Abstract
Objective
Retinal microvascular signs are associated with systemic conditions and cognitive decline. We studied the associations of microvascular changes, measured by retinal signs, with disability in performing activities of daily living (ADL).
Design
Prospective cohort study.
Setting
Community.
Participants
1487 participants in the Cardiovascular Health Study (mean age 78 years) who were free of ADL disability and had available data on retinal signs and carotid intima-media thickness (IMT) at the 1998–99 visit.
Main Outcome Measure
Incident ADL disability, defined as self-reported difficulty in performing any ADLs, by the presence of retinal signs and advanced carotid atherosclerosis, defined by carotid IMT ≥ 80th percentile or ≥ 25% stenosis; and potential mediation by cerebral microvascular disease on brain imaging or by executive dysfunction, slow gait, and depressive mood that are symptoms of frontal subcortical dysfunction.
Results
During the median follow-up of 3.1 years (maximum 7.8 years), participants with ≥ 2 retinal signs had a higher rate of disability than those with < 2 retinal signs (10.1% versus 7.1%; adjusted hazards ratio, 1.45; 95% confidence interval, 1.24–1.69; P < 0.001). There was no evidence of interaction by advanced carotid atherosclerosis (P > 0.10). The association seemed to be partially mediated by executive dysfunction, slow gait, and depressive symptoms, but not by cerebral microvascular disease on brain imaging.
Conclusions
These results provide further support for the pathophysiologic and prognostic significance of microvascular disease in age-related disability. However, it remains to be determined how to best utilize retinal photography in the clinical risk prediction.
INTRODUCTION
Vascular disease and its risk factors are associated with functional impairment in older adults. Small vessel lesions in the brain which manifest as white matter disease and lacunar infarcts are associated with executive dysfunction, gait disorder, and urinary incontinence.1, 2 These lesions are viewed as the consequence of ischemic injury from vascular risk factors.3, 4 Moreover, large vessel lesions, which can be measured by carotid artery intima media thickness (IMT), are associated with poor cognitive and physical function.5, 6 It is evident that both microvascular and macrovascular disease play important roles in the development of functional impairment, but the importance of each process and their potential interaction are poorly understood.
Many studies have used the brain magnetic resonance imaging (MRI) to measure cerebral microvascular disease, but substantial evidence suggests that pathological changes in retinal microvasculature may reflect similar changes in the brain7–9 and predict incident and progression of cerebral microvascular disease and subcortical atrophy on brain MRI.9–12 Retinal signs have been shown to predict cardiovascular events13, 14 and cognitive decline.15 We have recently found that a higher burden of retinal signs was associated with poor executive function and physical function in a cross-sectional analysis.16
Based on this evidence, we hypothesized that retinal signs might predict future disability in performing activities of daily living (ADLs). Since ADL disability is closely associated with loss of independence, institutionalization, and mortality in older adults, ADL disability may reflect overall clinical consequences of microvascular and macrovascular disease. To this end, we examined whether retinal signs were associated with an increased risk of incident ADL disability and whether the risk was modified by the presence of advanced carotid atherosclerosis.
METHODS
Study Population
The Cardiovascular Health Study (CHS) is a population-based cohort study of cardiovascular disease in community-dwelling older adults.17 In CHS, 5201 adults who were 65 years of age and older were originally recruited in 1989–1990 and additional 687 eligible African Americans were recruited in 1992–1993.18 There were 1998 participants who had available data obtained from retinal photography (any of retinal arteriolar and venular caliber, retinopathy, arteriovenous nicking, and focal arteriolar narrowing) during the 1997–1998 visit and from carotid ultrasound during the 1998–1999 visit. Among those, 1609 who were free of ADL disability at the time of 1998–1999 visit, were eligible for this study. There were 122 with missing information on potential confounders, leaving a total of 1487 for our analysis. Because not all participants had complete data on retinal signs, sample size varied for the analyses of individual retinal signs.
Measurement of Retinal Microvascular Signs
The details of retinal photography procedures were described previously.19, 20 Briefly, a 45-degree retinal photograph, centered on the region of the optic disc and the macula, was taken of one randomly selected eye after 5 minutes of dark adaptation. Trained and certified graders who were masked to subject characteristics evaluated photographs using a standardized protocol.
For the measurement of retinal vascular calibers, photographs were digitized using a high-resolution scanner and the diameters of all arterioles and venules coursing through an area to 1 disc diameter from the optic disc margin were measured and summarized in central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), respectively.19, 21 These indices represent the average central arteriolar and venular caliber in that eye after taking into account the branching patterns. Retinopathy was considered present if any of the following findings (definite or probable) were noted: retinal microaneurysms, retinal hemorrhages, soft exudates, hard exudates, intraretinal microvascular abnormalities, venous beading, new vessels at the disk or elsewhere, vitreous hemorrhage, disk swelling, and laser photocoagulation scars. Retinal arteriovenous nicking and focal arteriolar narrowing were defined as present if they were graded definite or probable in any of the four quadrants.
Additionally, the overall burden of retinal microvascular changes was estimated using the total number of retinal signs present among generalized arteriolar narrowing (defined as < 10th percentile [142.29 μm] of CRAE), generalized venular widening (defined as ≥ 90th percentile [213.04 μm] of CRVE), retinopathy, arteriovenous nicking, and focal arteriolar narrowing. We used a priori definition of high burden as ≥ 2 retinal signs (versus low burden defined as < 2 signs), because this cutoff was associated with lower digit symbol substitution test (DSST) score (42.6, 41.8, and 39.8 points for 0, 1, and ≥ 2 retinal signs, respectively; p < 0.001) and gait speed (0.93, 0.92, and 0.84 m/sec; p = 0.047) in our recent work.16
Measurement of Disability
Participants were followed annually in the clinic or at home and semiannually by telephone calls through the 1998–1999 visit. Afterwards, telephone follow-ups were continued every six months. This study used ADL disability data collected until June 30, 2005. A modified version of the Health Interview Survey Supplement on Aging Questionnaire22 was used to assess disability by asking “Do you have difficulty or are you unable to … ?” in the following six ADLs: bathing, dressing, eating, toileting, walking around the home, and getting out of bed or chair. In addition, instrumental activities of daily living (IADLs) were assessed: walking up 10 steps, doing housework, shopping, preparing meals, paying bills, and using a telephone. Self-reported difficulty or inability to perform any activity without assistance was considered as disability.
Assessment of Vascular Risk Factors and Other Covariates
All the measurements performed during the 1997–1998 visit were used, except carotid ultrasound (1998–1999 visit), anthropometric measurements and blood chemistries (1996–1997 visit), and brain MRI (1997–1999 visit). The following risk factors were considered: sociodemographic factors, alcohol consumption, cigarette smoking, medical history, medications, body mass index (BMI), blood pressure, serum total cholesterol (mg/dl; multiply 0.0259 to convert to mmol/l), fasting glucose (mg/dl; multiply 0.0555 to convert to mmol/l), creatinine (mg/dl; multiply 88.4 to convert to μmol/l), and C-reactive protein (mg/l; multiply 9.524 to convert to nmol/l).17 Diabetes was defined as treatment with either oral hypoglycemic agents or insulin in the year before the examination; or fasting glucose ≥ 126 mg/dl in the past year.23 Advanced carotid atherosclerosis was defined as common carotid artery IMT ≥ 80th percentile (1.23 mm) or internal carotid artery IMT ≥ 80th percentile (2.29 mm) or carotid stenosis ≥ 25%.24, 25 Prevalent cardiovascular disease included ascertained coronary heart disease, myocardial infarction, congestive heart failure, or stroke.26 Functional measures included DSST for executive function, gait speed (m/sec) from a 15-foot walk, and the modified Center for Epidemiologic Studies – Depression (CES-D) scale.27 Ventricular size, white matter lesions, and infarcts were measured from brain MRI.28
Statistical Analysis
All analyses were performed in Stata SE version 11.2 (StataCorp, College Station, Tx). Baseline characteristics were compared using two-sample t-test, Wilcoxon ranksum test, and chi-square test. The outcome was the time to the first occurrence of ADL disability. Participants were followed until incident ADL disability or censored at the time of death or date of last known contact. Disability-free survival was compared by the burden of retinal signs, using Kaplan-Meier product estimator and log-rank test. In the main analysis, race-stratified Cox proportional hazards models were used to compute the hazard ratio (HR) and 95% confidence interval (CI) associated with high burden of retinal signs and with individual signs, after adjusting for sociodemographic characteristics, lifestyle, vascular risk factors, and IADL disability. The results did not change when IADL disability was not adjusted for. This stratified Cox model allows the underlying hazard function to vary by race, while adjusting for race effect. The proportionality assumption was satisfied. A potential correlation within CHS centers was accounted for by using a robust variance estimator.29 We examined the interactions with natural logarithm of carotid IMT for individual signs and high burden of retinal signs.
In the post hoc analyses, we repeated the analysis using the number of retinal signs (0, 1, or ≥ 2); explored whether particular combinations of retinal signs conferred a higher risk than others; and used advanced carotid atherosclerosis, as defined above, instead of natural logarithm of internal carotid IMT. We also examined whether the association attenuated after further adjusting for DSST score, gait speed, and CES-D score (after square root transformation) (n = 861). This examines the mediation by executive dysfunction, slow gait, and depressed symptoms that are characteristic of frontal subcortical dysfunction in the brain.2, 16 A similar analysis was done for cerebral microvascular disease on brain MRI by including ventricular size, white matter grade, and infarcts in the main model (n = 651). Finally, we conducted stratified analyses by diabetes and clinical cardiovascular disease at study baseline.
RESULTS
The prevalence of retinal signs was 7.1% (92/1294) for retinopathy, 7.5% (85/1135) for arteriovenous nicking, and 10.4% (111/1063) for focal arteriolar narrowing. Participants with ≥ 2 retinal signs, comprising 6.9% (61/880) of those with complete retinal data, were more likely to be current smokers and to have higher systolic blood pressure (Table 1).
Table 1.
Characteristics of Participants by the Number of Retinal Signs
| Characteristics | < 2 Retinal Signs (n = 819) | ≥ 2 Retinal Signs (n = 61) | P-value |
|---|---|---|---|
| Age, years | 77.5 ± 4.0 | 78.1 ± 4.2 | 0.281 |
| Male, % | 39.4 | 32.8 | 0.304 |
| White, % | 86.1 | 82.0 | 0.375 |
| Alcohol consumption, % | 43.8 | 42.6 | 0.854 |
| Current smoking, % | 6.2 | 14.8 | 0.011 |
| BMI, kg/m2 | 26.8 ± 4.3 | 27.4 ± 4.4 | 0.267 |
| Cardiovascular disease, %† | 25.4 | 23.0 | 0.671 |
| Systolic blood pressure, mmHg | 129.8 ± 18.3 | 140.0 ± 19.7 | < 0.001 |
| Diabetes, % | 14.3 | 19.7 | 0.251 |
| Total cholesterol, mg/dl | 203.5 ± 38.1 | 207.3 ± 40.4 | 0.458 |
| Fasting glucose, mg/dl | 101.6 ± 29.9 | 107.3 ± 32.8 | 0.157 |
| Creatinine, mg/dl | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.487 |
| C-reactive protein, mg/l | 2.2 [1.1, 5.1] | 2.5 [1.0, 5.5] | 0.851 |
| Internal carotid artery IMT, mm | 1.5 [1.0, 2.1] | 1.5 [1.2, 2.5] | 0.059 |
| Antihypertensive medication, % | 54.5 | 52.5 | 0.763 |
| Lipid-lowering medication, % | 18.1 | 14.8 | 0.514 |
Abbreviations: BMI, body mass index; IMT, intima-media thickness.
SI unit conversion: Multiply 0.0259 to convert serum total cholesterol from mg/dl to mmol/l; 0.0555 to convert fasting glucose from mg/dl to mmol/l; 88.4 to convert creatinine from mg/dl to μmol/l; and 9.524 to convert C-reactive protein from mg/l to nmol/l.
Data were presented in mean ± standard deviation, median [25th percentile, 75th percentile], or percent.
Cardiovascular disease includes prevalent coronary heart disease, myocardial infarction, congestive heart failure, and stroke at the 1997–1998 visit.
During the median follow-up time of 3.1 years (0.4–7.8 years), those with ≥ 2 retinal signs had a significantly lower disability-free survival than those with low burden (Figure). After adjusting for potential confounders, the presence of ≥ 2 retinal signs was associated with a 1.45-fold increased rate of disability, whereas individual retinal signs were not (Table 2). However, retinal arteriolar caliber (when analyzed as a continuous variable) appeared to be inversely associated with disability (HR, 0.95 per 1-standard deviation increase in CRAE [19.5 μm]; 95% CI, 0.91–1.00; P = 0.058), whereas retinal venular caliber was not (HR, 1.08 per 1-standard deviation increase in CRVE [17.8 μm]; 95% CI, 0.97–1.22; P = 0.172). When we examined the interaction with natural logarithm of carotid IMT, none of them were significant for individual retinal signs and for ≥ 2 retinal signs (P > 0.10 for all; data not shown).
Figure 1.
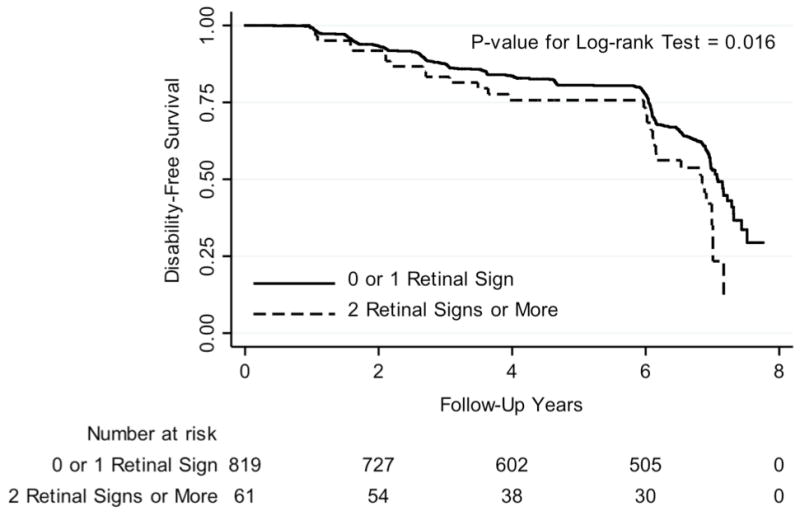
Kaplan-Meier Curve of Activity of Daily Living Disability-Free Survival By the Number of Retinal Signs
Table 2.
Retinal Microvascular Signs and the Rate of Incident Activity of Daily Living Disability
| Retinal Signs | Incidence Rate*
|
HR† | 95% CI | P-value | |
|---|---|---|---|---|---|
| Present n (%) | Absence n (%) | ||||
| Individual retinal sign | |||||
| Generalized arteriolar narrowing | 53 / 670 (7.9) | 434 / 5925 (7.3) | 1.22 | 0.99–1.50 | 0.068 |
| Generalized venular widening | 50 / 647 (7.7) | 437 / 5948 (7.3) | 1.24 | 0.96–1.60 | 0.097 |
| Retinopathy | 42 / 432 (9.7) | 446 / 6208 (7.2) | 1.22 | 0.82–1.80 | 0.321 |
| Arteriovenous nicking | 31 / 408 (7.6) | 401 / 5437 (7.5) | 1.10 | 0.61–1.97 | 0.750 |
| Focal arteriolar narrowing | 47 / 535 (8.8) | 356 / 4952 (7.2) | 1.22 | 0.85–1.76 | 0.284 |
| Total number of retinal signs | |||||
| ≥ 2 Retinal signs (versus < 2) | 30 / 298 (10.4) | 310 / 4380 (7.4) | 1.45 | 1.24–1.69 | < 0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Incidence rate was presented in the number of disability per total person-years (the number of disability per 100 person-years).
Adjusted for age, gender, white race, current smoking, body mass index, prevalent cardiovascular disease, systolic blood pressure, diabetes status, total cholesterol, natural logarithm of C-reactive protein, natural logarithm of internal carotid artery intima-media thickness, antihypertensive medication use, lipid-lowering medication use, and self-reported disability in performing instrumental activities of daily living at baseline.
In the post hoc analyses, the presence of ≥ 2 retinal signs was associated with disability (HR, 1.48; 95% CI, 1.31–1.68; P < 0.001) compared to having no sign, while having 1 retinal sign was not (1.08; 0.87–1.33; P = 0.489). We did not find any particular combinations of retinal signs that conferred a greater risk than others (data not shown). When advanced carotid atherosclerosis was used, the adjusted HR (95% CI) was 1.41 (1.23–1.61; P < 0.001) for ≥ 2 retinal signs and 1.28 (1.10–1.49; P = 0.002) for advanced carotid atherosclerosis.
In the post hoc mediation analysis, the association between retinal signs and disability was attenuated and became non-significant when DSST score, gait speed, and CES-D score were adjusted (Table 3). When ventricular size, white matter grade, and infarcts were adjusted for, the adjusted HR (95% CI) for ≥ 2 retinal signs did not change: 1.25 (0.98–1.59) before and 1.24 (0.97–1.60) after adjustment. The association between retinal signs and disability was similar by the presence of diabetes or clinical cardiovascular disease at baseline (Table 4).
Table 3.
Potential Mediation of the Relation Between Retinal Signs and Incident Activity of Daily Living Disability by Executive Function, Gait Speed, and Depressive Symptoms
| Model | Adjusted HR (95% CI), P-value*†
|
|||
|---|---|---|---|---|
| ≥ 2 Retinal Signs (versus retinal signs < 2) | DSST score (per 1-SD change) | Gait speed (m/sec) (per 1-SD change) | √CES-D score (per 1-SD change) | |
| Main model | 1.43 (1.13–1.81), P = 0.003 | - | - | - |
| Further adjusted for | ||||
| DSST score | 1.34 (1.02–1.76), P = 0.036 | 0.80 (0.67–0.94), P = 0.009 | - | - |
| Gait speed | 1.32 (1.03–1.68), P = 0.026 | - | 0.77 (0.64–0.92), P = 0.005 | - |
| √CES-D score | 1.36 (1.16–1.59), P < 0.001 | - | - | 1.26 (1.08–1.47), P = 0.004 |
| All three measures | 1.21 (0.99–1.46), P = 0.057 | 0.83 (0.70–0.99), P = 0.036 | 0.81 (0.67–0.99), P = 0.036 | 1.23 (1.04–1.46), P = 0.018 |
Abbreviations: CES-D, the Center for the Epidemiologic Studies – Depression scale; CI, confidence interval; DSST, digit symbol substitution test; HR, hazard ratio; SD, standard deviation.
Coefficients for three continuous functional measures were standardized. Standard deviation was 13 points for DSST score, 0.22 m/sec for gait speed, and 1.09 for √CES-D score.
Adjusted for age, gender, white race, current smoking, body mass index, prevalent cardiovascular disease, systolic blood pressure, diabetes status, total cholesterol, natural logarithm of C-reactive protein, natural logarithm of internal carotid artery intima-media thickness, antihypertensive medication use, lipid-lowering medication use, and self-reported disability in performing instrumental activities of daily living at baseline.
Table 4.
Stratified Analysis of Retinal Microvascular Signs and the Rate of Incident Activity of Daily Living Disability
| Disease Status at Baseline | Incidence Rate* by Number of Retinal Signs
|
HR† | 95% CI | P-value | |
|---|---|---|---|---|---|
| ≥ 2 Retinal Signs n (%) | < 2 Retinal Signs n (%) | ||||
| By Diabetes Status | |||||
| Absent | 24 / 241 (9.9) | 263 / 3812 (6.9) | 1.33 | 1.06–1.68 | 0.015 |
| Present | 6 / 57 (10.6) | 47 / 568 (8.3) | 1.54 | 0.65–3.68 | 0.325 |
| By Cardiovascular Disease Status | |||||
| Absent | 23 / 231 (10.0) | 229 / 3340 (6.9) | 1.35 | 1.00–1.82 | 0.051 |
| Present | 7 / 67 (10.4) | 81 / 1040 (7.8) | 1.25 | 0.62–2.50 | 0.531 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Incidence rate was presented in the number of disability per total person-years (the number of disability per 100 person-years).
Adjusted for age, gender, white race, current smoking, body mass index, systolic blood pressure, total cholesterol, natural logarithm of C-reactive protein, natural logarithm of internal carotid artery intima-media thickness, antihypertensive medication use, lipid-lowering medication use, and self-reported disability in performing instrumental activities of daily living at baseline, plus prevalent cardiovascular disease (for diabetes analysis) and diabetes status (for cardiovascular disease analysis).
COMMENTS
We found that high burden of retinal signs, defined as having ≥ 2 retinal signs, was associated with incident ADL disability, independently of vascular risk factors and carotid atherosclerosis, and even among those without diabetes or clinical cardiovascular disease. This association was partially explained by executive dysfunction, slow gait, and depressive symptoms, but not by prevalent cerebral microvascular disease on brain MRI. In addition, high burden of retinal signs appeared to be as important as advanced carotid atherosclerosis in its association with disability. Our study supports the hypothesis that microvascular disease accelerates age-related disability and retinal signs can be useful in understanding mechanisms and predicting outcomes.
In previous research, retinal signs have been linked to major risk factors of disability, including hypertension, diabetes, metabolic syndrome, heart disease, and stroke.30 We recently reported the association of retinal signs with prevalent functional impairment.16 The current study adds to the current literature by showing that retinal signs can predict future ADL disability, independently of major risk factors of disability at baseline. We speculate that retinal signs may be a marker of underlying microvascular disease leading to disability, rather than a direct cause. The presence of microvascular disease in retinal vessels may indicate a similar process in other systemic microvasculature, such as brain, heart, and kidney.7, 8, 31, 32 Microvascular disease in these organs has been implicated in incident cardiovascular and cerebrovascular events,13, 14 as well as cognitive and physical functional loss,33–36 all of which contribute to future disability. Although we could not empirically evaluate all these potential links, we found that executive dysfunction, slow gait, and depressive mood which have been recognized as clinical correlates of hypertensive brain microangiopathy1, 2 might partially mediate the association between microvascular disease and disability.
In our study, the rate of disability seemed to rise rapidly when there were ≥ 2 signs. This refers to a potential threshold effect that clinical phenotypes may not manifest until certain extents of subclinical physiologic derangements accumulate. Such a non-linear dose-response relationship has been observed in our cross-sectional analysis of retinal signs and functional impairments16 and others’ work on frailty.37 We also investigated whether certain retinal signs could provide further insights into a particular pathophysiologic process by examining individual signs and their combinations. Although the effect estimates were of a similar magnitude in the same direction for individual signs or their common combinations, our attempt was limited by insufficient power and, therefore, the possibility of differential effects by individual retinal signs remains open. Nonetheless, we do not think that the lack of power for individual signs should underestimate our main findings that the overall burden of retinal signs predicts future disability, because it can be viewed as a composite measure of physiologic changes and cumulative damage in retinal microvasculature from various insults, as evidenced by the distinct association profiles between each signs and vascular risk factors.16, 30, 38
In addition, there was no indication that the effect of retinal signs on disability differed by the presence of advanced carotid atherosclerosis. We have previously reported that high carotid IMT was associated with worse executive function when generalized arteriolar narrowing and arteriovenous nicking were present compared to when they were not.16 The lack of a significant interaction between retinal signs and carotid IMT on ADL disability may reflect the complex process leading to ADL disability that involves both cognitive and physical impairment.39, 40 We also found that high burden of retinal signs was more strongly associated with disability than advanced carotid atherosclerosis, although a direct comparison between the arbitrarily defined dichotomous variables is difficult. However, our definition of advanced carotid atherosclerosis has been associated with an increased short-term and long-term risk of cardiovascular events in previous CHS analyses.41, 42 Our findings imply that microvascular disease seems to have an independent role on the development of functional impairment and disability that is at least, as important as, yet distinct from macrovascular disease.
Another worthwhile finding is that retinal signs predicted disability among those without diabetes or clinical cardiovascular disease. This finding is important because retinal signs might be a preclinical marker of microvascular disease and endothelial dysfunction that precedes the diagnosis of major risk factors of disability, such as hypertension,43 diabetes,44 cardiovascular events,13, 14 lacunar infarcts,11 and cerebral atrophy.12 In multivariable analyses, adjusting for ventricular size, white matter disease, and infarcts on brain MRI did not change our estimates. The evidence from previous research and our results in all suggest that retinal signs may contain additional prognostic information on the risk of disability that are not readily captured by medical history, laboratory tests, and brain MRI abnormalities at the time of retinal photography.
The strengths of this study are prospective follow-up (up to 7.8 years); standardized assessments; and consistent findings from several post hoc analyses under different assumptions. A limitation of this study is selection bias, because participants who did not have gradable retinal photographs were more likely than those who did, to be older, female, black, and to have more vascular risk factors and diseases.20 If sicker individuals who did not have retinal photography were more likely to have retinal signs and to develop ADL disability during the follow-up, a selection bias could occur and underestimate the association between retinal signs and ADL disability. In CHS, retinal photography, carotid ultrasound, and brain MRI were taken near the end of in-person follow-up assessment in 1997–1999. Thus, we used prevalent measures of executive function, gait speed, and depressive mood, instead of incident measures. This is an important limitation in interpreting our mediation analysis. In addition, residual confounding cannot be excluded, despite our efforts to adjust for potential confounders that were selected based on biological plausibility. For instance, ankle arm index which may be associated with both retinal signs and ADL disability was not measured at baseline for this analysis. In addition, decreased vision could explain some of the association between retinal signs and disability. Finally, low prevalence of individual retinal signs resulted in 95% CIs that were too wide to exclude a modest association. Despite limited power, we found a significant association of high burden of retinal signs with disability. A larger study with more events is needed to evaluate the effects of individual retinal signs, which will further strengthen our study.
These limitations notwithstanding, our study suggests that the presence of ≥ 2 retinal signs may be an early marker of microvascular disease that portends an elevated risk for future ADL disability in community-dwelling older adults, independently of major risk factors for disability and microvascular disease on brain MRI. These findings lend further support for the role of microvascular disease in age-related functional loss and disability. Given low prevalence of retinal signs, our results do not justify the use of retinal examination as a routine population-wide screening strategy for disability. However, when retinal examination performed for other indications reveals ≥ 2 retinal signs, a thorough review and optimization of lifestyle habits and risk factors may be reasonable. Future research should investigate how to best utilize the information from retinal photography in the clinical risk prediction and whether slowing the progression of microvascular disease may prevent disability in high-risk older adults.
Acknowledgments
DHK has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding / Support: The research was supported by John A. Hartford Foundation Research Fellowship Award (DHK); PO1-AG-031720 (EN); P01-AG-004390, R37-AG-25037 (LAL), and the Harvard Claude D. Pepper Older Americans Independence Center P30-AG-028717 (DHK, LAL); R01-AG-023629 and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827 (ABN); R21-HL-077166, contract NHLBI-HC-97-06, and the Research to Prevent Blindness Senior Scientific Award (RK); and RO1-AG-027002 (MJS). The CHS was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The funding sources did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: None
References
- 1.Inzitari M, Pozzi C, Rinaldi LA, Masotti G, Marchionni N, Di BM. Cognitive and functional impairment in hypertensive brain microangiopathy. J Neurol Sci. 2007;257:166–173. doi: 10.1016/j.jns.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L, Simoni M. Pathophysiology of cerebral small vessels in vascular cognitive impairment. Int Psychogeriatr. 2003;15 (Suppl 1):59–65. doi: 10.1017/S1041610203008974. [DOI] [PubMed] [Google Scholar]
- 4.Geroldi C, Ferrucci L, Bandinelli S, et al. Mild cognitive deterioration with subcortical features: prevalence, clinical characteristics, and association with cardiovascular risk factors in community-dwelling older persons (The InCHIANTI Study) J Am Geriatr Soc. 2003;51:1064–1071. doi: 10.1046/j.1532-5415.2003.51353.x. [DOI] [PubMed] [Google Scholar]
- 5.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–2202. doi: 10.1161/01.STR.0000181752.16915.5c. [DOI] [PubMed] [Google Scholar]
- 7.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59:1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 9.Longstreth W, Jr, Larsen EK, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 2007;165:78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 10.Cheung N, Mosley T, Islam A, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010;133:1987–1993. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349–1355. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki R, Cheung N, Mosley T, et al. Retinal Microvascular Signs and 10-Year Risk of Cerebral Atrophy. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2010;41:1826–1828. doi: 10.1161/STROKEAHA.110.585042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. Jama. 2005;293:63–69. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- 15.Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology. 2009;73:862–868. doi: 10.1212/WNL.0b013e3181b78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Newman AB, Hajjar I, et al. Retinal microvascular signs and functional loss in older persons: the cardiovascular health study. Stroke. 2011;42:1589–1595. doi: 10.1161/STROKEAHA.110.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 22.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987;(21):1–115. [PubMed] [Google Scholar]
- 23.American Diabetes Association: clinical practice recommendations 1997. Diabetes Care. 1997;20 (Suppl 1):S1–70. [PubMed] [Google Scholar]
- 24.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 25.Kuller L, Borhani N, Furberg C, et al. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139:1164–1179. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Rodloff LS. The CES-D scale: a self-report depression scale for research in general population. Appl Pyschol Meas. 1977;1:385–401. [Google Scholar]
- 28.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 30.Wong TY, McIntosh R. Systemic associations of retinal microvascular signs: a review of recent population-based studies. Ophthalmic Physiol Opt. 2005;25:195–204. doi: 10.1111/j.1475-1313.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 31.Sharrett AR. A review of population-based retinal studies of the microvascular contribution to cerebrovascular diseases. Ophthalmic Epidemiol. 2007;14:238–242. doi: 10.1080/09286580701396712. [DOI] [PubMed] [Google Scholar]
- 32.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46:214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 33.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions an d cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42:2086–2090. doi: 10.1161/STROKEAHA.110.610360. [DOI] [PubMed] [Google Scholar]
- 35.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52:216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53:438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 38.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The Cardiovascular Health Study. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 39.Bennett HP, Corbett AJ, Gaden S, Grayson DA, Kril JJ, Broe GA. Subcortical vascular disease and functional decline: a 6-year predictor study. J Am Geriatr Soc. 2002;50:1969–1977. doi: 10.1046/j.1532-5415.2002.50608.x. [DOI] [PubMed] [Google Scholar]
- 40.Kuo HK, Leveille SG, Yu YH, Milberg WP. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Gerontology. 2007;53:102–110. doi: 10.1159/000096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuller LH, Arnold AM, Psaty BM, et al. 10-year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the Cardiovascular Health Study. Arch Intern Med. 2006;166:71–78. doi: 10.1001/archinte.166.1.71. [DOI] [PubMed] [Google Scholar]
- 42.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 43.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 44.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. Jama. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]


