Abstract
Background
The balANZ trial recently reported that neutral pH, low glucose degradation product (biocompatible) peritoneal dialysis (PD) solutions significantly delayed anuria and reduced peritonitis rates compared with conventional solutions. This article reports a secondary outcome analysis of the balANZ trial with respect to peritoneal membrane function.
Methods
Adult, incident PD patients with residual renal function were randomized to receive either biocompatible or conventional (control) PD solutions for 2 years. Peritoneal equilibration tests were performed at 1, 6, 12, 18 and 24 months. Peritoneal small solute clearances and ultra-filtration (UF) were measured at 3, 6, 9, 12, 18 and 24 months.
Results
Of the 185 patients recruited into the trial, 85 patients in the Balance group and 82 patients in the control group had peritoneal membrane function evaluated. Mean 4-h dialysate:plasma creatinine ratios (D:P Cr 4h) at 1 month were significantly higher in the Balance group compared with controls (0.67 ± 0.10 versus 0.62 ± 0.10, P = 0.002). Over the 2-year study period, mean D:P Cr 4 h measurements remained stable in the Balance group but increased significantly in controls [difference −0.004 per month, 95% confidence interval (95% CI) −0.005 to −0.002, P < 0.001]. Similar results were obtained for dialysate glucose ratios (D/D0 glucose). Peritoneal UF was significantly lower in the Balance group than in controls at 3 and 6 months. Over the 2-year study period, peritoneal UF increased significantly in the Balance group but remained stable in controls (difference 24 mL/day/month, 95% CI 9–39, P = 0.002). No differences in peritoneal small solute clearances, prescribed dialysate fill volumes or peritoneal glucose exposure were observed between the two groups.
Conclusions
Biocompatible and conventional PD solutions exert differential effects on peritoneal small solute transport rate and UF over time. Adequately powered trials assessing the impact of these differential membrane effects on PD technique and patient survival rates are warranted.
Keywords: biocompatibility, glucose degradation products, outcomes, peritoneal dialysis, peritoneal equilibration test
Introduction
Approximately 200 000 end-stage renal failure patients worldwide (or 11% of the global dialysis population) utilize peritoneal dialysis (PD) for life-sustaining maintenance renal replacement therapy [1]. Most published observational cohort studies suggest that the medium-term survival (up to 3–4 years) of patients treated with PD is at least comparable, and possibly superior, to that of patients receiving haemodialysis (HD) [2–6]. However, PD is associated with a higher rate of technique failure than HD [7].
A large body of basic research in animal models and peritoneal cell culture systems has suggested that a major contributor to the high technique failure rate of PD is the bio-incompatible nature of conventional PD fluids, particularly as a result of their acidic pH (5.0–5.8) and high concentration of glucose degradation products (GDP) generated during the heat sterilization process [8]. Such ‘unphysiological’ characteristics may have both a negative impact on peritoneal cell populations and a pro-fibrotic effect on the peritoneal membrane [9–13]. In particular, experimental and clinical exposure of the peritoneal membrane to conventional PD solutions engenders significant histopathological changes over time, including loss of the surface mesothelial cell layer, thickening of the sub-mesothelial compact zone and the development of a progressive vasculopathy [14, 15]. Most of these adverse effects have been largely abrogated by the use of neutral-buffered, low GDP fluids in in vivo studies [8, 11, 16, 17]. Subsequent short-term, small, clinical studies have demonstrated that the use of low GDP fluids in PD patients is accompanied by significant improvements in the effluent biomarkers of peritoneal membrane integrity, stable membrane function and reductions in peritoneal membrane inflammatory response [18–25]. However, evidence of a beneficial effect on the morphological and functional changes associated with long-term exposure to PD fluids is not yet available.
The recently published balANZ randomized controlled trial [26] found that the administration of a neutral pH, lactate-buffered, low GDP fluid (Balance®) to incident PD patients was associated with an appreciable reduction in peritonitis rates and a significant delay in the onset of anuria compared with conventional, standard, lactate-buffered PD solutions (stay.safe®). In order to further evaluate the impact of biocompatible fluid on PD outcomes, this secondary analysis aimed to determine whether neutral pH, low GDP (biocompatible) PD fluid exerted beneficial effects on peritoneal membrane permeability, small solute clearance and ultra-filtration (UF) over a 2-year period compared with conventional dialysate.
Materials and methods
The study design and methodology [27] and the main results of the balANZ trial [26] have been described previously. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Conference of Harmonization, and local regulatory requirements. It was approved by ethics committees at all participating centres and all patients provided written informed consent prior to trial participation.
The study was an investigator-initiated, prospective, open-label, randomized controlled Phase 4 trial involving 16 centres across Australia, New Zealand and Singapore. It included incident, adult PD patients who had both a residual measured glomerular filtration rate ≥5 mL/min/1.73 m2 and a measured urine volume ≥400 mL/day. Pregnant or breast-feeding patients, individuals expected to die within 12 months, patients participating in trials targeting residual renal function in PD or those with a significant cancer history in the past 5 years, acute infection at enrolment, contra-indications to PD, any physical or mental disorder that appreciably hampered study protocol compliance or known or suspected allergy to trial product or related products were excluded. Participants were randomized 1:1 to receive either neutral pH, lactate-buffered, low GDP solution (Balance®) or conventional, standard, lactate-buffered solution (stay.safe®). Randomization was performed centrally via a web-based system and was stratified for both centre and presence or absence of diabetic nephropathy. Patients in each trial arm were treated according to local PD unit management protocols. Icodextrin and automated PD were permitted in both groups. Each patient was followed for 24 months. The primary outcome measure of the study was residual renal function decline. This article focuses on the secondary outcome measures of peritoneal transport status, peritoneal small solute clearance and peritoneal UF.
Dialysate: plasma creatinine ratio at 4 h (D:P Cr 4 h) and the ratio of dialysate glucose concentrations at 4 and 0 h (D/D0 glucose) were determined by standard peritoneal equilibration test (PET) [28] at 1, 6, 12, 18 and 24 months. Weekly peritoneal creatinine clearance (CpCr) and urea clearance (CpUr) were calculated from 24-h dialysate collections at 3, 6, 9, 12, 18 and 24 months, normalized for body surface area (BSA) and expressed as L/week/1.73 m2. BSA was calculated using the Du Bois formula [29]. Peritoneal UF during the 24-h collection was also recorded and expressed as mL/day. Peritoneal glucose exposure was calculated according to the method described by Davies et al. [7]. Peritoneal UF was normalized for peritoneal glucose exposure on the day that the UF was measured.
Statistical analysis
Results were expressed as frequencies (percentages), mean ± standard deviation or median [range], depending on data distribution. Group comparisons were performed by χ2-test, unpaired t-test or Mann–Whitney test, as appropriate. For the outcome measures of changes in peritoneal membrane permeability, small solute clearance and UF over time, a mixed effects General Linear Model was fitted for each outcome variable with treatment group, centre and presence or absence of diabetic nephropathy as fixed effects terms. Patient identification number was fitted as a ‘random’ term in the model, along with time and intercept. In this way, the model provided estimates of the rate of change (slope) in the outcome measure for each patient allowing them to also have a different intercept (starting level). From these data, an overall estimate of the rate of change in each treatment group was determined, corrected for the fixed-effects terms. The data were assumed to be normally distributed and to change in a linear fashion. Differences in the rate of change between the intervention and control groups were analysed on an intention-to-treat basis. Mixed models assume missing at random patterns to cater for missed visits or withdrawal for any reason other than those related to treatment. Data were analysed by Statistical Revelations Pty Ltd (http://www.statisticalrevelations.com.au/). P-values <0.05 were considered statistically significant.
Results
Patient characteristics
One hundred and eighty-five patients were randomized to receive either Balance (n= 92) or control (stay.safe) fluid (n= 93). Of these, 85 patients in the Balance group and 82 patients in the control group had peritoneal membrane function tests. As previously reported [26], the two groups were well matched for all baseline characteristics, including age, gender, end-stage renal failure cause, presence of cardiovascular disease, body mass index, initial dialysis modality, prescribed medications, blood pressure, prescribed dialysate volumes and glucose exposure, residual renal function and urine volume and laboratory parameters (serum albumin, calcium and haemoglobin). At baseline, the median [range] prescribed dialysate fill volumes were 8000 [2000–10 000] mL/day in the Balance group and 8000 [2000–8700] mL/day in the stay.safe group (P = not significant). The peritoneal glucose exposures were 121.5 ± 35.3 and 123.6 ± 36.3 g/day, respectively (P = not significant).
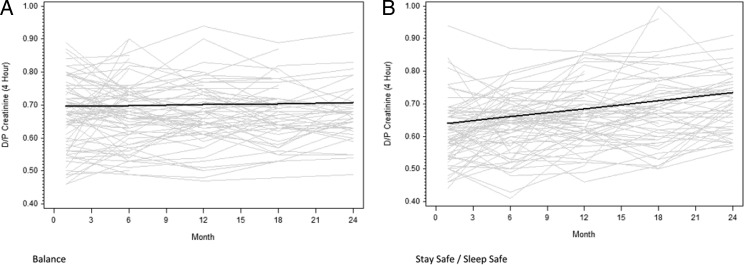
Peritoneal transport status
The results of PETs in each group at 1, 6, 12, 18 and 24 months are shown in Table 1. Mean D:P Cr 4 h values at 1 month were significantly higher in the Balance group compared with the control group (0.67 ± 0.10 versus 0.62 ± 0.10, P = 0.002). The respective proportions of high, high average, low average and low transporters were 8, 57, 29 and 6% in the Balance group and 5, 31, 58 and 7% in the stay.safe group (P = 0.001), respectively. Over the duration of the study, mean D:P Cr 4 h measurements remained stable in the Balance group [0.001 per month, 95% confidence interval (95% CI) −0.001 to 0.002] but increased significantly in the stay.safe group (0.004 per month, 95% CI 0.003–0.005) (Figure 1). The difference in D:P Cr 4h gradients over time between the two groups was statistically significant (−0.004 per month, 95% CI −0.005 to −0.002, P < 0.001, Figure 1). At 6 months, the respective proportions of high, high average, low average and low transporters were 9, 53, 35 and 3% in the Balance group and 1, 49, 44 and 6% in the stay.safe group (P = 0.11). No differences in peritoneal transport status were observed between the Balance and stay.safe groups at 12 months (P = 0.63) or 24 months (P = 0.65).
Table 1.
Measurements of peritoneal small solute clearance, UF and transport status over time in balANZ trial participants
| Parameter | 1 months |
3 months |
6 months |
9 months |
12 months |
18 months |
24 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balance (n= 85) | Stay.safe (n= 82) | Balance (n= 85) | Stay.safe (n= 80) | Balance (n= 76) | Stay.safe (n= 75) | Balance (n= 68) | Stay.safe (n= 68) | Balance (n= 62) | Stay.safe (n= 66) | Balance (n= 53) | Stay.safe (n= 59) | Balance (n= 42) | Stay.safe (n= 50) | |
| Weekly CpUr (L/week/1.73 m2) | N/A | N/A | ||||||||||||
| n | 85 | 78 | 75 | 74 | 68 | 68 | 62 | 65 | 53 | 56 | 40 | 48 | ||
| Median | 52 | 56 | 54 | 56 | 55* | 58 | 56 | 59 | 56 | 58 | 59 | 61 | ||
| [min, max] | [19, 70] | [6, 86] | [23, 71] | [13, 92] | [35, 90] | [20, 106] | [1, 71] | [1, 93] | [30, 76] | [32, 99] | [39, 77] | [26, 93] | ||
| Weekly CpCr (L/week/1.73 m2) | N/A | N/A | ||||||||||||
| n | 84 | 78 | 75 | 74 | 68 | 70 | 62 | 66 | 53 | 56 | 40 | 48 | ||
| Median | 40 | 39 | 41 | 43 | 39 | 43 | 43 | 44 | 42 | 46 | 44 | 49 | ||
| [min, max] | [11, 54] | [2, 67] | [11, 58] | [12, 71] | [20, 74] | [17, 79] | [21, 63] | [20, 77] | [25, 69] | [21, 69] | [23, 62] | [19, 70] | ||
| UF (mL/day) | N/A | N/A | ||||||||||||
| n | 85 | 79 | 75 | 74 | 68 | 70 | 62 | 66 | 53 | 56 | 40 | 48 | ||
| Median | 700* | 1090 | 850* | 1015 | 913* | 1233 | 955 | 1150 | 1100 | 951 | 900 | 993 | ||
| [min, max] | [−700, 3500] | [−400, 2800] | [−1040, 1966] | [−1716, 4040] | [−1082, 2300] | [−1000, 2900] | [−600, 2700] | [−400, 3000] | [−500, 2100] | [−1782, 3560] | [−100, 3340] | [−1338, 2568] | ||
| D:P Cr 4 h | N/A | N/A | N/A | N/A | ||||||||||
| n | 83 | 82 | 75 | 73 | 60 | 66 | 51 | 56 | 37 | 47 | ||||
| Mean ± SD | 0.67 ± 0.10* | 0.62 ± 0.10 | 0.67 ± 0.10* | 0.64 ± 0.09 | 0.67 ± 0.10 | 0.67 ± 0.09 | 0.67 ± 0.09 | 0.68 ± 0.11 | 0.67 ± 0.09 | 0.70 ± 0.08 | ||||
| D/D0 glucose 4 h | N/A | N/A | N/A | N/A | ||||||||||
| n | 83 | 82 | 75 | 73 | 59 | 66 | 50 | 56 | 37 | 47 | ||||
| Mean ± SD | 0.39 ± 0.08* | 0.43 ± 0.08 | 0.40 ± 0.08* | 0.43 ± 0.07 | 0.40 ± 0.08 | 0.40 ± 0.08 | 0.98 ± 0.15 | 0.42 ± 0.15 | 0.41 ± 0.07 | 0.39 ± 0.08 | ||||
| 4 h UF during PET (mL) | N/A | N/A | N/A | N/A | ||||||||||
| n | 83 | 82 | 75 | 73 | 60 | 66 | 51 | 56 | 37 | 47 | ||||
| Median | 300* | 354 | 300* | 400 | 260* | 400 | 300 | 345 | 300 | 350 | ||||
| [min, max] | [−200, 900] | [−100, 1085] | [−110, 900] | [−100, 1010] | [−200, 750] | [−80, 650] | [−350, 900] | [−250, 988] | [−270, 900] | [10, 863] | ||||
| 24 h dialysate fill volume (mL/day) | ||||||||||||||
| Median | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 8000 | 6000 | 8000 |
| [min, max] | [4000, 10 000] | [2000, 8700] | [4000, 10 500] | [2000, 13 500] | [3800, 12 300] | [2000, 14 995] | [6000, 12 000] | [4000, 14 500] | [5000, 12 300] | [4000, 15 194] | [4800, 12 300] | [4500, 14 685] | [8000, 14 500] | [4500, 15 000] |
| Peritoneal glucose exposure (g/day) | ||||||||||||||
| Mean ± SD | 139.1 ± 44.4 | 126.5 ± 35.5 | 142.1 ± 43.5 | 133.9 ± 36.9 | 143.7 ± 40.5 | 140.5 ± 43.5 | 149.2 ± 42.5 | 146.3 ± 46.2 | 150.0 ± 41.0 | 150.9 ± 48.5 | 158.4 ± 49.2 | 157.4 ± 52.3 | 161.7 ± 54.1 | 157.4 ± 52.3 |
Results are presented as mean ± SD or median [range], depending on data distribution.
*P < 0.05 versus Stay.safe (control).
CpCr, peritoneal creatinine clearance; CpUr, peritoneal urea clearance; D/D0 glucose, ratio of 4 h to initial dialysate glucose concentrations; D:P Cr 4 h, dialysate:plasma creatinine ratio at 4 h; N/A, not available; PET, peritoneal equilibration test; UF, ultrafiltration.
Fig. 1.
Change in D:P Cr 4 h over time in the balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was statistically significant (P < 0.001).
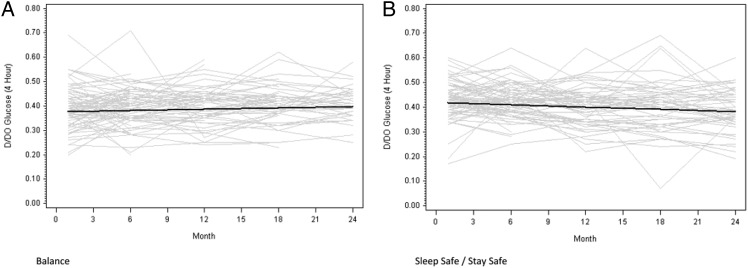
Similar results were observed for D/D0 glucose measurements. Mean values at 1 month were lower in the Balance group than in the stay.safe group (0.39 ± 0.08 versus 0.43 ± 0.08, P = 0.003). Over the duration of the study, mean D/D0 glucose measurements remained stable in the Balance group (0.001 per month, 95% CI −0.000 to 0.002) but decreased significantly in the stay.safe group (−0.002 per month, 95% CI −0.003 to −0.001) (Figure 2). The difference in D:P Cr 4 h gradients between the two groups was statistically significant (0.002 per month, 95% CI 0.001–0.004, P < 0.01).
Fig. 2.
Change in D/D0 glucose over time in the Balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was statistically significant (P < 0.01).
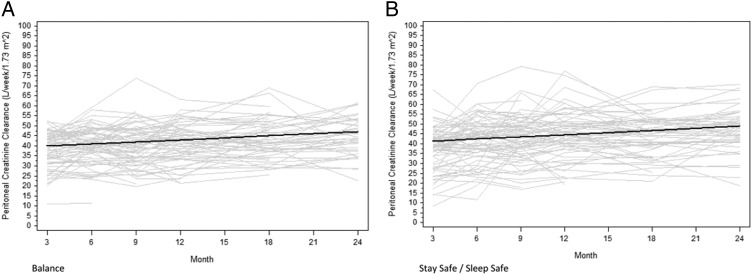
Peritoneal small solute clearance
The results of peritoneal small solute clearance measurements in each group at 3, 6, 9, 12, 18 and 24 months are shown in Table 1. Peritoneal CpCr measurements were comparable between the two groups over time. CpCr values increased over time in both the Balance group (0.33 L/week/1.73 m2/month, 95% CI 0.21–0.46) and the control group (0.37 L/week/1.73 m2/month, 95% CI 0.25–0.49) (Figure 3). The difference between the groups was not statistically significant (0.04 L/week/1.73 m2/month, 95% CI −0.21 to 0.14, P = 0.79).
Fig. 3.
Change in peritoneal CpCr over time in the Balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was not statistically significant (P = 0.69).
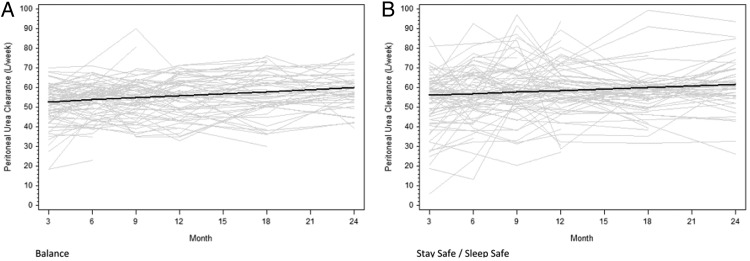
Similar results were observed for peritoneal CpUr measurements. CpUr values increased over time in both the Balance group (0.35 L/week/1.73 m2/month, 95% CI 0.19–0.50) and the control group (0.26 L/week/1.73 m2/month, 95% CI 0.11–0.41) (Figure 4). The difference between the groups was not statistically significant (0.08 L/week/1.73 m2/month, 95% CI −0.13 to 0.30, P = 0.45).
Fig. 4.
Change in peritoneal CpUr over time in the Balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was not statistically significant (P = 0.45).
Peritoneal ultra-filtration
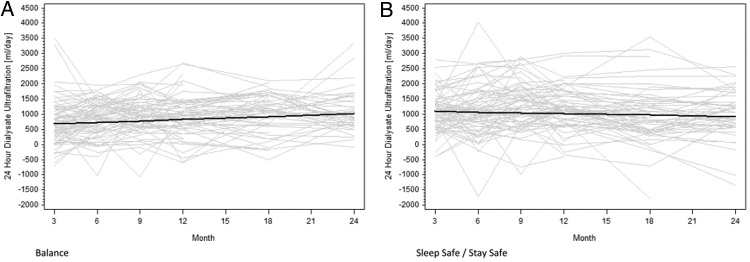
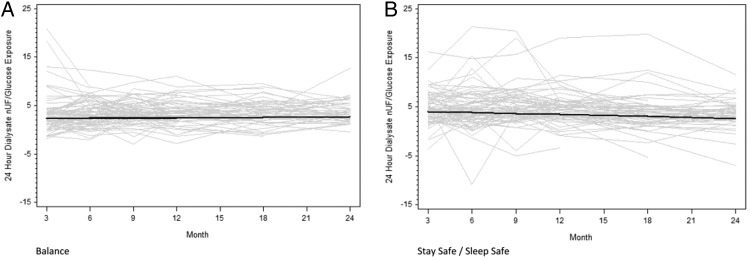
The results of peritoneal UF in each group at 3, 6, 9, 12, 18 and 24 months are shown in Table 1. Peritoneal UF was significantly lower in the Balance group than in controls at 3 and 6 months. These lower UF volumes in the biocompatible group coincided with higher urine volumes [26]. Over the course of the study, peritoneal UF increased significantly in the Balance group (16 mL/day/month, 95% CI 5–27) but remained stable in the stay.safe group (−8 mL/day/month, 95% CI −19 to 2) (Figure 5). The difference in gradients between the groups was statistically significant (24 mL/day/month, 95% CI 9–39, P = 0.002). This difference persisted after normalization of peritoneal UF for glucose exposure (0.08 mL/day/g/month, 95% CI 0.03–0.14, P = 0.004) (Figure 6).
Fig. 5.
Change in peritoneal UF over time in the Balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was statistically significant (P = 0.002).
Fig. 6.
Change in peritoneal UF, normalized for peritoneal glucose exposure, over time in the Balance (A) and control (B) groups over 2 years. Grey lines represent individual patient measurements while solid lines represent predicted gradients. The difference in gradients between the two groups was statistically significant (P = 0.004).
Discussion
This investigator-initiated, multi-centre, multi-country, prospective, open-label, randomized controlled Phase 4 trial involving 16 centres across Australia, New Zealand and Singapore demonstrated that prescription of neutral pH, lactate-buffered, low GDP (Balance) solution in PD patients was associated with an initially higher peritoneal membrane solute transport rate and lower peritoneal UF rate compared with controls. Moreover, whereas these parameters remained stable in the biocompatible group over the 2-year follow-up period of the study, there was a progressive increase in peritoneal membrane permeability in controls. Peritoneal small solute clearance increased at comparable rates over time in both groups.
Serial elevations in peritoneal solute transport characteristics and deterioration in peritoneal UF over time have been reported in patients treated with conventional dialysis fluids and have been attributed to the bio-incompatible nature of these solutions [30, 31]. However, while in vitro studies have reported that biocompatible fluid administration was associated with significant improvements in peritoneal cell viability, function and structure, clinical trials of biocompatible fluids on peritoneal membrane function have been both limited and conflicting. In keeping with the findings of the present investigation, the Euro Balance trial [19], a multi-centre, open-label, prospective randomized cross-over study of Balance versus standard PD fluid over two 12-week periods, observed significant increases in D:P Cr 4 h measurements in patients randomized to Balance. This rise in peritoneal permeability was associated with a significant fall in peritoneal UF. In a randomized controlled trial of a nutrineal, extraneal and physioneal (NEPP) regimen versus conventional dialysis fluid in 63 incident PD patients, Le Poole found that the lower GDP NEPP regimen was associated with an increase in peritoneal solute transport rate. Similarly, Kim et al. [23] observed significantly higher D:P Cr 4 h values in 48 patients randomly allocated to Balance solution compared with 43 control patients receiving continuous ambulatory peritoneal dialysis / Dialyse Péritonéale Continue Ambulatoire fluid (Balnet study). Moreover, D:P Cr 4 h remained stable in the biocompatible group over a period of 12 months. However, in contrast to the findings of the present study and those of other studies [32], Kim et al. reported a significant fall in D:P Cr 4 h over time in patients receiving conventional dialysis solutions. Peritoneal UF rates also tended to fall in the biocompatible group compared with controls, although this did not reach statistical significance (P = 0.09). Haag-Weber also observed a tendency to lower peritoneal UF in the biocompatible group (P = 0.10) without any significant changes in PET measurements, although these observations did not allow for the significant differences in overfill between biocompatible and standard PD solutions. On the other hand, two randomized controlled clinical trials [21, 33] have reported no changes in either PET measurements or peritoneal UF between biocompatible and conventional dialysates, while two other studies [34, 35] found no change in D:P Cr 4 h but a significant increase in peritoneal UF in association with biocompatible fluid use. The apparent disparities in findings between these studies and those of the present investigation may be explained by the fact that the other trials often suffered from a number of important limitations including insufficient statistical power due to small numbers, short-term follow-up, high drop-out rates, treatment-associated changes in fluid status, use of solutions with variable GDP content, sub-optimal methodological quality, lack of adjustment for peritoneal glucose exposure, enrolment of prevalent PD patients and single-centre designs. In contrast, our multi-centre study represents the largest and longest running randomized controlled trial to date evaluating the effects of biocompatible fluids on peritoneal membrane transport, small solute clearance and glucose-adjusted UF.
Similar to the results of the balANZ trial, the few previously published randomized controlled trials that have examined peritoneal small solute clearances have not observed a significant effect of biocompatible fluids [20, 23, 34]. However, Choi et al. [35] and Williams et al. [19] did report significant increases in peritoneal CpUr in patients receiving biocompatible fluids compared with controls, although peritoneal CpCrs were not different between the two groups. The similar overall findings between trials of comparable peritoneal small solute clearances in patients receiving biocompatible or conventional PD solutions in spite of variable observed differences in peritoneal solute transport rates may be potentially explained by the fact that PD patients’ prescriptions were titrated to achieve common small solute clearance targets between the two groups.
The observed higher initial peritoneal solute transport rate in patients receiving biocompatible fluids in the balANZ trial, as evidenced by higher initial D:P Cr 4 h and lower initial D/D0 glucose values, may be potentially explained by alterations in peritoneal vascular surface area. Previous studies in animals have observed that conventional, acidic pH, lactate-buffered 4.25% glucose resulted in a doubling of arteriolar flow and a 20% increase of perfused capillary length per area, while administration of a pH-neutral, bicarbonate-buffered, low GDP solution did not affect haemodynamic parameters [36]. Alternatively, administration of biocompatible fluids may influence local peritoneal membrane production of vasoactive cytokines, such as vascular endothelial growth factor and nitric oxide [19, 37]. The impact of these changes in peritoneal solute transport rate and UF on long-term patient-level outcomes remains uncertain. We have previously reported that technique and patient survival rates were comparable between the Balance and control groups in the balANZ trial [26], although the study had insufficient statistical power to exclude Type-2 statistical errors for these end-points.
The strengths of this study include its very large sample size, 2-year follow-up period, trial design and involvement of participants from a range of centres and countries with varying approaches to PD. This greatly enhanced the external validity of our findings. Randomization allocation was appropriately concealed and stratified for PD unit to mitigate against centre effects. The Balance and stay.safe groups were well balanced with respect to baseline demographic and clinical characteristics, thereby attesting to the success of the randomization process.
These strengths must be weighed up against the study's limitations, the principal one of which was that the relatively high drop-out rate (45% over 2 years) may have introduced informative censoring bias. However, the numbers of, and reasons for drop-out in each group were comparable. Moreover, missing peritoneal membrane tests due to withdrawal or non-treatment-related factors were catered for in the mixed effects general linear model analysis used in this study. The open-label design may have introduced the possibility of co-intervention bias. Observer bias could also not be excluded, although this was countered by the use of clearly defined, objective peritoneal membrane solute transport and UF measures. As with other studies in this area [19, 23, 30], a reciprocal relationship was observed in the balANZ trial between peritoneal UF and urine volume suggesting that some of the fall in peritoneal UF observed in the biocompatible group may have been explained by volume-driven changes. Overfill was not accounted for in the present study, although the difference in overfill between Balance and stay.safe is very small (∼20 mL).
In conclusion, administration of a neutral pH, lactate-buffered, low GDP fluid (Balance) to incident PD patients was associated with higher peritoneal solute transport rates, which then remained stable over the 2-year follow-up period. Peritoneal UF was initially lower but increased significantly over time. In contrast, patients receiving conventional PD solutions experienced progressive increases in peritoneal solute transport rate and stable peritoneal UF over time. Future, adequately powered randomized controlled trials investigating the impact of biocompatible fluid-induced changes in peritoneal membrane function on PD technique survival are warranted.
Acknowledgements
Collaborators (balANZ Investigators)
Australian Centres: G Rangan, L Liew, Blacktown Hospital, Sydney (NSW); U Steinwandel, Fremantle Hospital, Fremantle (WA); L Garvey, John Hunter Hospital, Newcastle (NSW); M Gilbert, Liverpool Hospital, Sydney (NSW); I Abraham, J Nandkumar Monash Medical Centre, Melbourne (VIC); A Coburn, V Bali, Princess Alexandra Hospital, Brisbane (QLD); S McDonald, S Frasca, M Hockley, C Russ, The Queen Elizabeth Hospital, Adelaide (SA); K Bannister, M Hockley, K Pirone, Royal Adelaide Hospital (SA); L Williams, Royal Brisbane Hospital, Brisbane (QLD); K Warr. G Smith, Perth (WA); S Pellicano, Sir Charles Gairdner Hospital, Perth (WA); E O'Flaherty, St Vincents Hospital, Melbourne (VIC.
New Zealand Centres: L Reed, L Anderson Dunedin Hospital, Dunedin; B Jagannathan, P Nicholls Middlemore Hospital, Auckland.
Singapore Centres: CK Tam, Singapore General Hospital, Singapore; R Lee, Tang Tock Seng Hospital, Singapore.
The invaluable assistance provided by Caro Badcock from Statistical Revelations Pty Ltd with regards to all statistical analyses in this study is gratefully acknowledged.
The invaluable assistance of Dr Feidhlim Woods (former Fresenius employee and current Fresenius consultant) and Ms Vanessa Wilson (current Fresenius employee) in providing advice regarding study design and co-ordination and critical review of the manuscript is gratefully acknowledged.
The results presented in this article have not been published previously in whole or part, except in abstract format.
Funding. The balANZ trial was funded by Fresenius Medical Care.
Conflict of interest statement. David Johnson is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers' honoraria and research grants from Fresenius Medical Care. He has previously been a consultant to Gambro Pty Ltd. He is an International Society of Peritoneal Dialysis Councillor and is a current recipient of a Queensland Government Health Research Fellowship. Fiona Brown is a consultant for Baxter and Fresenius. Margaret Clarke is an employee of Fresenius Medical Care. Neil Boudville has previously received research funds from Roche, travel grants from Roche, Amgen and Janssen-Cilag, and speaking honoraria from Roche. Michael Suranyi has participated in company sponsored research for both Baxter and Fresenius. In the past, but not currently, Michael Suranyi has been a member of the Clinical Advisory Boards of Baxter and Fresenius, and has attended sponsored meetings and received honoraria.
References
- 1.Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serkes KD, Blagg CR, Nolph KD, et al. Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: a multicenter study. Perit Dial Int. 1990;10:15–19. [PubMed] [Google Scholar]
- 3.Vonesh EF, Moran J. Mortality in end-stage renal disease: a reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 1999;10:354–365. doi: 10.1681/ASN.V102354. [DOI] [PubMed] [Google Scholar]
- 4.Fenton SS, Schaubel DE, Desmeules M, et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates [see comments] Am J Kidney Dis. 1997;30:334–342. doi: 10.1016/s0272-6386(97)90276-6. doi:10.1016/S0272-6386(97)90276-6. [DOI] [PubMed] [Google Scholar]
- 5.Tanna MM, Vonesh EF, Korbet SM. Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis. 2000;36:1175–1182. doi: 10.1053/ajkd.2000.19832. doi:10.1053/ajkd.2000.19832. [DOI] [PubMed] [Google Scholar]
- 6.Vonesh EF, Snyder JJ, Foley RN, et al. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006;70(Suppl 103):S3–S11. doi: 10.1038/sj.ki.5001910. doi:10.1038/sj.ki.5001910. [DOI] [PubMed] [Google Scholar]
- 7.Davies SJ, Phillips L, Naish PF, et al. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12:1046–1051. doi: 10.1681/ASN.V1251046. [DOI] [PubMed] [Google Scholar]
- 8.Topley N. In vitro biocompatibility of bicarbonate-based peritoneal dialysis solutions. Perit Dial Int. 1997;17:42–47. [PubMed] [Google Scholar]
- 9.Topley N. Membrane longevity in peritoneal dialysis: impact of infection and bio-incompatible solutions. Adv Ren Replace Ther. 1998;5:179–184. doi: 10.1016/s1073-4449(98)70030-5. [DOI] [PubMed] [Google Scholar]
- 10.Pecoits-Filho R, Stenvinkel P, Heimburger O, et al. Beyond the membrane—the role of new PD solutions in enhancing global biocompatibility. Kidney Int Suppl. 2003:S124–S132. doi: 10.1046/j.1523-1755.2003.08814.x. doi:10.1046/j.1523-1755.2003.08814.x. [DOI] [PubMed] [Google Scholar]
- 11.Witowski J, Jorres A. Effects of peritoneal dialysis solutions on the peritoneal membrane: clinical consequences. Perit Dial Int. 2005;25(Suppl 3):S31–S34. [PubMed] [Google Scholar]
- 12.Schambye HT. Effect of different buffers on the biocompatibility of CAPD solutions. Perit Dial Int. 1996;16(Suppl 1):S130–S136. [PubMed] [Google Scholar]
- 13.Nau B, Schmitt CP, Almeida M, et al. BIOKID: randomized controlled trial comparing bicarbonate and lactate buffer in biocompatible peritoneal dialysis solutions in children [ISRCTN81137991] BMC Nephrol. 2004;5:14. doi: 10.1186/1471-2369-5-14. doi:10.1186/1471-2369-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JD, Craig KJ, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479. doi: 10.1681/ASN.V132470. [DOI] [PubMed] [Google Scholar]
- 15.Dobbie JW, Anderson JD, Hind C. Long-term effects of peritoneal dialysis on peritoneal morphology. Perit Dial Int. 1994;14(Suppl 3):S16–S20. [PubMed] [Google Scholar]
- 16.Mortier S, Faict D, Schalkwijk CG, et al. Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int. 2004;66:1257–1265. doi: 10.1111/j.1523-1755.2004.00879.x. doi:10.1111/j.1523-1755.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 17.Mortier S, Faict D, Lameire NH, et al. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int. 2005;67:1559–1565. doi: 10.1111/j.1523-1755.2005.00237.x. doi:10.1111/j.1523-1755.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 18.Rippe B, Simonsen O, Heimburger O, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int. 2001;59:348–357. doi: 10.1046/j.1523-1755.2001.00497.x. doi:10.1046/j.1523-1755.2001.00497.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams JD, Topley N, Craig KJ, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004;66:408–418. doi: 10.1111/j.1523-1755.2004.00747.x. doi:10.1111/j.1523-1755.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 20.Szeto CC, Chow KM, Lam CW, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant. 2007;22:552–559. doi: 10.1093/ndt/gfl559. doi:10.1093/ndt/gfl559. [DOI] [PubMed] [Google Scholar]
- 21.Fan SL, Pile T, Punzalan S, et al. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008;73:200–206. doi: 10.1038/sj.ki.5002574. doi:10.1038/sj.ki.5002574. [DOI] [PubMed] [Google Scholar]
- 22.Haag-Weber M, Kramer R, Haake R, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010;25:2288–2296. doi: 10.1093/ndt/gfq087. doi:10.1093/ndt/gfq087. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Oh J, Kim S, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant. 2009;24:2899–2908. doi: 10.1093/ndt/gfp054. doi:10.1093/ndt/gfp054. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhary K, Khanna R. Biocompatible peritoneal dialysis solutions: do we have one? Clin J Am Soc Nephrol. 2010;5:723–732. doi: 10.2215/CJN.05720809. doi:10.2215/CJN.05720809. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DW, Williams JD. Impact of peritoneal dialysis solutions on outcomes. In: Molony DA, Craig JC, editors. Evidence-Based Nephrology. Oxford, UK: Blackwell; 2009. pp. 500–508. [Google Scholar]
- 26.Johnson DW, Brown FG, Clarke M, et al. Biocompatible versus standard peritoneal dialysis fluid - the balANZ trial. J Am Soc Nephrol. 2012;23:1097–1107. doi: 10.1681/ASN.2011121201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson DW, Clarke M, Wilson V, et al. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol. 2010;11:25. doi: 10.1186/1471-2369-11-25. doi:10.1186/1471-2369-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twardowski ZJ. PET—a simpler approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial. 1990;6:186–191. [PubMed] [Google Scholar]
- 29.Du Bois D, Du Bois EF. A formula to estimate the aproximate surface area if height and weight be known. Arch Int Med. 1916;17:863–971. doi:10.1001/archinte.1916.00080130010002. [PubMed] [Google Scholar]
- 30.Davies SJ. Preserving residual renal function in peritoneal dialysis: volume or biocompatibility? Nephrol Dial Transplant. 2009;24:2620–2622. doi: 10.1093/ndt/gfp313. doi:10.1093/ndt/gfp313. [DOI] [PubMed] [Google Scholar]
- 31.Devuyst O, Topley N, Williams JD. Morphological and functional changes in the dialysed peritoneal cavity: impact of more biocompatible solutions. Nephrol Dial Transplant. 2002;17(Suppl 3):12–15. doi: 10.1093/ndt/17.suppl_3.12. doi:10.1093/ndt/17.suppl_3.12. [DOI] [PubMed] [Google Scholar]
- 32.Davies SJ. Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int. 2004;66:2437–2445. doi: 10.1111/j.1523-1755.2004.66021.x. doi:10.1111/j.1523-1755.2004.66021.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss L, Stegmayr B, Malmsten G, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int. 2009;29:647–655. [PubMed] [Google Scholar]
- 34.Tranaeus A. A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. The Bicarbonate/Lactate Study Group. Perit Dial Int. 2000;20:516–523. [PubMed] [Google Scholar]
- 35.Choi HY, Kim DK, Lee TH, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int. 2008;28:174–182. [PubMed] [Google Scholar]
- 36.Mortier S, De Vriese AS, Van de Voorde J, et al. Hemodynamic effects of peritoneal dialysis solutions on the rat peritoneal membrane: role of acidity, buffer choice, glucose concentration, and glucose degradation products. J Am Soc Nephrol. 2002;13:480–489. doi: 10.1681/ASN.V132480. [DOI] [PubMed] [Google Scholar]
- 37.Combet S, Miyata T, Moulin P, et al. Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol. 2000;11:717–728. doi: 10.1681/ASN.V114717. [DOI] [PubMed] [Google Scholar]