Abstract
Background
Inflammation, hypoalbuminaemia and peritoneal protein clearance are important predictors of survival in patients treated with peritoneal dialysis (PD). We hypothesized that the common link is abnormal endothelial barrier function. To test this, we explored associations between hypoalbuminaemia, systemic albumin leak and soluble markers of systemic inflammation and endothelial injury.
Methods
This was a cross-sectional study of 41 prevalent PD patients. Endothelial barrier function was measured as transcapillary escape rate of 125I albumin [transcapillary escape rate of albumin (TERalb)]. Seventeen plasma biomarkers including pro-inflammatory cytokines, endothelial biomarkers and metalloproteinases were measured. Hierarchical clustering analysis (HCA) and principal component analysis (PCA) were used to explore the hypothesis.
Results
The mean TERalb was 13.7 ± 8.9 (%/h), higher than in non-uraemic subjects 8.22 ± 5.8 (%/h). Three patient clusters were defined from HCA according to their biomarker patterns. Cluster 1 was characterized by inflammation, hypoalbuminaemia, overhydration and intermediate TERalb. Cluster 2 was non-inflamed, preserved muscle mass and more normal TERalb. Cluster 3 had highest TERalb, platelet activation, preserved plasma albumin and intermediate high-sensitivity C-reactive protein levels. Two principal components (PCs) were identified from the biomarker matrix, PC1, indicating platelet activation and PC2, pro-inflammatory. TERalb was positively related to PC1 but not PC2. Diabetes and ischaemic heart disease were associated with PC1 and PC2, respectively.
Conclusions
This exploratory analysis indicates that endothelial barrier function is decreased in PD patients and is associated with diabetic status and markers of platelet activation more than inflammation. In contrast, hypoalbuminaemia is associated more with inflammation and atherosclerotic disease indicating a more complex relationship between systemic endothelial barrier function, inflammation and hypoalbuminaemia which requires further validation.
Keywords: biomarker pattern, body composition, inflammation, platelet activation, transcapillary escape rate
Introduction
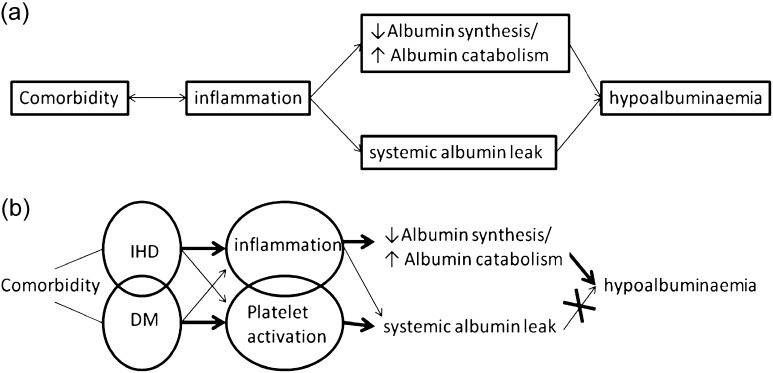
Inflammation and hypoalbuminaemia are both important predictors of survival in patients treated with dialysis for advanced renal failure [1–3]. Endothelial dysfunction is a feature of uraemia [4], also present in complicated diabetes and cardiovascular co-morbidities that commonly occur in these patients. Indeed, it may represent the link between increased systemic and/or peritoneal protein leak, inflammation, hypoalbuminaemia and worse survival through its association with reduced capillary barrier function [5] (see Figure 1a). However, the picture is further complicated in peritoneal dialysis (PD) patients by the important daily losses of protein from the peritoneal cavity which likely explains why for a given degree of hypoalbuminaemia, survival in PD patients is superior to those on haemodialysis [1]. The observation that peritoneal protein clearance (the main determinant of absolute protein losses) predicts survival independent of effective peritoneal surface area [5–7] led us to test the hypothesis that the underlying common factor is impaired systemic endothelial barrier function.
Fig. 1.
(a) Simplified linear hypothesis linking co-morbidity to hypoalbuminaemia invoking reduced endothelial barrier function as the central unifying mechanism. (b) Modified relationship between co-morbidity, inflammation, systemic protein leak and hypoalbuminaemia in the light of current findings.
To do this, we explored the relationship between the systemic transcapillary escape rate of albumin (TERalb) as an indicator of endothelial barrier function, a panel of biomarkers of endothelial dysfunction and associated clinical phenotypes. We used the techniques of hierarchical clustering (HC) and principal component analysis (PCA) to generate patient groups from the patterns of biomarkers observed and then subsequently compared their clinical phenotypes so as to avoid any pre-conceived assumptions.
Materials and methods
Study design and patient population
This was a cross-sectional study of prevalent PD patients in a single centre. Sequential patients were approached to participate and studied while undergoing their routine 6-monthly assessments of peritoneal membrane function and dialysis adequacy tests, provided they were not acutely ill or within 1 month of peritonitis. The study was peer reviewed and approved by the local ethics committee and all patients signed the consent form.
TERalb and plasma volume
After a bolus intravenous injection of 10 mL 0.185 MBq 125I-human serum albumin (HSA), blood samples were taken at 10, 20 and 30 min at a remote venous site. The isotopic concentration in each case was plotted against time on a semi-logarithmic scale and the best linear fit line was performed from these points. TERalb, expressed as percentage loss per hour (%/h) was estimated from the disappearance rate of the 125I HSA from the gradient of this line. The plasma volume was calculated by extrapolating the line to time zero. The method was also applied for clinical purposes as the measurement of plasma volume in the normalization of red cell mass and diagnosis of primary polycythaemia. These patients who were mainly referred from haematologists during the time of this study served as non-uraemic contemporaneous ‘in-house’ controls.
Biomarker measurement
The plasma sample for biomarker analysis was collected just before 125I HSA injection on the study day and stored at −20°C. A panel of 17 biomarkers, which are involved in inflammation, endothelial function and tissue remodelling processes, [interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-1β, IL-10, monocyte chemotactic protein-1, inter-cellular adhesion molecule 1, vascular cell adhesion protein 1 (VCAM-1), E-selectin, P-selectin, CD40 ligand (CD40L), vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-1, MMP-2, MMP-3, MMP-8, MMP-9], was measured on a Luminex suspension array system (Bio-Plex 200TM platform; Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, UK) using commercially available multi-analyte cytokine kits [MILLIPLEX MAP; Millipore (UK) Ltd, Walford, Hertfordshire, UK] or Fluorokine multi-analyte profiling kits for MMPs (R&D Systems, Abingdon, UK). Assays were carried out according to the manufacturer's instructions.
Body composition, solute clearance, membrane function and blood biochemistry
Estimated total body water (TBW) and extra-cellular water were evaluated by bioimpedance (BIA, multi-frequency Xitron Hydra device, Model 4200; Xitron Technologies, San Diego, CA). At the same time, absolute TBW was measured by deuterium (D2O) dilution technique. The detailed methodologies have been described previously [8]. In brief, baseline and equilibrated blood samples were taken before and 2.5 h after an oral dose of 99.8% D2O (Cambridge Isotope Laboratories). The difference between the headspace HDO abundance of the two samples, measured by flowing-afterglow mass spectrometry, was used to determine TBWD after accounting for equilibration with dialysate and 4% D exchange with H in body proteins. The difference between BIA estimated (TBWBIA) and D dilution measured TBW (TBWD) reflects tissue overhydration [8, 9].
The dialysis dose and residual renal function were calculated as the weekly Kt/Vurea from the 24-h urinary and dialysate clearance by direct measurement of urea in urine and dialysate. Peritoneal dialysate protein loss was measured from the collection of 24-h dialysate effluent. A validated equation was used for the calculation of protein clearance, (Pcl), = 24-h dialysate protein loss/(serum albumin/0.4783).
Solute transport was measured by standard 4-h peritoneal equilibrium test with 2.27% glucose concentration 2-L exchange. The dialysate:plasma ratio of creatinine at the completion of the 4-h dwell period (D/P creatinine) was used to estimate low-molecular-weight solute transport.
Plasma albumin was estimated using the bromocresol purple colorimetric method, peritoneal dialysate and urine protein by the biuret method. C-reactive protein (CRP) was measured using a latex enhanced immunoturbidimetric method.
Statistical analysis
One-way analysis of variance and unpaired t-test were used to examine differences in normally distributed continuous data among groups, chi-square for categorical data. Univariate correlations were examined by Pearson correlation coefficient. Variables not normally distributed were log2 transformed for further analysis. Significance was considered at P-values <0.05. Statistical analyses were performed using IBM SPSS Statistics version 19 except for the HC analysis (HCA).
Hierarchical cluster analysis
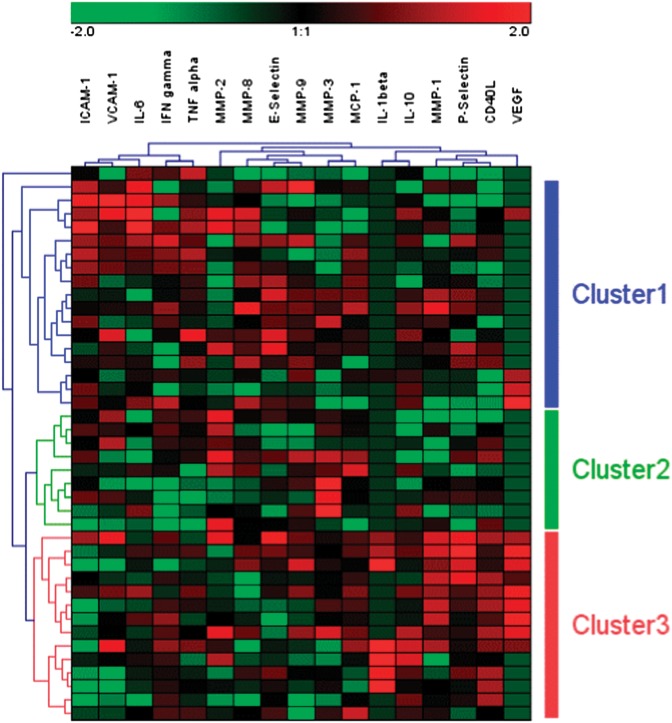
The biomarker levels were first converted to Log2 and expressed relative to the mean value for normalization. These measurements were used to generate heat map (Figure 2) using Genesis software (version 1.7.2, Alexander Sturn; Institute for Genomics and Bioinformatics, Graz University of Technology). The Genesis programme uses a two-dimensional HC method, using the average linkage clustering agglomerative rule that enables groups of variables with similar expression levels to be clustered together as well as grouping together patient samples with similar expression patterns.
Fig. 2.
Plasma concentration profiles of 17 biomarkers in 41 patients expressed as a HCA heat map. Plasma concentrations of biomarker close to higher and lower than the mean values are represented by black, red and green colours, respectively. Three patient clusters, Cluster 1 (blue bar), Cluster 2 (green bar), Cluster 3 (red bar) and one outlier are generated by HCA.
Principal component analysis
To decrease the dimensionality of the biomarker data set while retaining as much of the variance as possible, an exploratory PCA was employed. Principal components (PCs) were extracted using varimax rotation, with the factor selection based on an eigen value cut-off of 1.0. Because of limitations of the sample size, eight biomarkers only were processed in the final analysis. These biomarkers were selected from the first two PCs, which contributed the most variance of the whole matrix. Those biomarker variables not normally distributed after log transformation were converted to binary data.
Results
A total of 41 prevalent patients (M/F 22/19, mean age 61 ± 17 years, median time on PD 20 [9–33] months, diabetics 29%) were studied. The TERalb was higher in the PD patients 13.74 ± 8.8 (%/h) than the non-uraemic comparator group 8.22 ± 5.8 (%/h) (n = 13, P < 0.05).
Three clusters and one outlier were defined from the HCA (Figure 2). Patient characteristics, peritoneal membrane function and TERalb (Table 1) and absolute biomarker concentrations (Table 2) are shown by cluster. Cluster 1 was characterized by an inflammatory profile, associated with higher high-sensitivity CRP, lower albumin, intermediate TERalb and overhydration indicated by the big discrepancy between measured TBW (TBWD) and estimated (TBWBIA). Cluster 2 was non-inflamed according to the lowest CRP levels, had the lowest TERalb and the most normal body composition as evidenced by the highest body water by body weight but good agreement between D dilution and BIA measures, indicating well-preserved muscle mass but least overhydration. Cluster 3 was characterized by biomarkers consistent with platelet activation, had the highest TERalb but only moderate CRP levels, less inflammation and less overhydration than Cluster 1 patients.
Table 1.
Characteristics of the three patient clusters derived from HCAa
| Cluster 1 (n = 17) | Cluster 2 (n = 9) | Cluster 3 (n = 14) | Single outlier | |
|---|---|---|---|---|
| TERalb (%/h) | 13.88 ± 9.23 | 9.12 ± 5.29b | 17.29 ± 9.11 | 3 |
| Age (years) | 62.1 ± 17.2 | 61.4 ± 19.2 | 60.3 ± 15.7 | 60 |
| Gender (M, %) | 65 | 56 | 36 | M |
| BMI (kg/m2) | 26.1 ± 5.7 | 25.8 ± 3.9 | 28.3 ± 5.1 | 25.1 |
| PD duration (months) | 22.9 ± 20.3 | 27.6 ± 21.0 | 26.8 ± 21.2 | 10.5 |
| Solute transport (4 h D/P creatinine ratio) | 0.81 ± 0.13 | 0.78 ± 0.12 | 0.74 ± 0.17 | 0.7 |
| Albumin (g/L) | 29.1 ± 4.9c | 31.3 ± 3.3 | 33.2 ± 4.1 | 33 |
| CRP (mg/L) | 14.3 (4.8–21)d | 1.5 (0.6–4) | 4.6 (0.8–9.5) | 16.8 |
| Peritoneal Pcl (mL/day) | 95.6 ± 48.3 | 67.3 ± 21.7 | 69.0 ± 24.7 | 64.04 |
| Peritoneal protein loss (g/24 h) | 6.3 ± 3.2 | 4.9 ± 1.6 | 5.0 ± 1.8 | 4.28 |
| Urine protein loss (g/24 h) | 0.58 ± 0.5 | 0.88 ± 0.85 | 0.74 ± 0.51 | 1.12 |
| Total protein loss (g/24 h) | 7.11 ± 3.00 | 6.06 ± 2.06 | 6.02 ± 2.07 | 5.4 |
| Urine volume (mL) | 709 ± 631 | 1128 ± 626 | 1059 ± 759 | 997 |
| Adjusted PV (mL/m2) | 1439 ± 300 | 1441 ± 173 | 1446 ± 197 | 2158 |
| TBWD/Weight | 0.51 ± 0.04 | 0.53 ± 0.07b | 0.47 ± 0.06 | 0.52 |
| TBWBIA/Weight | 0.45 ± 0.05 | 0.53 ± 0.08d,e | 0.45 ± 0.06 | 0.56 |
| TBWD/Weight − TBWBIA/Weight | 0.049 ± 0.041 | 0.006 ± 0.025f | 0.023 ± 0.055 | − 0.046 |
| Ever smoked (%) | 44 | 22 | 23 | Yes |
| IHD (%) | 41 | 22 | 29 | No |
| Diabetes (%) | 17 | 22 | 50 | No |
aAdjusted PV, plasma volume adjusted by body surface area; BMI, body mass index; Pcl, protein clearance corrected for dialysis regime (modality, dry day and overfill); TBWD/Weight, TBW normalized to body weight as determined from deuterium (D) dilution; TBWBIA/Weight, TBW normalized to body weight as estimated by BIA. Clusters 1, 2 and 3 were compared using one-way analysis of variance (ANOVA) and when significant the post hoc between group differences are shown.
bCluster 2 versus Cluster 3, P < 0.05.
cCluster 1 versus Cluster 3, P < 0.05.
dCluster 1 versus Cluster 2, P < 0.01.
eCluster 2 versus Cluster 3, P < 0.01.
fCluster 1 versus Cluster 2, P < 0.05 (ANOVA).
Table 2.
Mean/median biomarker concentrations of the three patient clusters derived from HCAa
| Cluster 1 (n = 17) | Cluster 2 (N = 9) | Cluster 3 (n = 14) | Single outlier | P-value (ANOVA) | |
|---|---|---|---|---|---|
| ICAM-1 (μg/mL) | 0.49 ± 0.09 | 0.4 ± 0.07 | 0.34 ± 0.08 | 0.41 | <0.01 |
| VCAM-1 (μg/mL) | 0.83 ± 0.18 | 0.74 ± 0.16 | 0.72 ± 0.2 | 0.52 | NS |
| E-selectin (ng/mL) | 68.21 ± 31.07 | 44.16 ± 18.08 | 56.23 ± 27.51 | 26.13 | NS |
| P-selectin (ng/mL) | 94.63 ± 31.68 | 79.94 ± 27.23 | 126.52 ± 46.95 | 48.53 | <0.05 |
| MMP-1 (pg/mL) | 33.4 (15–104.8) | 10.1 (1–60.8) | 345 (35.9–821.8) | 1 | <0.05 |
| MMP-2 (μg/mL) | 0.24 (0.17–0.28) | 0.76 (0.34–1) | 0.29 (0.23–0.44) | 0.24 | 0.081 |
| MMP-3 (ng/mL) | 11.5 ± 5 | 22.76 ± 12.76 | 13.52 ± 4.49 | 9.04 | <0.01 |
| MMP-8 (ng/mL) | 3.69 ± 1.66 | 2.26 ± 0.8 | 2.04 ± 1.15 | 0.33 | <0.01 |
| MMP-9 (ng/mL) | 67.66 ± 38.23 | 55.54 ± 26.02 | 53.13 ± 30.4 | 30.7 | NS |
| IFN gamma (pg/mL) | 1.4 (0–4.6) | 0.5 (0–1.4) | 1.7 (1.1–2.3) | 2.1 | NS |
| IL-1β (pg/mL) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.4 (0.1–4.1) | 0.1 | <0.01 |
| IL-6 (pg/mL) | 5.4 (3–11.4) | 2.2 (1–4.2) | 3.5 (2.8–5.1) | 8.7 | <0.05 |
| IL-10 (pg/mL) | 2.7 ± 2 | 1.4 ± 1.9 | 4.5 ± 4 | 1.4 | <0.05 |
| MCP-1 (pg/mL) | 357.8 ± 156.9 | 377.5 ± 175.2 | 417.1 ± 131.2 | 61.7 | NS |
| CD40L (ng/mL) | 3.1 ± 2.3 | 4.16 ± 2.58 | 7.85 ± 2.61 | 1.16 | <0.01 |
| TNF-α (pg/mL) | 12.1 ± 4.9 | 6.4 ± 4 | 8.9 ± 3 | 19.5 | <0.01 |
| VEGF (pg/mL) | 3 (3–3) | 3 (3–3) | 50.9 (3–135.7) | 3 | <0.01 |
aANOVA, analysis of variance; ICAM-1, inter-cellular adhesion molecule 1; NS, non significant.
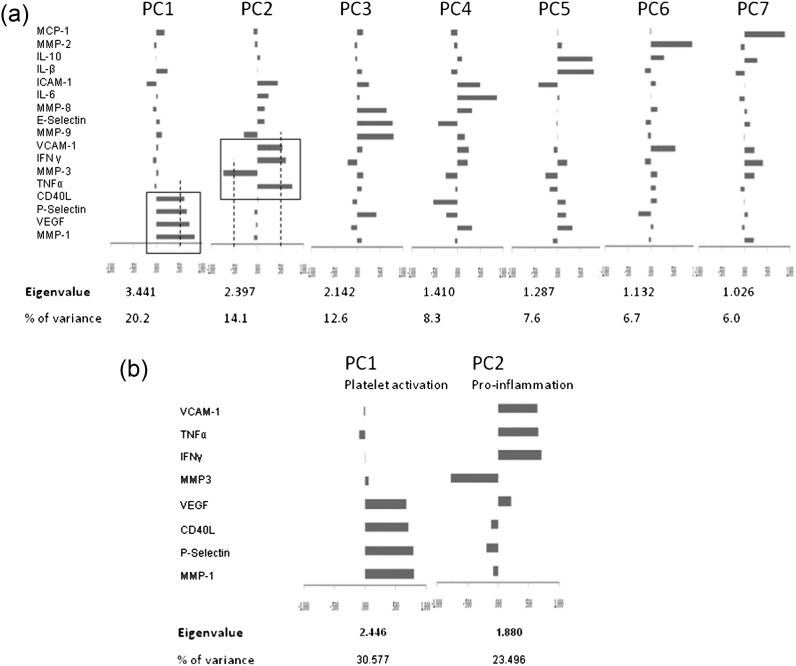
The PCA was first carried out in the whole panel of the 17 biomarkers. Seven PCs were identified, explaining 74.9% of the total variance, Figure 3a. The eight biomarkers that composed the two strongest PCs, contributing the majority of the variance, were selected for the final PCA and are shown with their eigen values (>1) in Figure 3b. The first PC (PC1-platelet activation) was composed mainly of MMP-1, P-selectin, CD40L and VEGF. The second PC (PC2 pro-inflammation) featured an inverse relationship between MMP-3 and positive association with IFN-γ, TNF-α and VCAM-1. This component showed a positive relationship with CRP (r = 0.31, P = 0.047) and was negatively correlated with plasma albumin (r = −0.50, P = 0.001).
Fig. 3.
(a) PCA from the whole panel of the 17 biomarkers eigen values and percentage contributing to the total sample variance of each of the seven PCs is presented. Individual composition feature is showed along with their rotated loading coefficients, a measure of the importance of each biomarker to the factor, displayed as a bar chart. (b) PCA from reduced number of biomarkers. The eight biomarkers that compose the two strongest PCs, contributing the majority of the total sample variance are selected for the final PCA. The composition of the 2 PCs derived from the final model is displayed along with their rotated loading coefficients expressed as a bar chart.
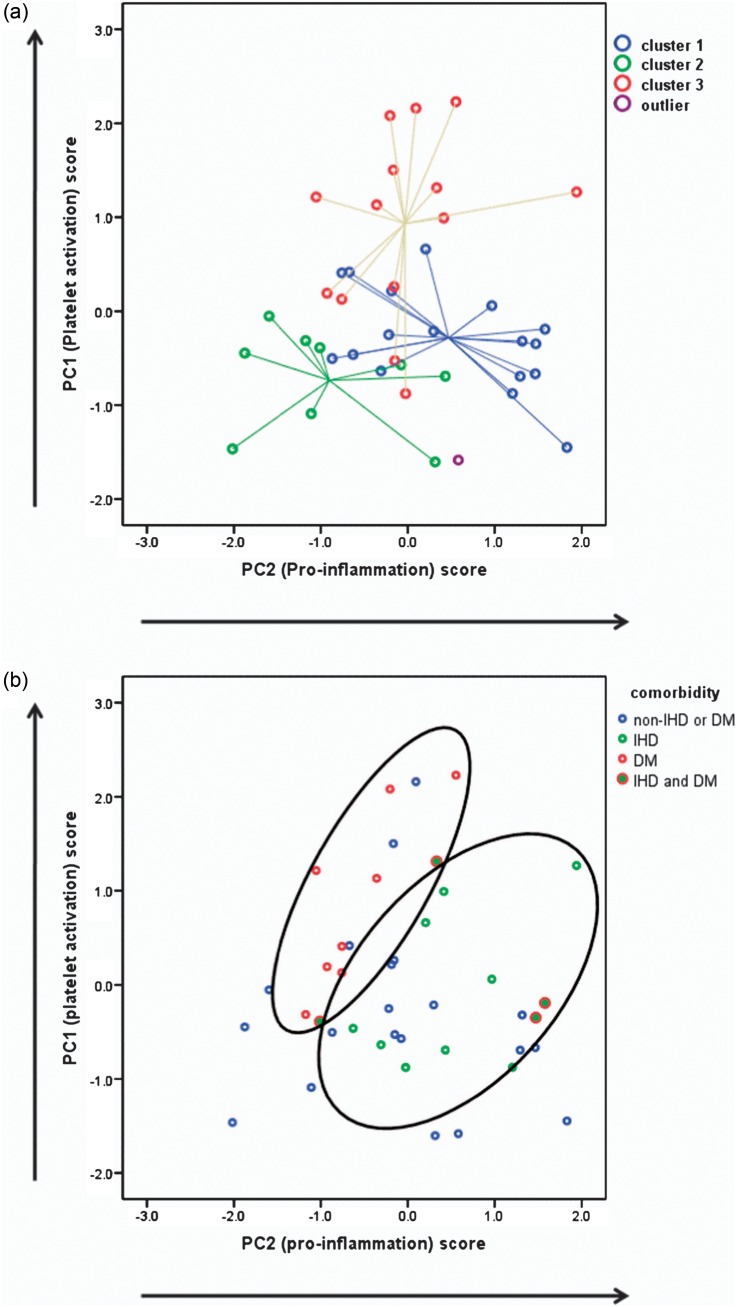
The relationship between the HCA and PCA is shown graphically in Figure 4a. It can be seen that despite differing dimensions that these two analytical techniques give patterns that are in agreement. Patients with different co-morbidities also showed differences in PCs such that patients with ischaemic heart disease (IHD) and diabetes mellitus (DM) have different, albeit overlapping, patterns of biomarkers (Figure 4b). Diabetes had higher PC1, while PC2 was higher in IHD (Table 3).
Fig. 4.
(a) Combined graphical representation of the HCA (centroid plot) and PCA (dimensionless weighted scores); there is good agreement between the methods showing association between Cluster 3 and increased platelet activation, Cluster 1 with inflammation and Cluster 2 with the absence of markers of endothelial dysfunction. (b) Combined graphical representation of PC scores and co-morbidity; IHD and DM are clustered according to their different pattern of PC scores.
Table 3.
PCs in different co-morbidity statusa
| IHD (n = 13) | Non-IHD (n = 28) | P-valueb | DM (n = 12) | Non-DM (n = 29) | P-valueb | PVD (n = 12) | Non-PVD (n = 29) | P-valueb | |
|---|---|---|---|---|---|---|---|---|---|
| PC1 | −0.01 ± 0.80 | 0.01 ± 1.09 | 0.951 | 0.62 ± 0.94 | −0.26 ± 0.92 | 0.009** | −0.01 ± 0.76 | 0.01 ± 1.10 | 0.96 |
| PC2 | 0.51 ± 0.89 | −0.23 ± 0.51 | 0.025* | −0.19 ± 0.97 | 0.08 ± 1.02 | 0.436 | −0.06 ± 0.85 | 0.02 ± 1.07 | 0.814 |
aPVD, Peripheral vascular disease.
bUnpaired t-test.
*P < 0.05.
**P < 0.01.
To further explore the relationship between clinical factors and biomarker profiles with our primary end point, TERalb, univariate regression analysis was undertaken. None of the clinical measures, including demographics, membrane function, PD duration, CRP or systemic dynamic factors (pulse pressure, mean blood pressure), showed significant correlation to TERalb whereas the biomarker derived PC1 score (platelet activation) showed a positive relationship (r = 0.33 P = 0.03) (Table 4). Multivariate analysis found this relationship to be independent of age, gender and PD duration.
Table 4.
Univariate correlation of TERalba
| Correlation coefficient | P-valueb | |
|---|---|---|
| Age (years) | −0.21 | 0.19 |
| BMI (kg/m2) | −0.09 | 0.57 |
| PD duration (months) | 0.26 | 0.11 |
| Pulse pressure (mmHg) | 0.10 | 0.54 |
| Albumin (g/L) | −0.09 | 0.56 |
| Log2CRP(mg/L) | −0.04 | 0.81 |
| D/P creatinine | 0.12 | 0.46 |
| UF capacity (mL) | −0.03 | 0.88 |
| Peritoneal protein loss (g/24 h) | 0.23 | 0.16 |
| Peritoneal Pcl (mL/day) | 0.15 | 0.36 |
| Urine protein loss (g/24 h) | −0.16 | 0.34 |
| Total protein loss (g/24 h) | 0.20 | 0.23 |
| Urine volume (mL) | −0.22 | 0.17 |
| Gender | 0.1 | |
| Ever smoking | 0.28 | |
| IHD | 0.39 | |
| Diabetes | 0.19 | |
| PVD | 0.71 | |
| PC 1 | 0.33 | 0.03* |
| PC 2 | 0.07 | 0.69 |
aPVD, Peripheral vascular disease; UF, ultrafiltration.
bPearson or Spearman correlation for continuous variables as appropriate according to the distribution and unpaired t-test for between group difference.
*P < 0.05.
Discussion
This is the first study to measure systemic endothelial barrier function in patients with chronic kidney disease (CKD) Stage 5 and relate this to a wide panel of circulating biomarkers of inflammation and endothelial dysfunction. It confirms that endothelial barrier function is abnormally decreased and suggests that this is not solely related to inflammation but more so to platelet activation in this clinically stable cross-sectional cohort. Furthermore, patients with IHD and DM have different, albeit overlapping, patterns of biomarkers, although both exhibit endothelial dysfunction (Figure 4b).
The TERalb is a widely accepted measurement of endothelial barrier function, first described as early as 1973 [10, 11]. TERalb is increased in several conditions associated with endothelial dysfunction such as diabetes, hypertension and atherosclerosis [12–15] and also sepsis where it is clearly associated with marked inflammation. However, endothelial barrier function has been less well investigated in end-stage renal disease. This study found that mean TERalb is higher in CKD Stage 5 patients than in non-uraemic controls who likely had other pathologies as they were undergoing investigation for polycythaemia. A previous study of 29 non-diabetic CKD patients (glomerular filtration rate 11–44 mL/min/1.73 m2) whose other markers of endothelial function (e.g. von Willebrand factor) were normal unless subjects were current smokers [16] showed no difference between CKD patients and normal controls. This suggests that severe renal failure is associated with more severe endothelial dysfunction and is in broad agreement with other rather small previously published studies in dialysis patients. TERalb was increased in 11 diabetics treated with PD (but not in non-diabetics) [17] and 9 pre-dialysis haemodialysis patients [18]. This is supported by in vivo experiments in which human uraemic plasma increased frog mesenteric micro- vascular permeability to both water and protein, [19] subsequently confirmed in other studies. [20] One of the strengths of our study, which demonstrated a relatively high median TERalb, was that we recruited sequential patients undergoing routine assessment of their therapy with <5% of patients declining investigation, so reducing selection bias.
Although a relationship between TERalb and blood pressure was seen in some early studies in hypertensive patients, we did not observe this to be the case in this cross-sectional study. The relationship may be more complex in this dialysis cohort with multiple co-morbidities and concomitant medication. A study comparing TERalb according to the angiotensin-converting enzyme (ACE) gene polymorphism in essential hypertension showed that ACE D/D homozygosis, which is associated with high risk of atherosclerotic vascular disease, is related to higher TERalb despite identical 24-h blood pressure readings [23]. Furthermore, endothelial function can be influenced by medications used in the patients with related effect on TERalb. High-dose simvastatin reduces low-density lipoprotein cholesterol by 39% and is reported to normalize TERalb [24]. No clear relationship between medications and TERalb was observed in this study.
The measurement of a wide range of biomarkers combined with hierarchical cluster and PCA allowed us to investigate the overall pattern of their association with endothelial barrier dysfunction. Using this approach, we were able to identify three main patient clusters and there was good agreement between these and the two main components identified through PCA; additional PCs were identified that warrant further investigation in a larger validation cohort but were excluded from further analysis here to avoid Type 1 statistical error. The advantage to this approach is that while biomarkers are likely to correlate to each other as they all reflect endothelial function, it enables identification of possible multiple biomarker functions or different metabolic or signalling pathways involved in endothelial dysfunction. For example, CD40L is expressed in lymph cells, epithelial cells, fibroblasts, endothelial cells and platelets in response to pro-inflammatory cytokines, platelet activators and nitric oxide signalling. After combining with CD40, it subsequently up-regulates the pro-inflammatory and pro-atherogenic genes [25]. Although results from our PCA should be taken as exploratory, we find that PC1 (HC3 phenotype) is composed primarily of MMP-1, P-selectin, CD40L and VEGF, all of which are released in significant amounts from platelets and reflect platelet activation [26]. PC2, composed by IFN-γ, TNF-α and VCAM-1 and negatively contributed by MMP-3, is mainly involved in pro-inflammatory pathways and defined the inflamed, overhydrated and hypoalbuminaemic HC1 phenotype. HC2 was a relatively healthy phenotype with most normal body composition and least abnormal TERalb. PC1 and PC2 could explain a significant proportion (34.3%) of the variance in the whole biomarker matrix.
One important finding of this study is the demonstration that the link between platelet activation and increased endothelial permeability may not be just through inflammation. The links between inflammations as a trigger for platelet activation in vascular lesions and between inflammation, atherosclerosis and endothelial dysfunction in uraemia are well established [27]. These pathways may, however, act either independently or sequentially; activated platelets can induce secretion of chemokines for monocyte recruitment [28] whereas single micro-vessel perfusion with TNF-α alone, without platelet activation, does not alter endothelial permeability [29]. In an aseptic animal injury model, systemic depletion of neutrophils with antibody failed to prevent the increase in vascular permeability, whereas anti-platelet pre-treatment reduced this by 25% [30]. These findings suggest that platelet activation may have a critical role in endothelial hyperpermeability that may not necessarily be through inflammation, especially in the low-grade inflammatory status seen in uraemia. Local release of VEGF by activated platelets, especially associated with HCA Cluster 3 and increased TERalb in this study, may explain this.
This study found that DM and IHD tend to cluster with different patterns of biomarkers associated with endothelial dysfunction and in keeping with other studies, we found that diabetics more commonly display an increase in platelet reactivity [31]. Worse cardiovascular outcomes in diabetics may reflect inadequate responses to anti-platelet therapy [32]. Another important finding of this study is that the systemic leak of albumin from the circulation to extravascular space is not correlated to hypoalbuminaemia and thus is unlikely to be causally related. It is well established that the single strongest predictor of plasma albumin concentration in PD patients is the daily peritoneal protein loss [33, 34], in turn strongly correlated to the rate of peritoneal small solute transport, an indicator of the effective vascular area in contact with dialysate. There is, however, residual variability in peritoneal protein losses which has now been shown in several studies to be an independent predictor of patient survival [6, 7]. This link between peritoneal protein leak and survival can at least in part be explained by an association with increasing age and cardiovascular co-morbidity, raising the possibility that some of the variability may reflect endothelial dysfunction. We did not see any clear relationship between systemic protein leak and peritoneal protein losses in this study probably because the latter is dominated by effective peritoneal vascular area in contact with dialysate. It is also possible, given that this was a study of prevalent patients, that some had acquired membrane changes that could influence protein losses. The reason that plasma albumin is more strongly associated with peritoneal protein losses than with systemic leak is that the former represents net daily losses (typically 5–10 g/day) whereas the latter reflects the re-circulation between intra- and extravascular pools of up to 10 times this amount daily. Net plasma albumin concentration will also be determined by the balance of synthesis and catabolism, and the former in relatively fit PD patients has been shown to be increased above normal.[33] The patients with the lowest albumin in this study were those with inflammation compared to markers of platelet activation, in keeping with previous observations that hypoalbuminaemia in inflamed patients is associated with reduced synthesis.
This study has a number of limitations. Firstly, the number of patients would ideally be greater to enable a more extensive correlation of the PCA with clinical phenotype. Despite this, it remains the largest study of TERalb in dialysis patients to date and the numbers were limited by the ethical permission which was primarily obtained to measure plasma volume in an adequately powered study of fluid status and hypoalbuminaemia [8]. Secondly, the biomarker patterns identified, while good evidence that the relationship in more complex that originally envisaged can only be considered as hypothesis generating at this stage. Further validation in a larger patient cohort of selected biomarkers is planned. Thirdly, we did not have ethics permission to study normal subjects, so limiting our comparison to a non-uraemic control group under clinical investigation, which might be expected to have worse endothelial function than normal subjects [35]. As acknowledged above, the cross-sectional design means that there may be confounding of the data by factors known and unknown that change with time on treatment.
In conclusion, this study elaborates a more complex relationship between abnormal endothelial dysfunction and clinical phenotype in patients on PD, emphasizing the importance of platelet activation as well as inflammation (Figure 1b). In addition, it further clarifies our understanding of the mechanisms of hypoalbuminaemia, an important predictor of survival in patients on PD.
Acknowledgement
We would like to thank Nicola Nixon (Senior Technician) for her help in running the multiplex assays.
Funding. The consumable costs for this research were funded by the North Staffordshire Medical Institute Renal Research Fund. The development of the Flowing Afterglow Mass Spectometer was supported by the Wellcome Trust (GR067160MA). Z.Y. is supported by a Dorothy Hodgkin Ph.D studentship grant from the UK Medical Research Council. B.K.T. was supported by the Baxter renal Discoveries Extramural grant programme. S.J.D. receives research funding from Baxter Healthcare and Fresenius.
Conflict of interest statement. None of the sponsors had any role in study design; collection, analysis and interpretation of data; writing the report and the decision to submit the report for publication.
(See related article by Heaf. Peritoneal transport: getting more complicated. Nephrol Dial Transplant2012; 27: 4248–4251.)
References
- 1.Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–428. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 3.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P, Pecoits-Filho R, Lindholm B. Coronary artery disease in end-stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol. 2003;14:1927–1939. doi: 10.1097/01.asn.0000069165.79509.42. [DOI] [PubMed] [Google Scholar]
- 5.Van Biesen W, Van Der Tol A, Veys N, et al. The personal dialysis capacity test is superior to the peritoneal equilibration test to discriminate inflammation as the cause of fast transport status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2006;1:269–274. doi: 10.2215/CJN.00820805. [DOI] [PubMed] [Google Scholar]
- 6.Perl J, Huckvale K, Chellar M, et al. Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol. 2009;4:1201–1206. doi: 10.2215/CJN.01910309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaf JG, Sarac S, Afzal S. A high peritoneal large pore fluid flux causes hypoalbuminaemia and is a risk factor for death in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:2194–2201. doi: 10.1093/ndt/gfi008. [DOI] [PubMed] [Google Scholar]
- 8.John B, Tan BK, Dainty S, et al. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1463–1470. doi: 10.2215/CJN.09411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan C, McIntyre C, Smith D, et al. Combining near-subject absolute and relative measures of longitudinal hydration in hemodialysis. Clin J Am Soc Nephrol. 2009;4:1791–1798. doi: 10.2215/CJN.02510409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parving HH, Gyntelberg F. Transcapillary escape rate of albumin and plasma volume in essential hypertension. Circ Res. 1973;32:643–652. doi: 10.1161/01.res.32.5.643. [DOI] [PubMed] [Google Scholar]
- 11.Ulrych M. Plasma volume decrease and elevated Evans Blue disappearance rate in essential hypertension. Clin Sci Mol Med. 1973;45:173–181. doi: 10.1042/cs0450173. [DOI] [PubMed] [Google Scholar]
- 12.Feldt-Rasmussen B. Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1986;29:282–286. doi: 10.1007/BF00452063. [DOI] [PubMed] [Google Scholar]
- 13.Nannipieri M, Rizzo L, Rapuano A, et al. Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care. 1995;18:1–9. doi: 10.2337/diacare.18.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Pedrinelli R, Penno G, Dell'Omo G, et al. Transvascular and urinary leakage of albumin in atherosclerotic and hypertensive men. Hypertension. 1998;32:318–323. doi: 10.1161/01.hyp.32.2.318. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JS. Renal and systemic transvascular albumin leakage in severe atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1324–1329. doi: 10.1161/01.atv.15.9.1324. [DOI] [PubMed] [Google Scholar]
- 16.Haaber A, Eidemak I, Jensen T, et al. Vascular endothelial cell function and cardiovascular risk factors in patients with chronic renal failure. J Am Soc Nephrol. 1995;5:1581–1584. doi: 10.1681/ASN.V581581. [DOI] [PubMed] [Google Scholar]
- 17.Graff J, Fugleberg S, Nielsen SL, et al. Transperitoneal transport in diabetic and non-diabetic patients on peritoneal dialysis. Clin Physiol. 1999;19:510–518. doi: 10.1046/j.1365-2281.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrandt P, Jensen HA, Henriksen JH. Studies on kinetics of albumin in uraemic patients on chronic haemodialysis: evidence of interstitial albumin wash-down. Clin Physiol. 1983;3:153–162. doi: 10.1111/j.1475-097x.1983.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 19.Harper SJ, Tomson CRV, Bates DO. Human uremic plasma increases microvascular permeability to water and proteins in vivo. Kidney Int. 2002;61:1416–1422. doi: 10.1046/j.1523-1755.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 20.Harper SJ, Bates DO. Endothelial permeability in uremia. Kidney Int. 2003;63:S41–S44. doi: 10.1046/j.1523-1755.63.s84.15.x. [DOI] [PubMed] [Google Scholar]
- 21.Pedrinelli R, Penno G, Dell'Omo G, et al. Microalbuminuria and transcapillary albumin leakage in essential hypertension. Hypertension. 1999;34:491–495. doi: 10.1161/01.hyp.34.3.491. [DOI] [PubMed] [Google Scholar]
- 22.Parving H-H, Rossing N, Jensen HA. Increased metabolic turnover rate and transcapillary escape rate of albumin in essential hypertension. Circ Res. 1974;35:544–552. doi: 10.1161/01.res.35.4.544. [DOI] [PubMed] [Google Scholar]
- 23.Dell'omo G, Penno G, Pucci L, et al. ACE gene insertion/deletion polymorphism modulates capillary permeability in hypertension. Clin Sci (Lond) 2006;111:357–364. doi: 10.1042/CS20060165. [DOI] [PubMed] [Google Scholar]
- 24.Dell'Omo G, Bandinelli S, Penno G, et al. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men[ast] Clin Pharmacol Ther. 2000;68:427–434. doi: 10.1067/mcp.2000.109787. [DOI] [PubMed] [Google Scholar]
- 25.Szmitko PE, Wang CH, Weisel RD, et al. New markers of inflammation and endothelial cell activation: part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 26.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 27.Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 29.He P, Zhang H, Zhu L, et al. Leukocyte-platelet aggregate adhesion and vascular permeability in intact microvessels: role of activated endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H591–H599. doi: 10.1152/ajpheart.01228.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kim MH, Curry FR, Simon SI. Dynamics of neutrophil extravasation and vascular permeability are uncoupled during aseptic cutaneous wounding. Am J Physiol Cell Physiol. 2009;296:C848–C856. doi: 10.1152/ajpcell.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 32.Geisler T, Anders N, Paterok M, et al. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care. 2007;30:372–374. doi: 10.2337/dc06-1625. [DOI] [PubMed] [Google Scholar]
- 33.Kaysen G. Biological basis of hypoalbuminemia in ESRD. J Am Soc Nephrol. 1998;9:2368–2376. doi: 10.1681/ASN.V9122368. [DOI] [PubMed] [Google Scholar]
- 34.Yeun JY, Kaysen GA. Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis. 1997;30:923–927. doi: 10.1016/s0272-6386(97)90105-0. [DOI] [PubMed] [Google Scholar]
- 35.Neunteufl T, Heher S, Stefenelli T, et al. Endothelial dysfunction in patients with polycythaemia vera. Br J Haematol. 2001;115:354–359. doi: 10.1046/j.1365-2141.2001.03092.x. [DOI] [PubMed] [Google Scholar]