Recent experiences in other fields of medicine show that more intensive treatment does not lead to better outcomes […] We may need to re-consider the value of careful monitoring and conservative treatment as a valid and independent option in the treatment of AKI.
Keywords: acute kidney injury, critically ill patients, intermittent haemodialysis, intermittent high-volume predilution on-line haemofiltration, mortality
Abstract
Background
The optimal modality of dialysis treatment in critically ill patients with acute kidney injury (AKI) remains unclear. Intermittent high-volume predilution on-line haemofiltration (HF) is not a well-established dialysis modality. The purpose of the study was to compare clinical outcomes between HF and standard intermittent haemodialysis (HD) in this specific population.
Methods
In this prospective, randomized, controlled single-centre clinical study, we compared mortality and recovery of kidney function between HF and HD in critically ill adult patients with AKI. The primary study outcome was 60-day all-cause mortality. Secondary study outcomes included 30-day and in-hospital all-cause mortality along with recovery of kidney function. Time to kidney function recovery and the number of required dialysis procedures were analyzed in the subgroup of patients with in-hospital recovery of kidney function.
Results
Baseline characteristics of the 273 patients in the two study groups were similar. All-cause mortality by Day 60 was 65.0% in the HF group and 65.5% in the HD group (hazard ratio, 0.98; 95% confidence interval, 0.71–1.33; P = 0.87). There were also no significant differences between the two groups in 30-day and in-hospital all-cause mortality or recovery of kidney function. Time to kidney function recovery and the number of required dialysis procedures were similar between the HF and the HD subgroup of patients with in-hospital recovery of kidney function.
Conclusions
Dialysis treatment with intermittent high-volume predilution on-line HF in critically ill patients with AKI did not decrease mortality, improve recovery of kidney function or reduce the need for dialysis support compared to standard intermittent HD.
Introduction
Acute kidney injury (AKI) develops in more than one-third of critically ill patients treated in intensive care units (ICUs) [1], predominantly due to acute tubular necrosis (ATN) and as part of multiple organ failure (MOF). Essential supporting treatment of severe AKI is acute dialysis support, which is required in 5–6% of critically ill ICU patients [1, 2]. As a result of development and widespread use of dialysis treatment, complications of AKI are nowadays uncommon cause of death. Even so, AKI represents an important risk factor of morbidity and mortality [3] and AKI as part of MOF is still associated with high in-hospital mortality rates of 50–80% [1–6]. Although >60 years have passed since the first successful clinical use of dialysis in AKI, many fundamental issues concerning the optimal approach to dialysis management of AKI in critically ill patients with MOF, including the selection of dialysis modality, are still controversial [7–9].
In this specific population, different dialysis modalities have been introduced and some are already well-established. However, despite long-term clinical experiences and numerous studies, there is no consistent evidence that any particular modality has advantage over the others due to better clinical outcomes [10–15]. Standard and most commonly prescribed dialysis modality is intermittent haemodialysis (HD). Haemofiltration (HF) has been established mainly as continuous modality [2, 9], while intermittent high-volume predilution on-line HF is relatively new and rarely applied in everyday clinical practice. Intermittent HF is presently well-established in chronic dialysis patients, in whom various studies proved important advantages of chronic HF compared to HD [16–19].
In AKI as part of MOF, high-volume HF is supposed to confer beneficial effects particularly in critically ill patients with sepsis or other systemic inflammatory response syndromes, owing to higher convective clearances for middle/high-molecular-weight humoural mediators involved in the development of MOF [20–22]. There are several small, observational non-randomized studies in severely ill ICU patients with advanced MOF that suggested improved patient outcomes when intermittent high-volume HF [23–26] or haemodiafiltration [27] was applied, but no relevant randomized controlled study has been published yet.
We performed a randomized controlled study to test the hypothesis that dialysis treatment with intermittent high-volume predilution on-line HF in critically ill patients with AKI can improve clinical outcomes, i.e. decrease mortality, increase recovery of kidney function and reduce the need for dialysis support compared to standard intermittent HD.
Materials and methods
Study design, setting and patients
This prospective, randomized single-centre clinical study comparing patient outcomes between intermittent high-volume predilution on-line HF and standard intermittent HD in critically ill ICU patients with AKI as part of MOF was undertaken at the Department of Nephrology, University Medical Centre Ljubljana, Slovenia.
Patients were eligible for enrolment if they were at least 18 years old, in ICU-treated critically ill patients, who had AKI due to ATN (based on clinical criteria) requiring acute dialysis support as well as a failure of at least one additional (non-renal) organ. Patients were excluded from the study if they had AKI due to other aetiology (not solely due to ATN), chronic kidney disease (baseline serum creatinine concentration ≥150 μmol/L), prior kidney or other organ transplantation, acute haematological or terminal stage malignancy, if they had received more than one intermittent or >24 h of continuous dialysis procedure prior to enrolment or if less than one study dialysis procedure was performed after enrolment (i.e. <50% of the first procedure due to patient death).
Patients were recruited from different ICUs of the University Medical Centre Ljubljana, Slovenia. Simple randomization was done. Eligible patients were randomly assigned to either the HF group or the HD group in 1:1 ratio according to randomization table by Fleiss [28]. The study group was written in closed numbered envelopes and thus blinded until enrolment of individual patient. In order to assess the adequacy of randomization, the following baseline patient characteristics at dialysis initiation were compared between the two modality groups: age, gender, weight, diuresis, serum concentration of creatinine, urea, potassium, bicarbonate and pH, presence of oliguria (diuresis < 400 mL/day), serum concentration of creatinine >300 μmol/L, urea >30 mmol/L, potassium >5.5 mmol/L, pH <7.2, fluid overload, mechanical ventilation, aetiological factors of AKI, number of failed non-renal organs and values of three different scoring systems, i.e. Acute Physiology and Chronic Health Evaluation II score (APACHE II) [29], Cleveland Clinic Foundation–Acute Renal Failure score [30] and Modified Organ System Failure score [31].
The study was performed in accordance with the ethical principles of the ‘Declaration of Helsinki’, and it was approved by The National Medical Ethics Committee of the Republic of Slovenia, which waived written patient informed consent for study enrolment. Due to the severity of the underlying critical illness and complications of AKI, patients were not legally competent and written informed consent could not be obtained from them. A decision about patient's enrolment was left to the discretion of the treating physicians taking into consideration the assumed patient's will according to opinion and principal consent from patient's relatives or legal representatives.
The study was registered in The Cochrane Renal Group registry (CRG030600055).
Interventions
Decision to initiate dialysis treatment was a specific inclusion criterion, so it had to be made ahead of patient's enrolment independently of the study. Indications for and the timing of dialysis initiation were not dictated by the study protocol but were determined in adherence to the generally accepted clinical practice. Dialysis modality was prescribed according to the assigned study group, i.e. either intermittent high-volume predilution on-line HF or intermittent HD. The modality assigned to individual patient at randomization was prescribed from the first dialysis procedure after enrolment to the last one.
General parameters of standard HD were not dictated by the protocol but were prescribed individually by the attending nephrologists with respect to temporary patient clinical characteristics and treatment goals. Several parameters of HF were dictated by the protocol, but others were prescribed individually. Further details of the prescribed parameters of intermittent HD and intermittent high-volume predilution on-line HF are described in the Appendix 1 and the Appendix 2, respectively. Special protocol for regional citrate anticoagulation for HF was designed and successfully applied in patients with increased bleeding risk [32]. Temporary untunnelled HD catheters were used as vascular access in all study patients [33].
Criteria for dialysis discontinuation were not dictated by the protocol but were the same as in regular practice, i.e. recovery of kidney function, patient death or withdrawal of life-sustaining therapy, including dialysis support. Recovery of kidney function was defined as improvement of kidney function to dialysis independence on the basis of clinical criteria. In patients with recovery of kidney function, time to recovery was considered to be equivalent to duration of dialysis treatment.
All study patients were followed up until the end of hospitalization, i.e. either until hospital discharge to home or death.
Study outcomes
The primary study outcome was mortality from any cause by Day 60 after randomization. Secondary outcomes included 30-day and in-hospital all-cause mortality along with 30-day and in-hospital recovery of kidney function. Time to kidney function recovery and the number of required dialysis procedures were analysed in the subgroup of patients with in-hospital recovery of kidney function.
Statistical analyses
Sample size calculation was based on the primary study outcome. To test the primary hypothesis, i.e. to detect a decrease in 60-day all-cause mortality from a priori estimated 65% (in the HD group) to 50% (in the HF group), at least 166 patients were required in each study group with an alpha risk set at 5% and a statistical power of at least 80%. Assuming a 10% dropout rate, we planned to enrol altogether 370 patients.
Because it was a single-centre study with a limited rate of patients' recruitment, interim assessments were performed in order to readjust the enrolment. When the number of the enroled patients had reached >80% of the required sample size, the interim analysis showed such a negligible difference in the primary study outcome between the two groups, that the detection of statistically significant difference could not be expected even if greater number of patients were enroled. For this reason, the investigators decided to end patients' recruitment prematurely.
All study outcomes were analysed according to the intention-to-treat principle. Normally distributed continuous variables were expressed as means ± SD and compared with the Student's t-test. Non-normally distributed variables were expressed as medians with interquartile ranges and compared with the non-parametric Mann–Whitney U-test. Proportions were compared with the chi-square test.
Rates of cumulative mortality and recovery of kidney function were calculated according to the Kaplan–Meier survival method. Comparisons of the Kaplan–Meier curves were performed with the log-rank test. Multivariate analyses of mortality and recovery of kidney function were performed using the Cox proportional hazard regression model, testing the effect of dialysis modality adjusted for the pre-specified variables considered to be of clinical significance, i.e. patient age, gender, APACHE II score, presence of oliguria, sepsis and major surgery. Additional subgroup analyses were performed with regard to the presence or absence of patient age >65 years, APACHE II score >25, oliguria and sepsis.
Two-sided P-value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using the SPSS statistical software (version 16.0; SPSS Inc., Chicago, IL).
Results
Enrolment
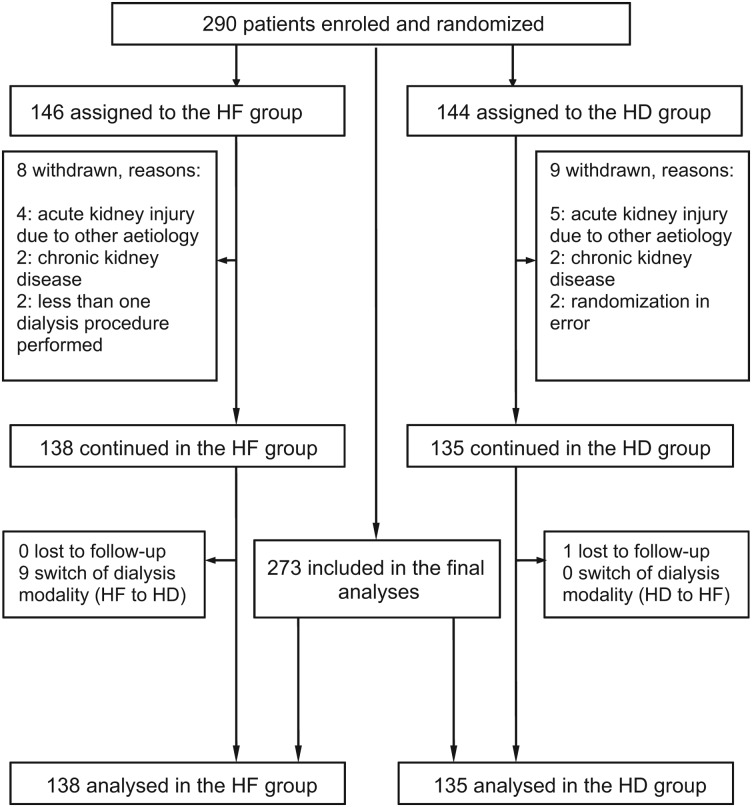
Between December 2004 and January 2008, altogether 290 critically ill adult patients with AKI as part of MOF treated in eight different ICUs of the University Medical Centre Ljubljana were enroled in the study, 146 patients were randomly assigned to the HF group and 144 patients to the HD group (Figure 1). A total of 17 (5.9%) patients (9 in the HD and 8 in the HF group) were withdrawn after randomization because they were subsequently found to be ineligible for the study. Finally, 273 patients (138 patients in the HF group and 135 patients in the HD group) continued the study and were included in the final analyses. Only one patient in the HD group was lost to follow-up, all other patients were followed up until the end of hospitalization (overall until the end of May 2008).
Fig. 1.
Diagram showing enrolment, randomization and follow-up of study patients.
Baseline characteristics
As shown in Table 1, baseline characteristics were similar between the two study groups, except for myoglobinuria, which was more frequent cause of AKI in the HD group. The mean (±SD) age was 68.7 ± 12.5 years, 66.3% of patients were male, 42.1% had oliguria and 75.1% required mechanical ventilation. AKI was most frequently attributed to sepsis (80.6%), ischaemia (69.2%) and major operation (39.2%). Other common aetiological factors were nephrotoxic antibiotics, myoglobinuria and radiocontrast agents. Multiple potential causes of AKI were present in 84.6% of patients.
Table 1.
Baseline characteristics of study patientsa
| Characteristic | Overall (N = 273) | HF group (N = 138) | HD group (N = 135) | P-value |
|---|---|---|---|---|
| Age (years) | 68.7 ± 12.5 | 67.9 ± 12.2 | 69.5 ± 12.8 | 0.30 |
| Male gender | 181 (66.3) | 91 (65.9) | 90 (66.7) | 0.90 |
| Weight (kg) | 79.7 ± 15 | 81.3 ± 14.1 | 77.8 ± 16.4 | 0.19 |
| Urine output (mL/day) | ||||
| Mean ± SD | 1135 ± 1176 | 1151 ± 1246 | 1118 ± 1094 | 0.83 |
| <400 | 115 (42.1) | 58 (42.0) | 57 (42.2) | 0.97 |
| Creatinine (μmol/L) | ||||
| Mean ± SD | 472 ± 165 | 481 ± 158 | 463 ± 171 | 0.37 |
| >300 | 237 (86.8) | 124 (89.8) | 113 (83.7) | 0.22 |
| Urea (mmol/L) | ||||
| Mean ± SD | 40.9 ± 15.5 | 39.4 ± 14.8 | 42.3 ± 16.1 | 0.14 |
| >30 | 187 (68.5) | 92 (66.7) | 95 (70.4) | 0.55 |
| Potassium (mmol/L) | ||||
| Mean ± SD | 4.9 ± 1.1 | 4.8 ± 0.9 | 5.0 ± 1.2 | 0.09 |
| >5.5 | 66 (24.2) | 28 (20.3) | 38 (28.1) | 0.10 |
| pH | ||||
| Mean ± SD | 7.29 ± 0.11 | 7.29 ± 0.11 | 7.29 ± 0.12 | 0.86 |
| <7.20 | 49 (17.9) | 27 (19.6) | 22 (16.3) | 0.54 |
| Bicarbonate (mmol/L) | 20.8 ± 5.4 | 20.5 ± 4.8 | 21.2 ± 6.1 | 0.36 |
| Fluid overload | ||||
| Yes | 145 (53.1) | 75 (54.3) | 70 (51.9) | 0.73 |
| Mechanical ventilation | 205 (75.1) | 107 (77.5) | 98 (72.6) | 0.33 |
| Cause of ATN | ||||
| Sepsis | 220 (80.6) | 109 (79.0) | 111 (82.2) | 0.50 |
| Ischaemia | 189 (69.2) | 90 (65.2) | 99 (73.3) | 0.15 |
| Major surgery | 107 (39.2) | 53 (38.4) | 54 (40.0) | 0.79 |
| Nephrotoxic antibiotics | 94 (34.4) | 44 (31.9) | 50 (37.0) | 0.37 |
| Myoglobinuria | 68 (24.9) | 26 (18.8) | 42 (31.1) | 0.03 |
| Radiocontrast agents | 58 (21.2) | 33 (23.9) | 25 (18.5) | 0.28 |
| Other | 23 (8.4) | 13 (9.4) | 10 (7.4) | 0.55 |
| Number of failed non-renal organs | 3.3 ± 1.1 | 3.4 ± 1.1 | 3.2 ± 1.2 | 0.28 |
| Severity of illness scoring system | ||||
| APACHE II score | 31.1 ± 7.1 | 31.4 ± 7.6 | 30.9 ± 6.7 | 0.56 |
| CCF–ARF score | 10.6 ± 3.3 | 10.9 ± 3.1 | 10.3 ± 3.4 | 0.15 |
| MOSF score | 8.7 ± 2.5 | 8.8 ± 2.3 | 8.5 ± 2.7 | 0.27 |
aData are presented as means ± SD or as total numbers (percentages). CCF–ARF score, Cleveland Clinic Foundation–Acute Renal Failure score; MOSF score, Modified Organ System Failure Score.
Management of study dialysis treatment
In the HF group, 129 (93.5%) patients received the assigned dialysis modality in >85% of all performed dialysis procedures. In nine patients (6.5%), HF was either switched to HD in all or was performed in <25% of all procedures. The most common reasons for the switch of HF to HD were technical problems, protocol violations and transfer of the patients from the University Medical Centre Ljubljana to another hospital. In the HD group, all patients received the assigned modality in all performed procedures.
Characteristics of study dialysis treatment and patients' follow-up are presented in Table 2. The number of dialysis procedures performed and the mean duration of dialysis treatment were similar between the two study groups, although the mean duration of the procedure was significantly longer in the HF group (4.8 versus 4.0 h; P < 0.001). The mean volume of infusate in the HF group was 81 ± 15 L, corresponding to the prescribed volume. Regional citrate anticoagulation was used in 33.7% of all procedures, more frequently in the HF group.
Table 2.
Characteristics of study dialysis treatment and follow-up of study patientsa
| Characteristic of study dialysis treatment | Overall (N = 273) | HF group (N = 138) | HD group (N = 135) |
|---|---|---|---|
| Dialysis procedures performed (number) | 2679 | 1321 | 1358 |
| Dialysis procedures/patient (number) | |||
| Mean ± SD | 9.8 ± 13.7 | 9.6 ± 13.8 | 10.0 ± 13.6 |
| Median (interquartile ranges) | 4 (2–13) | 4 (2–13) | 4 (2–13) |
| Duration of dialysis treatment (days) | |||
| Mean ± SD | 17.5 ± 30.7 | 17.0 ± 34.3 | 18.1 ± 26.7 |
| Median (interquartile range) | 7 (2–21) | 7 (2–18) | 7 (1–23) |
| Duration of dialysis procedure (hours) | |||
| Mean ± SD | 4.4 ± 0.94 | 4.8 ± 0.8 | 4.0 ± 0.9 |
| Median (interquartile range) | 4.5 (4.0–5.0) | 5.0 (4.5–5.5) | 4.0 (3.5–4.5) |
| Blood flow rate (mL/min) | 294 ± 48 | 316 ± 51 | 271 ± 31 |
| Dialysate flow rate (mL/min) | NA | NA | 500 |
| Volume of infusate (L) | |||
| Mean ± SD | NA | 81 ± 15 | NA |
| Median (interquartile range) | NA | 80 (72–96) | NA |
| Anticoagulation (%) | |||
| None | 4.8 | 0 | 9.6 |
| Heparin | 61.5 | 58.7 | 64.5 |
| Citrate | 33.7 | 41.3 | 25.9 |
| Surrogate markers of dialysis dose | |||
| Daily plasma urea (mmol/L)b | 29.1 ± 8.6 | 28.5 ± 8.3 | 29.8 ± 8.9 |
| Daily serum creatinine (μmol/L)b | 327 ± 117 | 321 ± 116 | 332 ± 120 |
| Follow-up of study patients (days) | |||
| Median (interquartile range) | 22 (7–56) | 21 (7–54) | 23 (6–58) |
aData are presented as total numbers, percentages, means ± SD or as medians (interquartile ranges). NA, not applicable.
bConcentrations were measured in routine morning blood samples.
Study outcomes
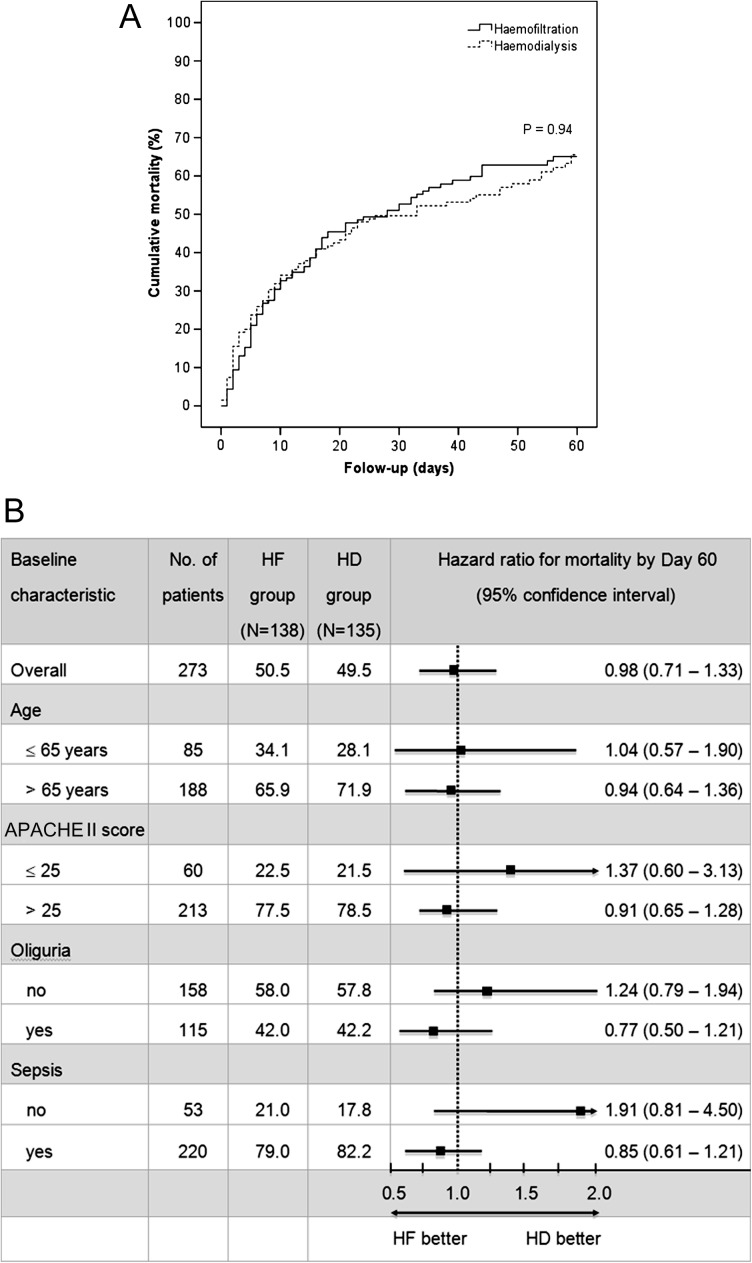
Total all-cause mortality by Day 60 was 65.3% and was similar between the HF and the HD study group (65.0 versus 65.5%, P = 0.94) (Figure 2A). Multivariate analysis adjusted for the pre-specified variables confirmed that dialysis treatment with HF was not significantly associated with 60-day mortality (hazard ratio, 0.98; 95% confidence interval, 0.71–1.33; P = 0.87) (Table 3). Mortality by Day 60 was also not significantly different between the two study groups regarding all pre-specified subgroups (Figure 2B).
Fig. 2.
Kaplan–Meier estimates of cumulative mortality (panel A) and hazard ratios for mortality by Day 60, according to baseline characteristics (panel B). Panel A shows the cumulative mortality from any cause in the HF and the HD study group. Panel B shows hazard ratios (and 95% confidence intervals) for mortality by Day 60 in the HF group as compared with the HD group. There was no significant interaction between dialysis modality and subgroup variables.
Table 3.
Primary and secondary study outcomes
| Outcome | Overall (N = 273) | HF group (N = 138) | HD group (N = 135) | Hazard ratioa (95% CI) | P-value |
|---|---|---|---|---|---|
| 60-Day mortalityb | 65.3 (59.2–71.4) | 65.0 (56.4–73.6) | 65.5 (56.774.3) | 0.98 (0.71–1.33) | 0.87 |
| 30-Day mortalityb | 51.2 (45.1–57.3) | 52.7 (44.3–61.1) | 49.6 (41.0–58.2) | 0.93 (0.66–1.31) | 0.69 |
| In-hospital mortalityc | 193 (70.7) | 95 (68.8) | 98 (72.6) | 0.97 (0.72–1.29) | 0.82 |
| 30-Day recovery of kidney functionb | 60.6 (52.6–68.6) | 65.4 (54.0–76.8) | 55.5 (43.9–67.1) | 1.25 (0.84–1.85) | 0.28 |
| In-hospital recovery of kidney functionc | 124 (45.4) | 65 (47.1) | 59 (43.7) | 1.12 (0.77–1.61) | 0.56 |
aHazard ratios (95% CI) in the HF group as compared with the HD group were calculated using the Cox proportional hazard regression model adjusted for the following pre-specified covariates: patient age, gender, APACHE II score, presence of oliguria, sepsis and major surgery. CI, confidence interval.
bData are presented as percentages (95% CI); analysis with the Kaplan–Meier survival method.
cData are presented as total numbers (percentages).
Total 30-day and in-hospital all-cause mortality was 51.2 and 70.7%, respectively. Total 30-day and in-hospital recovery of kidney function was 60.6 and 45.4%, respectively. Multivariate analyses showed no significant differences in any of the secondary outcomes between both modality groups (Table 3).
Kidney function has recovered during hospitalization in 124 (45.4%) patients, which is in all hospital survivors as well as in 44 (22.8%) hospital non-survivors. There was a trend towards fewer required dialysis procedures and faster recovery of kidney function in the HF subgroup compared to the HD subgroup of patients with in-hospital recovery of kidney function; however, these differences were non-significant (Table 4).
Table 4.
Subgroup of patients with in-hospital recovery of kidney functiona
| Characteristic of study dialysis treatment | Overall (N = 124) | HF subgroup (N = 65) | HD subgroup (N = 59) | P-value |
|---|---|---|---|---|
| Dialysis procedures performed (number) | 1227 | 517 | 710 | NA |
| Dialysis procedures/patient (number) | ||||
| Mean ± SD | 9.9 ± 12.2 | 8.0 ± 7.8 | 12.0 ± 15.6 | 0.06 |
| Median (interquartile range) | 5 (2–13) | 4 (2–12) | 7 (2–13) | 0.38 |
| Duration of dialysis treatment (days) | ||||
| Mean ± SD | 17.7 ± 24.2 | 14.9 ± 21.3 | 20.7 ± 27.0 | 0.18 |
| Median (interquartile range) | 10 (3–24) | 8 (3–20) | 14 (3–30) | 0.20 |
aData are presented as total numbers, means ± SD or as medians (interquartile ranges). NA, not applicable.
Complications of dialysis treatment
Hypotension, defined as an intratreatment reduction in mean arterial blood pressure of >20% from the pre-treatment value in at least one study dialysis procedure, was reported in altogether 124 patients (45.4%), among whom 73 patients (26.7%) had hypotension that required intratreatment introduction or escalation of vasopressors. Hypotension developed in lower proportion of patients receiving HF treatment (40.6%) than HD treatment (50.4%); however, the difference was not statistically significant (Table 5). Hypokalaemia (at least one measurement of serum potassium <3.8 mmol/L) was detected in 42.8% of patients in the HF group as compared with 35.6% in the HD group (P = 0.22) and hypophosphataemia (at least one measurement of serum phosphate <0.8 mmol/L) in 10.1 and 7.4%, respectively (P = 0.43). The proportions of patients who underwent at least one episode of any serious adverse event requiring discontinuation of dialysis procedure were not significantly different between the two modality groups (Table 5).
Table 5.
Summary of complications associated with study dialysis treatmenta
| Complication | Overall (N = 273) | HF group (N = 138) | HD group (N = 135) | P-value |
|---|---|---|---|---|
| Hypotension (>20% reduction in MAP) | 124 (45.4) | 56 (40.6) | 68 (50.4) | 0.13 |
| Requiring introduction or escalation of vasopressors | 73 (26.7) | 32 (23.2) | 41 (30.4) | 0.18 |
| Electrolyte disturbanceb | ||||
| Hypokalaemia | 107 (39.2) | 59 (42.8) | 48 (35.6) | 0.22 |
| Hypophosphataemia | 24 (8.8) | 14 (10.1) | 10 (7.4) | 0.43 |
| Other serious adverse eventsc | ||||
| Arrhythmia causing haemodynamic instability | 24 (9.5) | 11 (8.0) | 13 (9.6) | 0.63 |
| Bleeding | 9 (3.3) | 4 (2.9) | 5 (3.7) | 0.71 |
| Filter clotting | 11 (4.0) | 7 (5.1) | 4 (3.0) | 0.38 |
aData are presented as total numbers (percentages) of patients with at least one complication episode. MAP, mean arterial pressure.
bConcentrations were measured in routine morning blood samples.
cRequiring discontinuation of dialysis procedure.
Discussion
In this randomized, controlled single-centre clinical study, intermittent high-volume predilution on-line HF did not significantly decrease mortality of critically ill ICU patients with AKI as part of MOF compared to standard intermittent HD. There were also no significant differences in the rate of kidney function recovery or the need for dialysis support. Complications associated with dialysis treatment developed in similar proportions of patients in both modality groups.
Our results disagree with those from small, observational non-randomized studies that suggested improved clinical outcomes in severely ill ICU patients with advanced MOF, who were treated with intermittent high-volume HF [23–26] or haemodiafiltration [27]. However, no relevant randomized controlled study has been published beforehand. Mortality rate in the present study is higher than the rates reported in several other studies involving critically ill ICU patients with AKI as part of MOF [10, 34–39]. Higher values of prognostic scores and advanced MOF indicate worse prognosis of our study patients at enrolment compared to these studies. The main reason is probably the design of our study, namely non-requirement of written patient informed consent as inclusion criterion, which allowed more extensive and uniform enrolment of the most severely ill patients (including the patients requiring immediate initiation of dialysis) as well. Another study by Gastaldello et al. [40], which investigated the influence of different types of dialysis membranes on the clinical outcomes of AKI in critically ill ICU patients, used similar approach. Difficulties in acquiring written informed consent represent one of the major obstacles to recruitment of critically ill ICU patients. Studies have shown that in this particular population, restricting a study sample only to patients with obtained consent may lead to selection bias and thus limit generalizability of study results [41–43]. Time constraints associated with obtaining the consent disproportionately prevent from study enrolment mostly patients with the highest and the earliest mortality and may result in a ‘death before consent’ bias [41]. Avoiding such potential selective enrolment due to non-requirement of written consent has presumably contributed substantially to high mortality in our patient cohort but on the other hand enabled our study sample to be highly representative of the broad spectrum of critically ill patients with AKI as part of MOF. An additional reason for high mortality is sepsis, which was the most frequent cause of AKI (potentially contributing to development of AKI in 80.6% of all study patients) and is the leading cause of mortality in critically ill ICU patients [3, 6, 44].
Our results of kidney function recovery are comparable with those reported in previous studies, showing partial or complete recovery of kidney function following AKI as part of MOF in up to 95% of surviving as well as in up to 20% of non-surviving patients [10, 11, 35–37]. Kidney function has recovered in all our hospital survivors, which confirms a high potential for recovery of kidney function in case of ‘de novo’ AKI due to ATN also in critically ill patients with MOF, providing their survival and recovery from critical illness.
To our knowledge, this is the first randomized controlled study that compared clinical outcomes between intermittent high-volume predilution on-line HF and standard intermittent HD in critically ill ICU patients with AKI as part of MOF. Nevertheless, the lack of statistical power remains an important limitation of our study. Recruitment of study patients ended before the required sample size was reached; consequently, the study is underpowered to detect statistically significant reduction in mortality in the HF as compared with the HD group. Although our results cannot exclude the possibility that high-volume predilution on-line HF confers better clinical outcomes in critically ill patients with AKI as part of MOF, they imply that potential survival benefit, if present at all, would not be clinically relevant. In addition, our study is subject to several other limitations. Firstly, indications for and the timing of dialysis initiation were not dictated by the study protocol but were equal as in everyday clinical practice, based on broader patient clinical characteristics and trends of standard parameters, i.e. uraemia, hyperkalaemia, metabolic acidaemia, oliguria and/or fluid overload. There were no differences regarding these initiation criteria between the two groups. Except for emergency indications, clinical and biochemical parameters pointing to the optimal time to initiate dialysis support in critically ill patients with AKI as part of MOF remain undefined [45]. There are only few suitably designed high-quality studies, which have not confirmed better patient outcomes with ‘early’ versus ‘late’ dialysis initiation [37, 46, 47], therefore, we assume that the timing of initiation did not significantly influence our results. Secondly, dialysis dose or the intensity of dialysis treatment was not standardized in the protocol but was prescribed individually with respect to temporary treatment goals based on everyday patients' assessment. Likewise, actual delivered dose was not estimated and compared between the two study groups by means of standard parameters. However, the issue of dialysis dose and the validation of adequacy parameters are controversial in many aspects. Above all, critically ill patients with severe AKI differ substantially from stable chronic dialysis patients, in whom standard parameters were established and validated [48–51]. Furthermore, in contrast to guidelines concerning chronic dialysis (recommending high doses), there is no consensus on the optimal dialysis dose in this specific group of patients [48, 49]. Few earlier studies suggested superior clinical outcomes with higher doses [34–36], but many recent studies, including two multicentre, so far the largest randomized controlled studies among critically ill ICU patients with AKI as part of MOF, proved no significant improvements in survival or recovery of kidney function with higher compared to conventional doses, regardless of dialysis modality [37–39, 52, 53]. These studies present relatively firm evidence that in this population, increasing the intensity of dialysis support beyond the standard level provides no additional clinical benefit. Accordingly, we can assume that even though dialysis dose was possibly different between the two study groups, the intensity of dialysis treatment did not significantly affect our results. Mean daily urea and creatinine concentration as the main blood markers of uraemic retention/dialytic clearance were not significantly different between the HF and the HD groups, which suggests similar dialysis dose in both modality groups, although we agree with many other authors that concerning various complex problems in patients with AKI as part of MOF small solute clearance or surrogate markers alone cannot adequately reflect dialysis efficiency or cover the wide-ranging goals of dialysis support. Finally, patients with pre-existing chronic kidney disease were excluded from our study because they represent unique population with distinctively different prognosis (that is with both lower mortality and lower potential for kidney function recovery) compared to patients with ‘de novo’ AKI [54–56]. Still, patients with acute-on-chronic kidney injury constitute a considerable proportion of critically ill ICU patients requiring acute dialysis support. Because of their exclusion, our results cannot be generalized to such patients but are limited only to patients with true ‘de novo’ AKI due to ATN.
In critically ill patients with AKI as part of MOF, there is no consistent evidence that particular dialysis modality is superior to the others owing to better clinical outcomes, therefore, the selection of the optimal modality is still questionable. Although our study did not show improved clinical outcomes of intermittent high-volume predilution on-line HF compared to standard intermittent HD, it suggests that the application of high-volume on-line convective modality is feasible, safe and effective also in this specific population, in whom it is currently used only rarely in everyday clinical practice. Additional analyses addressing the technical issues, the workload required from dialysis staff and the costs are required to estimate whether this relatively novel dialysis modality could be added to the spectrum of modalities that are already established and applied routinely. In our dialysis centre, we have long-term clinical experiences with intermittent high-volume on-line HF in chronic dialysis patients, which is a valuable advantage. Very recently, Kron et al. [27] reported that having used the conventional on-line dialysis equipment already available in daily routine of dialysis facility (as it was in our study as well), the material costs per one intermittent high-volume on-line haemodiafiltration session were comparable to the costs per one standard HD session in chronic dialysis patient and thus much more cost-effective compared to conventional continuous modalities requiring expensive solution bags. We believe that the availability of larger choice of different modalities (diffusive and convective as well as intermittent and continuous), their exchange and combinations is beneficial because it facilitates more individual dialysis care, which might possibly be ‘the best dialysis modality’ or ‘the best approach to dialysis treatment’ in critically ill patients.
In conclusion, the present study indicates that dialysis treatment with intermittent high-volume predilution on-line HF in critically ill patients with AKI as part of MOF does not improve survival or recovery of kidney function compared to standard intermittent HD. Nevertheless, we have demonstrated that high-volume predilution on-line HF with individual reverse osmosis and regional citrate anticoagulation can be performed easily, safely and effectively also in the most severely ill ICU patients, which promises favourable options for further exploration of high-volume on-line convective modalities in the ICUs. The optimal approach to prescribe modality of dialysis treatment that could potentially improve the grave prognosis of these patients remains to be defined.
Acknowledgements
The authors express gratitude to the doctors and nurses of the Department of Nephrology and the ICUs, University Medical Centre Ljubljana, Slovenia, who participated in the treatment of study patients.
Conflict of interest statement. None declared.
(See related article by Lins. RRT treatment for AKI: is more always better? Nephrol Dial Transplant 2012; 27: 4252–4255.)
Appendix 1. Prescribed parameters of intermittent HD
Dialysis monitor: Gambro AK-200 ULTRA S (Gambro, Lund, Sweden) with individual water treatment system WRO 300 (Gambro).
Haemodialyser: biocompatible synthetic highly permeable hollow-fibre membrane (Polyflux; Gambro or FX; Fresenius Medical Care, Bad Homburg, Germany) of different areas with respect to patient's body surface (body weight and height).
Blood flow rate: 250–300 mL/min.
Dialysate flow rate: 500 mL/min.
Composition of dialysate (electrolyte concentrations in mmol/L): sodium 140–150, potassium 2–4, calcium 1.25–1.75, magnesium 0.5, bicarbonate 26–40, glucose 5.5, chloride 108.0–109.5, acetate 3.
Temperature of dialysate: prescribed individually; 35–38°C.
Neto ultrafiltration: prescribed individually; 0–500 mL/h.
Procedure duration: prescribed individually; generally 3–5 h.
Procedure schedule: prescribed individually; generally every day or every alternate day.
Anticoagulation: prescribed individually; standard heparin, regional citrate anticoagulation, no anticoagulation (heparin-free).
Vascular access: temporary untunnelled HD catheter; insertion site and type prescribed individually; femoral, jugular or subclavian; single-lumen or double-lumen.
Appendix 2. Prescribed parameters of intermittent high-volume predilution on-line HF
Dialysis monitor: Gambro AK-200 ULTRA S (Gambro) with individual water treatment system WRO 300 (Gambro).
Haemofilter: biocompatible synthetic highly permeable hollow-fibre membrane, with an area of 2.4 m2 (Polyflux 24S; Gambro) or 2.2 m2 (FX 100; Fresenius Medical Care).
Blood flow rate: 250–400 mL/min.
Volume of infusate (replacement fluid): ∼1.3 times the dry body weight, i.e. 60 L at dry body weight <50 kg, 60–100 L at dry body weight of 50–80 kg and 100 L at dry body weight >80 kg.
Degree of predilution (infusate flow rate/blood flow rate ratio): 1–1.5.
Composition of infusate: equivalent to composition of dialysate.
Temperature of infusate: prescribed individually; 35–38°C.
Neto ultrafiltration: prescribed individually; 0–500 mL/h.
Procedure schedule: prescribed individually; generally every day or every alternate day.
Anticoagulation: prescribed individually; standard heparin, regional citrate anticoagulation.
Vascular access: temporary untunnelled HD catheter; insertion site and type prescribed individually; femoral, jugular or subclavian; single-lumen or double-lumen.
References
- 1.Liaño F, Junco E, Pascual J, et al. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl. 1998;66:S16–S24. [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock—a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23:904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 4.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 5.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 7.Davenport A, Bouman C, Kirpalani A, et al. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? Clin J Am Soc Nephrol. 2008;3:869–875. doi: 10.2215/CJN.04821107. [DOI] [PubMed] [Google Scholar]
- 8.Pannu N, Klarenbach S, Wiebe N, et al. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 9.Ricci Z, Ronco C, D'Amico G, et al. Practice patterns in the management of acute renal failure in the critically ill patient: an international survey. Nephrol Dial Transplant. 2006;21:690–696. doi: 10.1093/ndt/gfi296. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, McDonald B, Gabbai FB, et al. A randomized clinic trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 11.Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent hemodialysis for acute renal failure patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 12.Uehlinger DE, Jakob SM, Ferrari P, et al. Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrol Dial Transplant. 2005;20:1630–1637. doi: 10.1093/ndt/gfh880. [DOI] [PubMed] [Google Scholar]
- 13.Augustine JJ, Sandy D, Seifert TH, et al. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44:1000–1007. doi: 10.1053/j.ajkd.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24:512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 15.Bagshaw SM, Berthiaume LR, Delaney A, et al. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36:610–617. doi: 10.1097/01.CCM.0B013E3181611F552. [DOI] [PubMed] [Google Scholar]
- 16.Quellhorst E, Scheunemann B, Hildebrand U. Hemofiltration—an improved method of treatment for chronic renal failure. Contrib Nephrol. 1985;44:194–211. doi: 10.1159/000410211. [DOI] [PubMed] [Google Scholar]
- 17.Altieri P, Sorba G, Bolasco P, et al. Predilution haemofiltration—the Second Sardinian Multicentre Study: comparisons between haemofiltration and haemodialysis during identical Kt/V and session times in a long-term cross-over study. Nephrol Dial Transplant. 2001;16:1207–1213. doi: 10.1093/ndt/16.6.1207. [DOI] [PubMed] [Google Scholar]
- 18.Beerenhout CH, Luik AJ, Jeuken-Mertens SG, et al. Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–1163. doi: 10.1093/ndt/gfh775. [DOI] [PubMed] [Google Scholar]
- 19.Santoro A, Mancini E, Bolzani R, et al. The effect of on-line high-flux hemofiltration versus low-flux hemodialysis on mortality in chronic kidney failure: a small randomized controlled trial. Am J Kidney Dis. 2008;52:507–518. doi: 10.1053/j.ajkd.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Honoré PM, Joannes-Boyau O. High volume hemofiltration (HVHF) in sepsis: a comprehensive review of rationale, clinical applicability, potential indications and recommendations for future research. Int J Artif Organs. 2004;27:1077–1082. doi: 10.1177/039139880402701211. [DOI] [PubMed] [Google Scholar]
- 21.Ronco C, Kellum JA, Bellomo R, et al. Potential interventions in sepsis-related acute kidney injury. Clin J Am Soc Nephrol. 2008;3:531–544. doi: 10.2215/CJN.03830907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouman CS, Oudemans-van Straaten HM, Schultz MJ, et al. Hemofiltration in sepsis and systemic inflammatory response syndrome: the role of dosing and timing. J Crit Care. 2007;22:1–12. doi: 10.1016/j.jcrc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Oudemans-van Straaten HM, Bosman RJ, van der Spoel JI, et al. Outcome of critically ill patients treated with intermittent high-volume haemofiltration: a prospective cohort analysis. Intensive Care Med. 1999;25:814–821. doi: 10.1007/s001340050957. [DOI] [PubMed] [Google Scholar]
- 24.Honoré PM, Jamez J, Wauthier M, et al. Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the haemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med. 2000;28:3581–3587. doi: 10.1097/00003246-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Cornejo R, Downey P, Castro R, et al. High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med. 2006;32:713–722. doi: 10.1007/s00134-006-0118-5. [DOI] [PubMed] [Google Scholar]
- 26.Ratanarat R, Brendolan A, Piccinni P, et al. Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care. 2005;9:R294–R302. doi: 10.1186/cc3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kron J, Kron S, Wenkel R, et al. Extended daily on-line high-volume haemodiafiltration in septic multiple organ failure: a well-tolerated and feasible procedure. Nephrol Dial Transplant. 2012;27:146–152. doi: 10.1093/ndt/gfr269. [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL, editor. The Design and Analysis of Clinical Experiments. New York, NY: John Wiley & Sons; 1986. pp. 49, 390. [Google Scholar]
- 29.Knaus W, Draper E, Wagner D, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 30.Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: the introduction of a new model. Clin Nephrol. 1996;46:206–211. [PubMed] [Google Scholar]
- 31.Chen YC, Yap HHJ, Chang MY, et al. Modified organ system failure score for critically ill patients with acute renal failure requiring dialysis. Nephrol Dial Transplant. 2003;32:125–132. [Google Scholar]
- 32.Gubenšek J, Buturović-Ponikvar J, Škofic N, et al. Regional citrate anticoagulation for intermittent predilution online hemofiltration. Ther Apher Dial. 2009;13:306–309. doi: 10.1111/j.1744-9987.2009.00729.x. [DOI] [PubMed] [Google Scholar]
- 33.Škofic N, Buturović-Ponikvar J, Kovač J, et al. Hemodialysis catheters with citrate locking in critically ill patients with acute kidney injury treated with intermittent online hemofiltration or hemodialysis. Ther Apher Dial. 2009;13:327–333. doi: 10.1111/j.1744-9987.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 34.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 35.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 36.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 37.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–2211. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Palevsky PM, Zhang JH, O'Connor TZ, et al. VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellomo R, Cass A, Cole L, et al. RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 40.Gastaldello K, Melot C, Kahn RJ, et al. Comparison of cellulose diacetate and polysulfone membranes in the outcome of acute renal failure. A prospective randomized study. Nephrol Dial Transplant. 2000;15:224–230. doi: 10.1093/ndt/15.2.224. [DOI] [PubMed] [Google Scholar]
- 41.Chertow GM, Pascual MT, Soroko S, et al. Reasons for non-enrollment in a cohort study of ARF: the Program to Improve Care in Acute Renal Disease (PICARD) experience and implications for a clinical trials network. Am J Kidney Dis. 2003;42:507–512. doi: 10.1016/s0272-6386(03)00745-5. [DOI] [PubMed] [Google Scholar]
- 42.Crowley ST, Chertow GM, Vitale J, et al. Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2008;3:955–961. doi: 10.2215/CJN.05621207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803–810. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 44.Vincent JL, Atalan HK. Epidemiology of severe sepsis in the intensive care unit. Br J Hosp Med (Lond) 2008;69:442–443. doi: 10.12968/hmed.2008.69.8.30739. [DOI] [PubMed] [Google Scholar]
- 45.Gibney N, Hoste E, Burdmann EA, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol. 2008;3:876–880. doi: 10.2215/CJN.04871107. [DOI] [PubMed] [Google Scholar]
- 46.Bagshaw SM, Uchino S, Bellomo R, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–140. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricci Z, Bellomo R, Ronco C. Dose of dialysis in acute renal failure. Clin J Am Soc Nephrol. 2006;1:380–388. doi: 10.2215/CJN.00520705. [DOI] [PubMed] [Google Scholar]
- 49.Mehta RL, Bouchard J. Dialysis dosage in acute kidney injury: still a conundrum? J Am Soc Nephrol. 2008;19:1046–1048. doi: 10.1681/ASN.2008040379. [DOI] [PubMed] [Google Scholar]
- 50.Himmelfarb J, Evanson J, Hakim RM, et al. Urea volume of distribution exceeds total body water in patients with acute renal failure. Kidney Int. 2002;61:317–323. doi: 10.1046/j.1523-1755.2002.00118.x. [DOI] [PubMed] [Google Scholar]
- 51.Kanagasundaram NS, Greene T, Larive AB, et al. Dosing intermittent haemodialysis in the intensive care unit patient with acute renal failure—estimation of urea removal and evidence for the regional blood flow model. Nephrol Dial Transplant. 2008;23:2286–2298. doi: 10.1093/ndt/gfm938. [DOI] [PubMed] [Google Scholar]
- 52.Faulhaber-Walter R, Hafer C, Jahr N, et al. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2009;24:2179–2186. doi: 10.1093/ndt/gfp035. [DOI] [PubMed] [Google Scholar]
- 53.Van Wert R, Friedrich JO, Scales DC, et al. High-dose renal replacement therapy for acute kidney injury: systematic review and meta-analysis. Crit Care Med. 2010;38:1360–1369. doi: 10.1097/CCM.0b013e3181d9d912. [DOI] [PubMed] [Google Scholar]
- 54.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liangos O, Wald R, O'Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 56.Prescott GJ, Metcalfe W, Baharani J, et al. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007;22:2513–2519. doi: 10.1093/ndt/gfm264. [DOI] [PubMed] [Google Scholar]