Abstract
Background
Measurement of hospital quality has traditionally focused on processes of care and post-procedure outcomes. Appropriateness measures for percutaneous coronary intervention (PCI) assess quality as it relates to patient selection in the context of anticipated benefits relative to potential harm. The association, if any, between patient selection for PCI and processes of care and post-procedural outcomes is unknown. Defining whether these measures are redundant or complementary can inform the optimal range of metrics for monitoring quality.
Methods
We included patients undergoing non-acute (elective) PCI within the NCDR CathPCI Registry® between July 2009 and April 2011. We examined the association between a hospital’s proportion of non-acute PCIs categorized as inappropriate by the 2009 Appropriate Use Criteria (AUC) for Coronary Revascularization and in-hospital mortality, bleeding complications, and use of optimal guideline-directed medical therapy at discharge (i.e. aspirin, thienopyridines, and statins).
Results
A total of 203,531 non-acute PCIs from 779 hospitals were classified by the AUC. Of these, 101,779 (50.0%) were classified as appropriate, 77,220 (35.5%) as uncertain, and 24,532 (12.1%) as inappropriate. When categorized as hospital tertiles, the range of inappropriate PCI was 0.0 to 8.1% in the lowest-tertile, 8.1 to 15.2% in the middle-tertile, and 15.2 to 58.6% in the highest-tertile. Compared with lowest-tertile hospitals, mortality was not significantly different at middle-tertile (adjusted odds ratio [OR] 0.93; 95% confidence interval [CI] 0.73 to 1.19) or highest-tertile hospitals (OR 1.12; 95% CI 0.88 to 1.43; p=0.35 for differences between any tertile). Similarly, risk-adjusted bleeding did not vary significantly (middle-tertile OR 1.13; 95% CI 1.02 to 1.16; highest-tertile OR 1.02; 95% CI 0.91 to 1.16; p=0.07 for differences between any tertile) nor did use of optimal therapy at discharge after PCI (85.3% vs. 85.7% vs. 85.2%; P=0.58).
Conclusions
In a national cohort of non-acute PCIs, a hospital’s proportion of inappropriate PCIs was not associated with in-hospital mortality, bleeding, or medical therapy at discharge. These findings suggest that PCI appropriateness measures aspects of hospital PCI quality that are independent of, and complementary to, traditional quality metrics.
Keywords: Appropriateness criteria, Coronary artery disease, Percutaneous coronary intervention, Utilization, Hospital, Quality of care, Health services research
Achieving high quality percutaneous coronary intervention (PCI) requires efforts to minimize the potential for significant complications and maximize patient benefit. Traditionally, measuring the quality of PCI has focused upon processes of care and post-procedural outcomes, such as in-hospital mortality, bleeding and vascular complication rates, and provision of guideline-recommended medications.1 These metrics have been useful in supporting quality improvement.2, 3
Despite their importance, assessing processes of care and post-procedural outcomes fail to account for a key aspect of high quality care – proper patient selection. Recently, multiple major cardiology organizations collaborated to create Appropriate Use Criteria (AUC) for Coronary Revascularization; a guidelines-based approach for assessing procedural “appropriateness” for a range of clinical scenarios.4 In the AUC, coronary revascularization was considered “appropriate” for a given clinical scenario when the expected benefits, in terms of survival or quality of life, exceeded the expected negative consequences of the procedure and “inappropriate” when the risks were perceived to outweigh the benefits. Therefore, these criteria represent an assessment of PCI quality as it relates to patient selection and the decision to perform PCI, in contrast to processes of care and post-procedural outcomes that represent how well the PCI was performed.
Assessment of procedural appropriateness using the AUC is rapidly being incorporated into PCI registries and quality improvement programs in the hopes of facilitating high-quality PCI that is both effective and efficient.5, 6 However, the association between the AUC and traditional quality metrics is unknown. It is possible that PCI appropriateness correlates with other aspects of care, such as the provision of optimal medical therapy and low complication and mortality rates. Alternatively, PCI appropriateness may measure a different and independent component of PCI quality, such that hospitals with the lowest rate of PCI complications may not excel at patient selection. In fact, there is potential for an inverse relationship to be compounded by lower complication rates in the setting of inappropriate PCI given that such patients often have lower clinical risk (e.g. less ischemic burden, lower severity coronary disease). As a result, failure to account for patient selection in the measurement of PCI quality may lead to erroneous conclusions. We sought to determine whether these measures are redundant or complementary to help inform the optimal range of metrics for monitoring quality. Accordingly, we analyzed data from a large, contemporary, national PCI registry to determine the association between a hospital’s proportion of inappropriate PCIs and processes of care and post-procedural outcomes.
METHODS
Data source
The National Cardiovascular Data Registry (NCDR) CathPCI Registry, sponsored by the American College of Cardiology (ACC) and the Society for Cardiovascular Angiography and Interventions (SCAI), is the largest national registry of diagnostic cardiac catheterization and PCI, with more than 1,000 participating centers across the United States.7, 8 Captured data includes detailed patient and hospital characteristics, procedural findings, interventions, and outcomes based on pre-specified data elements defined by an NCDR committee.5 Data quality assurance is achieved through automatic system validation and reporting of data completeness, education and training for site data managers, and random on-site auditing.9 Only institutions whose data submissions meet NCDR quality criteria for reporting are included.
Study population
Patients who underwent PCI for non-acute indications in the period from July 2009 through April 2011 were included. This study period follows implementation of version 4.0 of the NCDR data collection tool, which included the data necessary for assigning the AUC to each procedure. We excluded PCI for acute indications (ST-segment elevation myocardial infarction, non-ST segment elevation myocardial infarction, and unstable angina with high-risk features) as prior work found these indications were nearly uniformly appropriate with minimal hospital-level variation.10 Each non-acute PCI was mapped to an AUC clinical indication using previously developed algorithms, based upon the 2009 publication of the AUC.10 We excluded non-acute PCI procedures that were missing the necessary data elements for mapping to the AUC, such as Canadian Cardiovascular Society (CCS) class for angina severity and stress test results. Additionally, we excluded PCIs from sites with annual non-acute procedure volume ≤ 50 to ensure that the rates of inappropriate PCI were not inflated by small numbers.
Outcomes Measures
To compare appropriateness with standard performance measures, we assessed the use of secondary prevention medications and peri-procedural complications. Guideline directed medications at discharge after PCI was created as an ‘all-or-none’ measure and defined as documentation of the prescription of all clinically indicated medications (i.e. aspririn, thienopyridine, and statin) after accounting for patient exclusions. Periprocedural bleeding was defined, in accordance with the NCDR CathPCI data definition, as bleeding requiring a blood transfusion, prolonged hospital stay for management, or bleeding associated with a >3 g/dL decrease in hemoglobin level. In-hospital mortality is documented as part of the NCDR record.
Statistical Analysis
For each hospital, we determined the proportion of PCIs for non-acute indications classified as inappropriate by the AUC. We compared patient and hospital level characteristics across tertiles of hospitals’ proportions of inappropriate PCIs using linear trend test for continuous variables and Mantel-Haenszel trend test for categorical variables.
We then evaluated the relationship between a hospital’s proportion of inappropriate PCI with guideline-directed medications at discharge and post-procedural outcomes, including in-hospital mortality and periprocedural bleeding. The unadjusted association between hospital tertiles of inappropriate PCIs and in-hospital mortality, bleeding complications, and guideline-directed medications at discharge was determined using the Mantel-Haenszel trend test. Next, using covariates from predictive models previously developed and validated within NCDR for in-hospital mortality and periprocedural bleeding,11, 12 we used multivariable logistic regression to model the risk-adjusted association between hospital tertiles of inappropriate PCIs and in-hospital mortality and periprocedural bleeding. The association of hospital tertiles of inappropriate non-acute PCI with risk-adjusted in-hospital mortality and bleeding was then assessed using the Wald chi-square test.
Because the primary reason for excluding non-acute cases in our analysis was due to missing data on stress test results, we performed sensitivity analyses with best- and worst-case scenarios in which all missing stress tests results were assumed to be high-risk and low-risk, respectively. We explored the impact of these assumptions on the categorization of hospital tertile of inappropriate PCI in addition to the primary analyses described above.
All statistical analyses were performed with SAS 9.2 (SAS Institute, Inc, Cary, NC) and evaluated at a significance level of 0.05. The institutional review board at Saint Luke’s Mid America Heart Institute granted a waiver of written informed consent and provided authorization for this study.
RESULTS
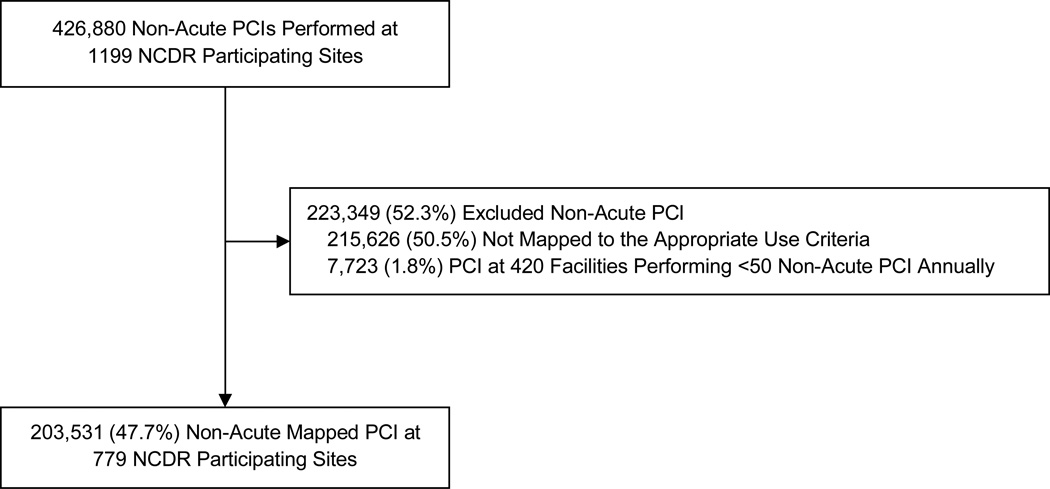
From July 2009 to April 2011, we identified 426,880 patients who underwent PCI for non-acute indications at one of 1199 NCDR participating sites. Of these, we were unable to map 215,626 (50.5%) patients to the AUC, largely because no stress test was performed or stress test results were missing. We also excluded 7,723 (1.8%) PCIs at 420 sites performing fewer than 50 non-acute PCIs annually. Our final cohort was comprised of 203,561 patients at 779 participating centers (Figure 1).
Figure 1.
Identification of the Study Cohort
Of the non-acute PCIs which were categorized by the AUC, 101,779 (50.0%) were classified as appropriate, 77,220 (35.5%) as uncertain, and 24,532 (12.1%) as inappropriate. The median hospital proportion of inappropriate PCI was 10.9%, with a range from 0.0% to 58.6%. Categorized as hospital tertiles, the median proportion of inappropriate PCI in the lowest hospital tertile was 5.3% (range 0.0% to 8.1%), as compared with a median proportion of inappropriate PCI of 10.9% (range 8.1% to 15.2%) in the middle-tertile, and 20.0% (range 15.2% to 58.6%) in the highest-tertile (Table 1).
Table 1.
Patient Characteristics by Hospital Tertile of Inappropriate PCIs for Non-Acute Indications
| Patient Characteristics | All Hospitals n = 779 |
Hospital Tertile of Inappropriate PCI | P-Value | ||
|---|---|---|---|---|---|
| 1 (Lowest) n = 259 |
2 n = 260 |
3 (Highest) n = 260 |
|||
| Median Proportion of Inappropriate PCI (Range) | 10.9 (0.0, 58.6) | 5.2 (0.0, 8.1) | 10.9 (8.1, 15.2) | 20.0 (15.2, 58.6) | |
| Patients | 203531 | 73753 | 71782 | 57996 | |
| Demographics | |||||
| Age, mean (SD), y | 65.4 (11.1) | 65.0 (11.2) | 65.5 (11.1) | 65.6 (11.0) | < 0.001 |
| Male sex | 135615 (66.6%) | 48289 (65.5%) | 48147 (67.1%) | 39179 (67.6%) | < 0.001 |
| White | 181070 (89.0%) | 66031 (89.5%) | 62874 (87.6%) | 52165 (89.9%) | 0.18 |
| Insurance | |||||
| Private | 137771 (67.8%) | 48182 (65.4%) | 48985 (68.3%) | 40604 (70.1%) | <0.001 |
| Public only | 59639 (29.3%) | 22993 (31.2%) | 20657 (28.8%) | 15989 (27.6%) | |
| Non-US | 107 (0.1%) | 30 (0.0%) | 39 (0.1%) | 38 (0.1%) | |
| None | 5764 (2.8%) | 2444 (3.3%) | 1993 (2.8%) | 1327 (2.3%) | |
| Clinical Risk Factors and Comorbidities | |||||
| Use of tobacco | 44665 (22.0%) | 17310 (23.5%) | 14887 (20.7%) | 12468 (21.5%) | < 0.001 |
| Hypertension | 175645 (86.3%) | 63936 (86.7%) | 61906 (86.3%) | 49803 (85.9%) | < 0.001 |
| Dyslipidemia | 175401 (86.3%) | 63321 (85.9%) | 62176 (86.7%) | 49904 (86.1%) | 0.25 |
| Family history of CAD | 50598 (24.9%) | 19776 (26.8%) | 17469 (24.3%) | 13353 (23.0%) | < 0.001 |
| Prior MI | 58687 (28.8%) | 21452 (29.1%) | 20660 (28.8%) | 16575 (28.6%) | 0.04 |
| Heart Failure | 22590 (11.1%) | 8645 (11.7%) | 7756 (10.8%) | 6189 (10.7%) | < 0.001 |
| Prior Valve Surgery | 2715 (1.3%) | 848 (1.2%) | 1067 (1.5%) | 800 (1.4%) | < 0.001 |
| Prior PCI | 90710 (44.6%) | 34257 (46.5%) | 31612 (44.0%) | 24841 (42.8%) | < 0.001 |
| Prior CABG | 28455 (14.0%) | 9565 (13.0%) | 10257 (14.3%) | 8633 (14.9%) | < 0.001 |
| Hemodialysis | 4256 (2.1%) | 1469 (2.0%) | 1551 (2.2%) | 1236 (2.1%) | 0.06 |
| Cerebrovascular Disease | 25689 (12.6%) | 9323 (12.6%) | 8962 (12.5%) | 7404 (12.8%) | 0.56 |
| Peripheral Arterial Disease | 27365 (13.5%) | 10058 (13.6%) | 9182 (12.8%) | 8125 (14.0%) | 0.13 |
| Chronic Lung Disease | 30234 (14.9%) | 11803 (16.0%) | 10069 (14.0%) | 8362 (14.4%) | < 0.001 |
| Diabetes Mellitus | 77301 (38.0%) | 27826 (37.7%) | 27236 (38.0%) | 22239 (38.3%) | 0.02 |
| Clinical Presentation | |||||
| Clinical symptoms None Atypical angina Stable angina Unstable angina without high-risk features |
27875 (13.7%) 14370 (7.1%) 91682 (45.0%) 69604 (34.2%) |
5433 (7.4%) 4039 (5.5%) 30481 (41.3%) 33800 (45.8%) |
9758 (13.6%) 4879 (6.8%) 34145 (47.6%) 23000 (32.0%) |
12684 (21.9%) 5452 (9.4%) 27056 (46.7%) 12804 (22.1%) |
< 0.001 |
| Angina No symptoms CCS I CCS II CCS III CCS IV |
29023 (14.3%) 23799 (11.7%) 70613 (34.7%) 65190 (32.0%) 14906 (7.3%) |
4899 (6.6%) 6350 (8.6%) 22100 (30.0%) 33812 (45.8%) 6592 (8.9%) |
10045 (14.0%) 8080 (11.3%) 26881 (37.4%) 20968 (29.2%) 5808 (8.1%) |
14079 (24.3%) 9369 (16.2%) 21632 (37.3%) 10410 (17.9%) 2506 (4.3%) |
< 0.001 |
| Any anti-anginal medication | 146693 (72.1%) | 54295 (73.6%) | 52401 (73.0%) | 39997 (69.0%) | < 0.001 |
| No. of anti-anginal medications 0 1 ≥ 2 |
56940 (28.0%) 91531 (45.0%) 55045 (27.0%) |
19488 (26.4%) 31988 (43.4%) 22274 (30.2%) |
19415 (27.1%) 32739 (45.6%) 19619 (27.4%) |
18037 (31.1%) 26804 (46.2%) 13152 (22.7%) |
< 0.001 |
| Stress test results Low risk Intermediate risk High risk |
39637 (35.2%) 47462 (42.2%) 25466 (22.6%) |
9232 (29.5%) 12899 (41.2%) 9170 (29.3%) |
13436 (32.4%) 17780 (42.9%) 10234 (24.7%) |
16969 (42.6%) 16783 (42.2%) 6082 (15.2%) |
<0.001 |
| Coronary artery stenoses 1 2 3 |
97779 (48.0%) 67593 (33.2%) 36709 (18.0%) |
35908 (48.7%) 24383 (33.1%) 12995 (17.6%) |
33959 (47.3%) 24043 (33.5%) 13220 (18.4%) |
27912 (48.1%) 19167 (33.0%) 10494 (18.1%) |
0.02 |
| Significant proximal LAD stenosis | 55316 (27.2%) | 20699 (28.1%) | 19509 (27.2%) | 15108 (26.1%) | < 0.001 |
Continuous variables were compared using linear trend test. Categorical variables were compared using Mantel-Haenszel trend test.
Most patients were white and male, the mean age was 65.4 ± 11.1 years, and the vast majority had hypertension or dyslipidemia. Nearly 40% of patients had diabetes mellitus and one-quarter were smokers, had a prior myocardial infarction, or a family history of CAD. Approximately half had prior PCI and nearly 15% had prior bypass surgery. Comparisons across hospital tertiles of inappropriate PCI were statistically significant for the majority of comorbidities and risk factors given our large sample size; however, most of these differences were small (see Table 1). Consistent with the variables that determine classification of PCI appropriateness for non-acute indications, patients from hospitals in the lowest-tertile of inappropriate PCI had greater severity of angina (CCS III or IV; 54.7% vs. 37.3% vs. 22.2% from lowest to highest-tertile, p<0.001 for trend), higher risk stress tests results (intermediate or high-risk; 70.5% vs. 67.6% vs. 57.4%, p<0.001 for trend), and were on more anti-anginal medications (at least 2 anti-anginals; 30.2% vs. 27.4 vs. 22.7%, p<0.001 for trend) before the procedure (see Table 1).
In the evaluation of hospital factors, most hospitals were private or community-based and located in urban settings. Nearly half were dedicated teaching hospitals. Notably, there were no significant trends in hospital characteristics across tertiles of inappropriate PCIs (Table 2).
Table 2.
Hospital Characteristics by Hospital Tertile of Inappropriate PCI for Non-Acute Indications
| Hospital Characteristics | All Hospitals n = 779 |
Hospital Tertile of Inappropriate PCI | P- Value |
||
|---|---|---|---|---|---|
| 1 n = 259 |
2 n = 260 |
3 n = 260 |
|||
| Median Proportion of Inappropriate PCI (Range) | 10.9 (0.0, 58.6) | 5.3 (0.0, 8.1) | 10.9 (8.1, 15.2) | 20.0 (15.2, 58.6) | |
| Hospital Location Rural Suburban Urban |
114 (14.6%) 253 (32.5%) 412 (52.9%) |
47 (18.1%) 73 (28.2%) 139 (53.7%) |
31 (11.9%) 82 (31.5%) 147 (56.5%) |
36 (13.8%) 98 (37.7%) 126 (48.5%) |
0.89 |
| Hospital Type Government Private/Community University |
8 (1.0%) 689 (88.4%) 82 (10.5%) |
4 (1.5%) 236 (91.1%) 19 (7.3%) |
2 (0.8%) 221 (85.0%) 37 (14.2%) |
2 (0.8%) 232 (89.2%) 26 (10.0%) |
0.23 |
| Teaching Hospital | 360 (46.2%) | 117 (45.2%) | 127 (48.8%) | 116 (44.6%) | 0.90 |
| Public Hospital | 435 (55.8%) | 141 (54.4%) | 143 (55.0%) | 151 (58.1%) | 0.40 |
Hospital characteristics were compared using Mantel-Haenszel trend test.
A total of 453 (0.2%) patients suffered in-hospital death, 3,699 (1.8%) suffered periprocedural bleeding, and 173,847 (85.4%) were discharged on optimal medical therapy after PCI (Table 3). Outcomes were similar after non-acute PCIs among patients excluded from the primary analysis (in-hospital mortality n=747 [0.3%]; periprocedural bleeding n=4,579 [2.1%]; optimal discharge medications n=188,763 [84.5%]). The hospital tertile of inappropriate PCIs was not associated with unadjusted or risk-adjusted mortality. Similarly, hospital tertile was not associated with unadjusted or risk-adjusted periprocedural bleeding, nor the provision of guideline-directed medications at discharge after PCI.
Table 3.
Post-procedural Outcomes and Processes of Care by Hospital Tertile of Inappropriate PCI for Non-Acute Indications
| All Hospitals n = 779 |
Hospital Tertile of Inappropriate PCI | P-Value | |||
|---|---|---|---|---|---|
| 1 (Lowest) n = 259 |
2 n = 260 |
3 (Highest) n = 259 |
|||
| Median Proportion of Inappropriate PCI (Range) | 10.9 (0.0, 58.6) | 5.3 (0.0, 8.1) | 10.9 (8.1, 15.2) | 20.0 (15.2, 58.6) | |
| Total Number of PCI | 203531 | 73753 | 71782 | 57996 | |
| Total Number of Inappropriate PCI | 24532 (12.1%) | 3810 (5.2%) | 8056 (11.2%) | 12666 (21.8%) | |
| In-hospital mortality | |||||
| Total deaths | 453 (0.2%) | 156 (0.2%) | 150 (0.2%) | 147 (0.3%) | 0.13 |
| Adjusted Odds Ratios | Reference | 0.93 (0.73–1.19) | 1.12 (0.88–1.43) | 0.35 | |
| Periprocedural Bleeding | |||||
| Total periprocedural bleeding | 3699 (1.8%) | 1331 (1.8%) | 1407 (2.0%) | 961 (1.7%) | 0.08 |
| Adjusted Odds Ratios | Reference | 1.13 (1.02–1.26) | 1.02 (0.91–1.16) | 0.07 | |
| Discharge Medications | |||||
| Aspirin | 100% | 100% | 100% | 100% | 0.99 |
| Thienopyridine | 100% | 100% | 100% | 100% | 0.99 |
| Statin | 173847 (85.4%) | 62940 (85.3%) | 61497 (85.7%) | 49410 (85.2%) | 0.58 |
| All Medications | 173847 (85.4%) | 62940 (85.3%) | 61497 (85.7%) | 49410 (85.2%) | 0.58 |
Unadjusted outcomes were compared using Mantel-Haenszel trend test. Adjusted odds ratios were compared using Wald test. In additional multivariable logistic regression with hospital tertile modeled for trend, there was no association between hospital tertile and in-hospital mortality (odds ratio [OR] for single increase in tertile 1.05, 95% confidence interval [CI] 0.93 to 1.19) or periprocedural bleeding (OR 1.02, 95% CI 0.96 to 1.08).
The sensitivity analyses assigning stress test results when the results were missing increased the number of non-acute PCIs that could be classified by the AUC and influenced the proportion of inappropriate PCIs in each hospital tertile. In these analyses, several statistically significant associations were identified. However, there were no consistent or clinically important trends between hospital tertiles of inappropriate PCIs and post-procedural processes and outcomes (Online Supplemental Material). Compared with the primary analysis, when missing stress test results were assumed high-risk, the category of hospital tertile changed for 167 (21.4%) hospitals. When missing stress test results were assumed low-risk, the category of hospital tertile changed for 367 (47.1%) hospitals.
DISCUSSION
In this large, national registry of PCI procedures, we evaluated the association between a hospital’s proportion of inappropriate PCI in non-acute settings – as defined by the AUC – and traditional performance measures of processes of care and post-procedural outcomes. In 203,561 PCIs from 779 hospitals, we found no relationship between hospital tertiles of inappropriate PCIs and in-hospital mortality, periprocedural bleeding, or medical therapy at discharge. These findings suggest that PCI appropriateness, relative to processes of care and post-procedural outcomes, measures a different aspect of PCI quality. Furthermore, the large hospital-level variation in the proportion of inappropriate PCIs for non-acute indications suggests that there are significant differences in the quality of patient selection for PCI across facilities that are unrelated to how well the procedure is performed. Therefore, measurement of PCI appropriateness and post-procedural outcomes are equally important to informing PCI quality.
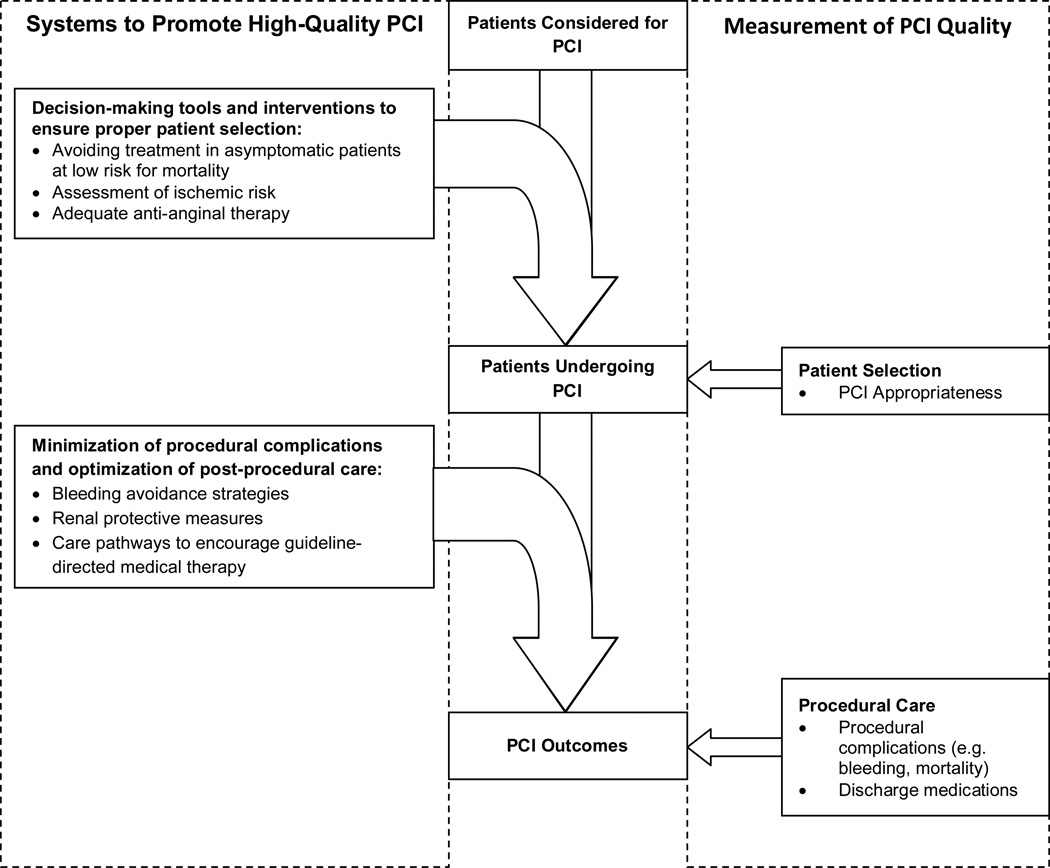
Although AUC have been developed for a number of diagnostic and therapeutic procedures in cardiovascular medicine,4, 13–15 to our knowledge, this is the first study to assess the relationship between facility-level procedural appropriateness and traditional metrics of procedural quality. Although appropriateness assessment, processes of care, and post-procedural outcomes are all quality measures for PCI, the systems required to improve quality in these domains are likely very different (Figure 2). Hospital systems to ensure proper patient selection likely include decision-making tools and interventions prior to patient arrival in the cardiac catheterization laboratory. Among patients being considered for non-acute PCI, this may include ensuring an adequate assessment of ischemic risk, a trial of robust anti-anginal medications prior to PCI, since medications alone may alleviate patients’ angina,16 and the avoidance of revascularization in asymptomatic patients. Furthermore, proper patient selection to avoid inappropriate PCI may be disincentivized by monetary reimbursement, referral structures and the expectation of colleagues, or abundance of catheterization facilities and interventional cardiologists.17 Systems to support high-quality patient selection are likely unrelated to systems that ensure minimization of procedural complications and promote high-quality post-procedural care. Procedural systems may include optimization of bleeding avoidance strategies (e.g. radial access site and bivalrudin), renal protective measures for chronic kidney disease, and development of care pathways to improve adherence to guideline-directed medications.18–23
Figure 2.
Conceptual Framework for Systems and Measurement of High-Quality PCI
In this context, our study has several important implications. First, the lack of association between hospital level appropriateness classification and PCI outcomes indicates that measures of appropriateness alone are inadequate in determining PCI quality, as they do not describe hospitals with higher or lower rates of procedural complications. In fact, both hospitals with high and low proportions of PCIs classified as inappropriate perform the procedures with relatively low mortality and bleeding rates. Second, the considerable hospital-level variation in inappropriate PCIs suggests there exists a substantial opportunity to explore upstream PCI quality metrics to ensure patients are expected to benefit from the procedure. Notably, among PCIs performed at hospitals in the highest-tertile of inappropriate PCIs, nearly 25% were for asymptomatic patients where there is no expectation for clinical benefit.24–26 Similarly, 75% of patients undergoing PCI at hospitals in the highest-tertile were not on maximal anti-anginal therapy prior to the procedure, precluding the opportunity for adequate medical therapy to cost-effectively control patients’ symptoms.16, 24, 25, 27 Thus, a higher proportion of patients undergoing PCI at hospitals in the highest-tertile of inappropriate PCI are exposed to the clinical risk of PCI without reasonable expectation of greater benefit as compared with more conservative management strategies. If these inappropriate PCIs represent unnecessary procedures, then their identification represent an opportunity to improve PCI quality by reducing unnecessary complications and the resource utilization associated with these procedures.
One particular area of concern when evaluating AUC involves the issue of missing stress test data. We therefore conducted extensive sensitivity analyses to explore the impact of missing stress test data on classification of procedural appropriateness on our findings. Importantly, the association between hospital tertile of PCI classified as inappropriate and post-procedural processes of care and outcomes was not meaningfully influenced by assumed stress test results. However, the categorization of hospital tertile changed depending on assumptions about missing stress test data. Given the implications of missing stress test data on the site level assessment of PCI appropriateness, reducing site level variation in PCI performed without adequate documentation is an important corollary goal to reducing hospital variation in inappropriate PCI. In the interim, site-to-site comparisons of PCI appropriateness must account for the distribution of PCI without documentation of preprocedural stress testing to ensure equitable conclusions.
Strengths of our analysis include the large number of participating facilities and non-acute PCIs from a nationwide registry. However, our findings should be considered in the context of the following limitations. First, participation in NCDR is often voluntary and observed results may not reflect non-NCDR PCI hospitals. However, analysis from a statewide quality improvement program that includes non-NCDR hospitals suggests similarity of PCI appropriateness across NCDR participation status.28 Second, there are limitations in the application of the AUC for Coronary Revascularization, most notably due to missing results for non-invasive stress testing. However, our sensitivity analyses that assumed the highest and lowest risk for missing stress tests did not alter our conclusions. Additional limitations in the application of AUC have been described,29 however it is unclear these limitations importantly influence the assessment of patient selection for PCI across broad practice settings. Third, our study does not address the potential association between hospital PCI appropriateness and long-term outcomes. In addition to in-hospital complications, PCI incurs long-term risk such as bleeding related to dual anti-platelet therapy, acute thrombosis, and the need for repeat revascularization. It is possible that patient factors not accounted for in the AUC, but associated with increased risk of these long-term complications (e.g. prior bleeding event), are less frequently considered at facilities that also perform more inappropriate PCI. As a result, facilities performing more inappropriate PCI may have higher long-term complication rates. Finally, although our risk-adjusted analyses considered key variables identified from contemporary models developed and validated within NCDR, residual confounding is possible given the observational nature of our study.
In conclusion, in this large national registry, we found significant variation in the hospital proportion of non-acute PCIs classified as inappropriate. The hospital proportion of inappropriate PCI was not associated with other measures of PCI quality, including in-hospital mortality, periprocedural bleeding, and medication treatment after PCI. Our findings suggest that PCI appropriateness measures unique and important information that complements traditional PCI metrics to more fully inform quality. Additionally, these findings suggest hospitals with low rates of PCI complications do not necessarily provide high-quality PCI in settings where suboptimal patient selection results in more frequent use of PCI for inappropriate clinical indications. Hospital-based systems are needed to both ensure proper patient selection to maximize anticipated procedural benefit and to minimize post-procedural complications.
Acknowledgments
FUNDING/SUPPORT
The NCDR CathPCI Registry is an initiative of the ACC Foundation and the Society for Cardiovascular Angiography and Interventions. This study was supported by the NCDR. Dr. Steven Bradley is a Career Development Grant Awardee (1 IK2 HX000779-01, funding pending) from the VA HSR&D. Dr. Paul Chan is supported by a Career Development Grant Award (K23HL102224) from the NHLBI. Dr. John Spertus is supported by a Clinical and Translational Science Award (1UL1RR033179).
Footnotes
DISCLOSURES
Dr. Bradley had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: The authors report no relevant disclosures.
Role of the Sponsor: Because the ACC oversees the NCDR, it funded the collection of data in the CathPCI registry, and representatives of the CathPCI Research and Publications committee approved the final manuscript.
Disclaimers: The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. Additionally, the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
REFERENCES
- 1.Frey P, Connors A, Resnic FS. Quality measurement and improvement in the cardiac catheterization laboratory. Circulation. 2012;125:615–619. doi: 10.1161/CIRCULATIONAHA.111.018234. [DOI] [PubMed] [Google Scholar]
- 2.Bradley EH, Nallamothu BK, Herrin J, Ting HH, Stern AF, Nembhard IM, Yuan CT, Green JC, Kline-Rogers E, Wang Y, Curtis JP, Webster TR, Masoudi FA, Fonarow GC, Brush JE, Jr, Krumholz HM. National efforts to improve door-to-balloon time results from the Door-to-Balloon Alliance. J Am Coll Cardiol. 2009;54:2423–2429. doi: 10.1016/j.jacc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Dehmer GJ, Elma M, Hewitt K, Brindis RG. Bringing measurement and management science to the cath laboratory: the National Cardiovascular Data Registry (ACC-NCDR) and the Cardiac Catheterization Laboratory Continuous Quality Improvement Toolkit (ACC-CathKIT) J Cardiovasc Manag. 2004;15:20–26. [PubMed] [Google Scholar]
- 4.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.NCDR® CathPCI Registry®. [Accessed: January 12, 2012]; Available at: https://www.ncdr.com/webncdr/DefaultCathPCI.aspx.
- 6. [AccessedJanuary 1, 11];Clinical Outcomes Assessment Program (COAP): A Program of the Foundation for Health Care Quality. COAP 11 A.D. January 1. Available at: URL: http://www.coap.org. A.D.
- 7.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub WS, McKay CR, Riner RN, Ellis SG, Frommer PL, Carmichael DB, Hammermeister KE, Effros MN, Bost JE, Bodycombe DP. The American College of Cardiology National Database: progress and challenges. American College of Cardiology Database Committee. J Am Coll Cardiol. 1997;29:459–465. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 9.CathPCI Registry Companion Guide to Your NCDR Data Quality Report. Washington, DC: American College of Cardiology Foundation; 2008. [Google Scholar]
- 10.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 12.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Parker WR, Weiner RB. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229–267. doi: 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–e555. doi: 10.1161/CIR.0b013e3181fcae66. [DOI] [PubMed] [Google Scholar]
- 15.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119:e561–e587. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 17.Lange RA, Hillis LD. Use and overuse of angiography and revascularization for acute coronary syndromes. N Engl J Med. 1998;338:1838–1839. doi: 10.1056/NEJM199806183382509. [DOI] [PubMed] [Google Scholar]
- 18.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 20.Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. %19; [DOI] [PubMed] [Google Scholar]
- 22.Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA, Tumlin J. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98:59K–77K. doi: 10.1016/j.amjcard.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De MM, Moltrasio M, Grazi M, Rubino M, Veglia F, Fabbiocchi F, Bartorelli AL. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150:170–177. doi: 10.7326/0003-4819-150-3-200902030-00006. [DOI] [PubMed] [Google Scholar]
- 24.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 25.Boden WE, O'Rourke RA, Teo KK, Maron DJ, Hartigan PM, Sedlis SP, Dada M, Labedi M, Spertus JA, Kostuk WJ, Berman DS, Shaw LJ, Chaitman BR, Mancini GB, Weintraub WS. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial) Am J Cardiol. 2009;104:1–4. doi: 10.1016/j.amjcard.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 26.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, Spertus JA, Hartigan P, Veledar E, Jurkovitz C, Bowen J, Maron DJ, O'Rourke R, Dada M, Teo KK, Goeree R, Barnett PG. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes. 2008;1:12–20. doi: 10.1161/CIRCOUTCOMES.108.798462. [DOI] [PubMed] [Google Scholar]
- 28.Bradley SM, Maynard C, Bryson CL. Appropriateness of Percutaneous Coronary Interventions in Washington State. Circulation:Cardiovascular Quality and Outcomes. 2009 May; doi: 10.1161/CIRCOUTCOMES.111.964320. Abstract. [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Teirstein PS, Kereiakes DJ, Moses J, Lasala J, Grantham JA. Percutaneous Coronary Intervention Use in the United States Defining Measures of Appropriateness. JACC Cardiovasc Interv. 2012 doi: 10.1016/j.jcin.2011.12.004. [DOI] [PubMed] [Google Scholar]