depression treatment.
In evaluating the course of insomnia symptoms with depression treatment, we found that insomnia improved significantly, from over 3/4ths of participants at baseline to less than half of participants. The post treatment rates of insomnia were similar to those of other reports of sleep disturbance in MS samples[3, 7]. The greatest improvement in insomnia was seen in those with the greatest improvement in symptoms of depression and anxiety. Specifically, it was the symptoms of sleep onset insomnia that were the most responsive to changes in depression and anxiety. When evaluated alone, middle of the night awakenings and early morning awakenings were not associated with improvement in depression and anxiety symptoms.
Contrary to our expectations, there was no difference between the two psychotherapies- T-CBT and T-SEFT for improvement in insomnia symptoms. This result may be due to the fact that neither psychotherapy specifically addressed sleep related thoughts and behaviors, such as sleep restriction therapy, stimulus control therapy, and sleep hygiene to improve sleep latency, sleep efficiency and sleep quality.[36] There is some evidence suggesting that increasing daytime activity alone may have a significant impact on sleep. An multidisciplinary “energy conservation program” in patients with MS improved physical and mental fatigue, depression, and sleep quality.[37] In addition, participants significantly reduced their average time in bed from 8 hours 36 minutes to 8 hours 6 minutes post treatment, which is consistent with techniques to improve sleep consolidation used in treating insomnia.
Despite the improvements in depression and insomnia in this study, a high number of participants without major depressive disorder at post treatment continued to report clinically significant insomnia symptoms (33% of those without depression). Part of the residual insomnia is due to the multifactoral nature of insomnia in MS. The intervention was focused on addressing depression, therefore insomnia due to restless legs, pain or bladder symptoms would not be expected to improve with depression.
Given the high rates of insomnia symptoms and other sleep disorders in patients with MS, results of this study demonstrate the utility of sleep disorders screening and interventions to improve quality of life and possibly additional outcomes for patients with MS. There is evidence that CBT interventions are effective in improving insomnia comorbid with other medical conditions, including osteoarthritis, coronary artery disease, and chronic obstructive pulmonary disease.[38] In addition, emerging evidence suggests that CBT for insomnia may improve response to antidepressant medications in patients with depression.[17]
Results of this study are limited by our use of a simple and measurement of insomnia from a depression measure rather than measures more specific to sleep (e.g., sleep times, sleep quality, restless legs) Therefore, we were not able to determine the cause of insomnia or the aspects of insomnia that improved with treatment of depression. Our measurement also did not take into account daytime dysfunction related to sleep, therefore it was an assessment of insomnia symptoms rather than the disorder of insomnia as defined by the International Classification of Sleep Disorders Second Edition (ICSD-2).[39] There also was no treatment specifically provided to target sleep symptoms in either psychotherapy group. There was no placebo or no-treatment control condition in this study. Thus, while we believe it is unlikely, it is possible that these results reflect the natural history of the symptoms reported.
In conclusion, this study demonstrates high prevalence of insomnia in patients with both MS and depression. Insomnia symptoms, particularly sleep onset insomnia, improved with psychotherapy for depression, with the greatest improvement seen in those with most improvement in their depression and anxiety. However, a considerable percentage of participants continued to experience insomnia despite remission of major depressive disorder and low symptoms of anxiety. These results suggest that treatment of depression and anxiety has the potential to greatly improve insomnia in MS but may not be sufficient to address the heterogeneous etiology of insomnia in many MS patients. Due to the multiple psychological, physiological, and behavioral factors that cause insomnia in MS, a multi-faceted intervention addressing each of the relevant factors is likely to be necessary in the treatment of insomnia, including screening for other primary sleep disorders (e.g., obstructive sleep apnea and restless legs syndrome), the management of depression and anxiety as well as pain.
Table 6.
| Variable | Estimate | Standard Error |
p |
|---|---|---|---|
| Insomnia Total Score | |||
| Intercept | 1.58 | .36 | <.0001 |
| Time | −.35 | .13 | <.01 |
| Therapy group | −.18 | .27 | .50 |
| Baseline HADS | .11 | .03 | <.01 |
| Change in HADS | −.02 | .04 | .62 |
| Therapy group × time | −.03 | .17 | .85 |
| Change in HADS × time | .05 | .02 | <.05 |
| Early Insomnia | |||
| Intercept | −1.71 | .52 | <.01 |
| Time | −.19 | .16 | .24 |
| Therapy group | −.31 | .37 | .40 |
| Baseline HADS | .16 | .05 | <.01 |
| Change in HADS | .008 | .05 | .88 |
| Therapy group × time | −.03 | .24 | .91 |
| Change in HADS × time | .07 | .03 | <.05 |
| Middle Insomnia | |||
| Intercept | −1.16 | .53 | <.05 |
| Time | −.56 | .21 | <.01 |
| Therapy group | −.11 | .36 | .77 |
| Baseline HADS | .12 | .05 | <.05 |
| Change in HADS | .01 | .05 | .77 |
| Therapy group × time | −.30 | .27 | .26 |
| Change in HADS × time | −.004 | .03 | .90 |
| Late Insomnia | |||
| Intercept | −3.06 | .70 | <.0001 |
| Time | .01 | .35 | .98 |
| Therapy group | .28 | .51 | .58 |
| Baseline HADS | .08 | .06 | .15 |
| Change in HADS | −.08 | .07 | .27 |
| Therapy group × time | .06 | .41 | .88 |
| Change in HADS × time | .11 | .05 | <.05 |
Acknowledgements
The project described was supported by Award Number K12HD055884 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development, NIH T32 HS00078, R01-MH059708, R01-HD043323. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health.
References
- 1.Brass SD, Duquette P, Proulx-Therrien J, Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev. 2009;14(2):121–129. doi: 10.1016/j.smrv.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Bamer AM, Cetin K, Johnson KL, Gibbons LE, Ehde DM. Validation study of prevalence and correlates of depressive symptomatology in multiple sclerosis. General Hospital Psychiatry. 2008;30(4):311–317. doi: 10.1016/j.genhosppsych.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Tachibana N, Howard RS, Hirsch NP, Miller DH, Moseley IF, Fish D. Sleep problems in multiple sclerosis. Eur Neurol. 1994;34(6):320–323. doi: 10.1159/000117070. [DOI] [PubMed] [Google Scholar]
- 4.Ferini-Strambi L, Filippi M, Martinelli V, Oldani A, Rovaris M, Zucconi M, et al. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. Journal of the Neurological Sciences. 1994;125(2):194–197. doi: 10.1016/0022-510x(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 5.Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Archives of Neurology. 2004;61(4):525–528. doi: 10.1001/archneur.61.4.525. [DOI] [PubMed] [Google Scholar]
- 6.Merlino G, Fratticci L, Lenchig C, Valente M, Cargnelutti D, Picello M, et al. Prevalence of 'poor sleep' among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep Med. 2009;10(1):26–34. doi: 10.1016/j.sleep.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Multiple Sclerosis. 2006;12(4):481–486. doi: 10.1191/135248506ms1320oa. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–S353. [PubMed] [Google Scholar]
- 9.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 10.Patten SB, Beck CA, Williams JV, Barbui C, Metz LM. Major depression in multiple sclerosis: a population-based perspective. Neurology. 2003;61(11):1524–1527. doi: 10.1212/01.wnl.0000095964.34294.b4. [DOI] [PubMed] [Google Scholar]
- 11.Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862–1868. doi: 10.1176/appi.ajp.159.11.1862. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety.[see comment] Sleep. 2005;28(11):1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 13.Brown RF, Valpiani EM, Tennant CC, Dunn SM, Sharrock M, Hodgkinson S, et al. Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother. 2009;82(Pt 1):41–56. doi: 10.1348/147608308X345614. [DOI] [PubMed] [Google Scholar]
- 14.Bruce JM, Arnett P. Clinical correlates of generalized worry in multiple sclerosis. J Clin Exp Neuropsychol. 2008:1–8. doi: 10.1080/13803390802484789. [DOI] [PubMed] [Google Scholar]
- 15.Pigeon WR, Hegel M, Unutzer J, Fan MY, Sateia MJ, Lyness JM, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146(2):105–114. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 17.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohr DC, Hart SL, Julian L, Catledge C, Honos-Webb L, Vella L, et al. Telephone-administered psychotherapy for depression. Archives of General Psychiatry. 2005;62(9):1007–1014. doi: 10.1001/archpsyc.62.9.1007. [DOI] [PubMed] [Google Scholar]
- 19.Sharrack B, Hughes RA. The Guy's Neurological Disability Scale (GNDS): a new disability measure for multiple sclerosis. Mult Scler. 1999;5(4):223–233. doi: 10.1177/135245859900500406. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Second Edition: Manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis 1 Disorders: Patient Edition (SCID-I/P), Version 2.0. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 23.Potts MK, Daniels M, Burnam MA, Wells KB. A structured interview version of the Hamilton Depression Rating Scale: evidence of reliability and versatility of administration. J Psychiatr Res. 1990;24(4):335–350. doi: 10.1016/0022-3956(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale: Third Edition. San Antonio: TX: Psychological Corporation; 1997. [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version. San Antonio: TX: Psychological Corporation; 1987. [Google Scholar]
- 27.Lezak MD. Neuropsychological Assessment. Third Edition ed. New York: Oxford University Press Inc.; [Google Scholar]
- 28.Beck AT. Cognitive Therapy: Basics and Beyond. New York: Guilford Press; 1995. [Google Scholar]
- 29.Mohr DC. The stress and mood management program for individuals with multiple sclerosis: Therapist's guide. New York: Oxford Press; 2010. [Google Scholar]
- 30.Mohr DC. The stress and mood management program for individuals with multiple sclerosis: Workbook. New York: Oxford Press; 2010. [Google Scholar]
- 31.Greenberg LS, Rice LN, Elliott R. Facilitating Emotional Change: The Moment by Moment Process. New York: Guilford Press; 1993. [Google Scholar]
- 32.De Pauw A, Dejaeger E, D'Hooghe B, Carton H. Dysphagia in multiple sclerosis. Clin Neurol Neurosurg. 2002;104(4):345–351. doi: 10.1016/s0303-8467(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 33.Howard RS, Wiles CM, Hirsch NP, Loh L, Spencer GT, Newsom-Davis J. Respiratory involvement in multiple sclerosis. Brain. 1992;115(Pt 2):479–494. doi: 10.1093/brain/115.2.479. [DOI] [PubMed] [Google Scholar]
- 34.Autret A, Lucas B, Mondon K, Hommet C, Corcia P, Saudeau D, et al. Sleep and brain lesions: a critical review of the literature and additional new cases. Neurophysiol Clin. 2001;31(6):356–375. doi: 10.1016/s0987-7053(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 35.Thomas FJ, Wiles CM. Dysphagia and nutritional status in multiple sclerosis. J Neurol. 1999;246(8):677–682. doi: 10.1007/s004150050431. [DOI] [PubMed] [Google Scholar]
- 36.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 37.Sauter C, Zebenholzer K, Hisakawa J, Zeitlhofer J, Vass K. A longitudinal study on effects of a six-week course for energy conservation for multiple sclerosis patients. Multiple Sclerosis. 2008;14(4):500–505. doi: 10.1177/1352458507084649. [DOI] [PubMed] [Google Scholar]
- 38.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164–1174. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 39.International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]