Abstract
Ovarian cancer is the leading cause of death among all gynecological cancers. Some women choose bilateral oophorectomy as means of cancer prevention. In most patients, by the time this cancer is diagnosed, it has already metastasized. Treatment involves oophorectomy followed by radiation, chemo-, and immuno-therapies. However, oopherectomy results in infertility and fails to eliminate all cancer cells. Radiation and chemotherapy cause severe side effects and may lead to genetic mutations in DNA of the ova.
The ultimate goal of this project is development of a therapy which would target a therapeutic gene specific to ovarian cancer cells causing their apoptosis, but which would leave ova and other cells unharmed.
Herein, we report the proof of concept for such a therapy, in which genetically engineered single chain variable fragment (scFv) antibodies against HER2/ neu, RON, and NK1R, guide the delivery of the therapeutic transgenes into the cancer cells of the ovaries. Under ovary specific promoters (OSP), the transgene expression generates the intracellular scFv antibodies, which quench cell antioxidative enzymes, thus raising levels of reactive oxygen species (ROS), inflicting oxidative stress, activating apoptotic signaling pathways, and causing cancer cell deaths.
Keywords: Ovarian cancer, anti-oxidative enzymes, signal transduction, genetically engineered, single chain variable fragment (scFv) antibodies, ovary specific promoters (OSP), human epidermal growth factor receptor 2 (HER2), RON receptor tyrosine kinase (RON), neurokinin-1 receptor (NK1-R)
INTRODUCTION
Ovarian cancer is the leading cause of deaths among all gynecological cancers. In the USA in 2007, 22430 women would be newly diagnosed with cancers of the ovaries, and 15280 of them would die according to the predictions of the American Cancer Society (Jemal et al. 2007). While the exact causes of ovarian cancer have yet to be determined, BRCA1,2 gene mutations, which lead to dysfunctional gene products, put their carriers at high risk (Quinn et al 2007, Silva et al 2007). Therefore, some women carrying these mutations choose bilateral oophorectomy as a preventive measure.
The combinations of current diagnoses and therapies are less than adequate because the cancers’ origins, growth and metastases may remain non-symptomatic and hard to detect within abdominal cavity for a long time prior to diagnosis (Badgwell and Bast 2007). At the time of diagnosis, cancers spread beyond the ovaries in more than 68% of patients, reaching stage III – advanced invasion of the neighboring tissues and/or stage IV – distant metastases according to the WHO classification. Hence, ovarian cancer is often termed a “silent killer”. Currently, initial diagnosis often relies upon detection of the MUC16 gene expression product CA125 in blood (Suzuki et al. 2007). Computer assisted tomography (CAT) and magnetic resonance imaging (MRI) may help in diagnostic and therapeutic processes by showing images of tumor topography (Milam et al. 2007). However, the final diagnosis relies upon laparascopy and immunohistopathological examination of the biopsies. At that time, an evidence of HER2/neu oncogene being amplified and overexpressed is often demonstrated (Slamon et al. 1989, Shin et al. 2007, Tuma 2007). The level of its expression is associated with cancer malignancy (Berchuck et al. 2007, King et al. 1992). The ovarian or breast cancer cells may display approximately 1.5 × 106 HER2/neu receptors on their surfaces expressed from multiple copies of the gene, while healthy cells display only approximately 2 ×104 of these receptors. Moreover, we have recently demonstrated that over-expression of preprotachykinin and RON genes led to the significant increase of NR1K and RON. These features of cancer cell surface biomolecules are the driving force for developing non-invasive means of diagnosis - in vivo immunocytochemistry - using scFv antibodies, which are genetically engineered for detection in gamma, single photon emission tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI) (Malecki 2007). Clinically, overexpression of HER2/neu leads to a large increase in stimulation of signal transduction pathways, accelerated cell cycles, and increased cell proliferation. As a consequence, HER2/neu positive cancers are the most invasive and have the worst prognosis.
The preferred therapy is radical surgery, which involves oophorectomy and hysterectomy. However, in advanced stages, metastases are often missed and become sources for relapses. Radiation and chemotherapy, while intentionally targeting cancer cells, indiscriminately affect healthy cells and cause severe side effects (Cannistra et al. 2007). Therefore, the majority of the ovarian cancer survivors become infertile, while the others risk carrying various mutations of genetic information in oocytes (Kesic 2007). Emerging therapies involve inhibitors of enzyme receptor HER2/neu (Kaye 2007, Palayekar and Herzog 2007), silencing RNA (Numnum et al. 2007), and Hsp70 (Chang et al. 2007). Immunotherapy trials, with antibodies targeting HER2/neu, are in progress; however, multiple rounds of monoclonal antibodies sooner or later elicit the patient’s immune response and suppress the therapeutic outcome. These therapies are further complicated by mutations in the signaling pathways within ovarian cancer cells (Estep et al. 2007). In summary, current therapeutic approaches suffer from serious problems: multiple rounds of therapy with concomitant side effects, indiscriminate, non-specific delivery of therapy hurting healthy cells, toxic effects of dead cells, risk of developing mutations in oocytes, and risks of causing women’s infertility.
The ultimate goal of this project is development of a therapy, which would progressively eliminate only cancer cells, while leaving oocytes and all other healthy cells unharmed. Here, we tested the hypothesis, that if therapeutics would be delivered specifically to cancer cells only, then not only would healthy oocytes remain unharmed, but also the patient’s immune system (itself unaffected by therapy) would be capable of continued elimination of cancer cells. Furthermore, if only ovarian cancer cells were eliminated in progressive stages of apoptosis, then toxic effects of the therapy would be reduced. In addition, a targeted, single dose delivery of therapeutic transgenes would be far more advantageous than conventional multiple rounds of therapies. Finally, all these measures would protect oocytes, thus protecting the women’s ability for healthy procreation.
To attain this goal, the DNA constructs for antioxidative enzymes and for single chain variable fragment (scFv) antibodies against antioxidative enzymes were genetically engineered with the gene products being scFv antibodies of high specificity and affinity (Malecki et al. 2002). The targeted antioxidative enzymes were manganese superoxide dismutase (SODMn), copper-zinc superoxide dismutase (SODCuZn), catalase (CAT), glutathione reductse (GR), and glutathione peroxidase (GPX). The scFv antibodies were capable of quenching these antioxidative enzymes; thus disabling their ability to perform redox reactions and manage reactive oxygen species. Expressed from the transgenes, intracellularly, under the CMV and OSP promoters, these scFv inflicted oxidative stress and caused activation of apoptosis signaling pathways. Using the same genetic engineering platform, the constructs were engineered for scFv antibodies targeting domains of HER2/neu, EGFR and EGFRvIII, NK1R, RON, and TfR displayed on the surfaces of cancer cells. They were expressed in myeloma cells and used to guide receptor-targeted delivery of the transgenes (Malecki 2007).
Herein, we report proof of concept for the scFv antibody-guided targeting of the receptors displayed on the ovarian cancer cells, effective delivering of the transgenes for the scFv antibodies against antioxidative enzymes, expressing them in targeted cells, inducing oxidative stress, and causing ovarian cancer cells selective, suicidal deaths.
METHODS
Cell cultures
The cell line NIH OVCAR-3 HTB-161 (ATCC) was derived from the cells in ascites of a patient with malignant adenocarcinoma of the ovary. The cell line was grown in RPMI-1640 Medium (ATCC) supplemented with 0.01 mg/ ml bovine insulin and donor bovine serum to the final concentration of 20%. The cells were positive for both estrogen and progesterone receptors. They formed colonies and spheroids when grown in soft agars and were tumorigenic in nude mice.
The cell line TOV-112D CRL-11731(ATCC) was derived from primary malignant adenocarcinoma of the ovary at grade 3, stage IIIC. It was cultured in 85% of a 1:1 mixture of MCDB 105 medium and Medium 199 and 15% donor bovine serum (ATCC). When cultured in soft agar, these cells formed colonies and spheroids. The cells tested positive for HER2/neu expression and the p53 mutation. The cells also induced tumors in nude mice.
Genetically engineered single chain variable fragment (scFv) antibodies against antioxidative enzymes
The DNA constructs for the single chain variable fragment (scFv) antibodies were genetically engineered with the high specificity and affinity of the gene expressed scFv antibodies – as previously demonstrated (Malecki et al 2002). The four targeted antioxidative enzymes were: manganese superoxide dismutase (SODMn), copper-zinc superoxide dismutase (SODCuZn), catalase (CAT), and glutathione peroxidase (GPX). Coding sequences for the scFv antibodies targeting HER2/neu and EGFR were selected from the surface displayed libraries and cloned into pM vectors designed with CMV and OSP immediate early promoter and SV40 poly(A) termination with hexahistidine and pentaglutamate and neomycin-resistance coding sequences (Malecki 1996). The DNA constructs for these antibodies were electroporated into human myelomas. Expressions of these constructs resulted in secretion of hetero-specific, poly-functional, monovalent antibodies. For therapeutic endeavors, the constructs were modified to specifically target the transgene expression products to ER, mitochondria, peroxisomes, cytoplasm, or nuclei by introducing organelle specific targeting signal coding sequences as described earlier (Malecki et al. 2002). Chelating sites were saturated with the metal ions Gd and Eu. Purification from non-bound metal was performed on affinity columns. The antibodies were produced in modified roller bottles.
Affinities of these antibodies were determined on Biacore loaded with either the recombinant or purified enzymes: SOD, CAT, or GPX. Specificities were determined on Western blots from denaturing PAGE-SDS and in-native-gel immunolabels (Malecki 2007). Abilities of these scFv antibodies for quenching the antioxidative enzymes were determined in colorimetric reactions. The constructs coding the non-specific intracellular scFv antibodies against the main groups of blood served as the controls (Malecki 2007).
Effects of the transgene expression products exerted upon the ovarian cells were determined by evaluation of the cell membrane organization (LASCFM, FESEM), reactive oxygen species (ROS) levels (LSCFM), and reorganization of genome (pulse-electrophoresis, LSCFM, EFTEM).
Genetically engineered, superparamagnetic, single chain variable fragment (scFv) antibodies against HER2/neu and TfR
Details of the plasmid DNA constructs were described previously (Malecki et al. 2002). Coding sequences for scFv antibodies targeting HER2/neu were selected from the surface displayed libraries and cloned into pM vectors designed with CMV and OSP promoters and SV40 poly(A) termination. The vectors also contained coding sequences for hexahistidine and pentaglutamate, biotin or digoxigenin, and neomycin-resistance. Transgenes with the coding sequences for the antibodies against the main groups of blood inserted into excised sites of antioxidative scFv antibody sequences served as controls. These constructs were electroporated into human myelomas, which were then grown in the modified roller bottles. Expressions of these constructs resulted in secretion of hetero-specific, poly-functional, mono-valent antibodies. The scFv antibodies were absorbed onto superparamagnetic, core-shell Fe Au nanoparticles and purified from non-bound antibodies with magnets.
Rapid freezing and freeze-drying
The details for cryoimmobilization and follow-up molecular imaging techniques were described earlier (Malecki 1996, Malecki et al. 2002). The cells injected into the chambers were rapidly frozen in nitrogen slurry down to −196°C. The frozen samples were further processed in two ways: freeze-drying for field emission scanning electron microscopy (FESEM) on LEO 1530 or Hitachi 3400 and freeze-substitution for energy filtering transmission electron microscopy (EFTEM) on Zeiss or Philips. For FESEM, the frozen cells were freeze-dried followed by fast atom beam sputter coating (freeze-drying and cryocoating, oil-free vacuum station unit built based upon NSF grant). For EFTEM, the cells were freeze-substituted at −90°C, −35°C, and 0°C and infiltrated with Lowicryl followed by polymerization with UV at −35°C and ultramicrotomy.
Native electrophoresis
An agarose gel was poured at 2% using a 10 mM Tris, 31 mM NaCl buffer of varying pH that did not contain any denaturing agents. The samples in their native state were loaded after mixing with glycerol to add density without denaturing the proteins. The gel was run in the same buffer that was used for pouring agarose at 60 mAmps until the desired separation was reached. The gel was then stained for 30 minutes in Sypro Tangerine Gel Stain (Invitrogen) diluted in the running buffer prior to imaging on Storm 840 (Amersham) or FluorImager (Molecular Dynamics).
SDS-PAGE
Electrophoresis was run on 12% polyacrylamide gel. Standard cell culture lysates were loaded into 0.75 mm × 2 mm wells. The samples, after mixing with SDS and DTT containing sample buffers (Sigma) were loaded into the wells. The gels were run using Tris/Glycine/SDS/DTT running buffers. After each run, the gels were stained with colloidal silver or Sypro Tangerine for imaging using FluorImager (Molecular Dynamics).
Electrotransfer
After electrophoresis, the samples were immediately transferred onto PVDF. Immunoblotting was performed with the Mini Trans-Blot Cell (Bio-Rad) within CAPS: 10 mM 3-[Cyclohexylamino]-1-propanesulfonic acid (CAPS) and Tris/glycine transfer buffer (25 mM Tris base, 192 mM glycine, pH 8.3). Prior to the transfer the cooling units were stored with deionized water at −20°C. Immediately after electrophoresis the gel, membrane, filter papers and fiber pads were soaked in transfer buffer for 5–10 min. The pre-cooled transfer units were filled with cooled transfer buffer, and electrotransfer proceeded at 350 mA.
Antibody guided receptor mediated transfection and magnetoporation
AntiHer2/neu, antiEGFR, EGFRvIII, and antiTfR scFv antibodies carried the plasmid constructs for transgenic, intracellular expression of SODMn, SODCuZn, CAT, or GPX. After docking into the ovarian cancer cell surface receptor sites, some were subjected to an electromagnetic field which forced the transgenic complexes to enter the cytoplasm without passing through lysosomal compartments. The nuclear localization signal enhanced their retention or delivery into the nuclei for transient expression.
Laser scanning confocal microscopy
Z-stacks of the immuno-labeled cells were acquired with an Odyssey laser scanning confocal system on an Olympus inverted microscope. The lines were generated by blue diode, helium and argon lasers at 337, 488, 543, and 588 nm. Images were acquired with Kernel averaging followed by deconvolution for the three-dimensional rotation or gallery display for analysis.
Scanning electron microscopy and energy dispersive x-ray spectral imaging
Analysis of the cell three-dimensional structure and distribution of antibodies were determined with LEO 1530 Field Emission Scanning Electron Microscope (FESEM) and Hitachi 3400 FESEM both equipped with Energy Dispersive X-Ray Spectral Imaging systems (EDXSI). Complete elemental spectra were acquired for every pixel of the scans to create the elemental databases. From these, after selecting an element specific energy window, the map of this element atoms distribution was calculated with ZAF correction (NIST). As the antibodies were tagged with atoms of either Gd or Eu (exogenous elements incorporated into their structure), the location of antibodies, thus the receptors, could be determined based upon the elemental maps as described in details earlier (Malecki 1996).
Energy filtering transmission electron microscopy
Supramolecular organization was determined with energy filtering transmission electron microscopy (EFTEM) using either the 912 Zeiss with the Omega in-column energy filter or the 430 Phillips with the Gatan post-column energy filter. Ultrathin sections were maintained in the cryo-holder and protected with double-bladed cryo-traps. The instruments were operated at zero-loss or at a C-energy edge.
RESULTS
The success of this experimental therapy was contingent upon two main factors: specificity of transgenes’ delivery and efficiency of blocking of antioxidative enzymes.
The targets for gene therapy were the antioxidative enzymes manganese superoxide dismutase (SODMn), copper-zinc superoxide dismutase (SODCuZn), catalase (CAT), glutathione peroxidase (GPX). The genetically engineered scFv antibodies against SODMn, SODCuZn, CAT and GPX had very high affinities and specificities towards their antigens as demonstrated in previous studies (Malecki et al. 2002). They were capable of blocking the functions of these enzymes as demonstrated in native-in-gel functional studies. In this endeavor, the transgenically expressed scFv antibodies were redirected from the cell secretion pathways toward intracytoplasmic and intraorganellar sites as determined on Z-stacks from laser scanning confocal microscopy after three-dimensional reconstructions. The data were confirmed on organelle fractions with gradient centrifugation studies as demonstrated earlier (Malecki 1996). Likewise, the specificity of the targeting of genetically engineered scFv antibodies for HER2/neu and other EGFR family receptors, as well as, for TfR were all shown in preceding studies (Malecki 2007). Since they were guiding the delivery of transgenes to receptors, their high specificity and affinity were essential for specific delivery of the therapeutics. In this study, the effects which these genetically engineered scFv antibodies had upon cancer cells were evaluated based upon the results of three basic, functional assays specific for detection of apoptosis and presented in this paper: formation of apoptotic blebs (Figure 1), phosphatidylserine extracellular flip and chromatin structure collapse (Figure 2), and genome disintegration (Figure 3).
Figure 1.
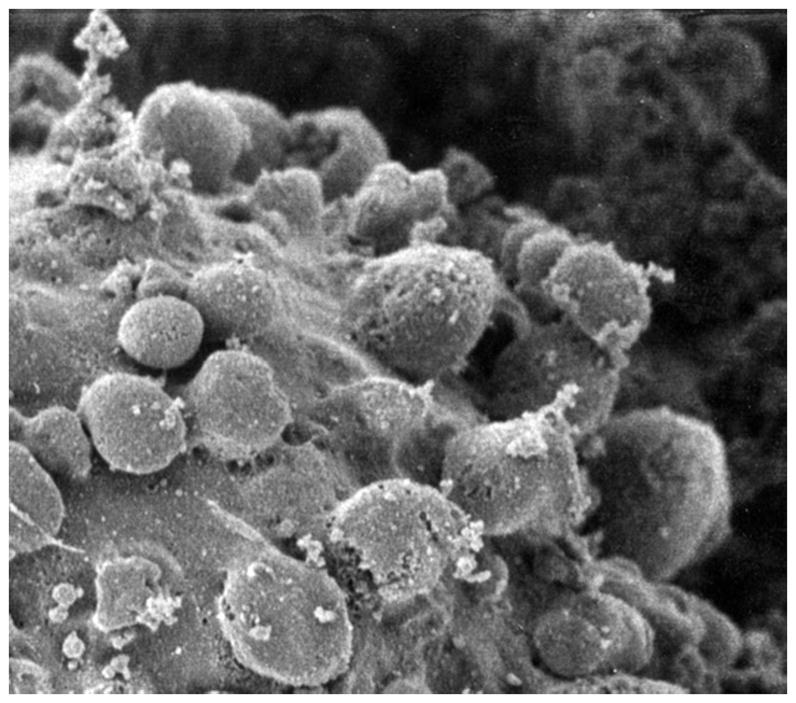
Membrane blebs on the surface of the apoptotic ovarian cancer cell in the field emission scanning electron microscope. Horizontal field width = 2 microns.
Figure 2.

Apoptotic ovarian cancer cells as seen in the laser scanning confocal fluorescent microscope after staining with bisbenzimide and propidium iodide. Phase-contrast showing a group of cells (left), which retained their cell membrane integrity assessed upon propidium iodide stain exclusion (middle), and disintegration of the genomic DNA presented as pyknotic figures revealed with bisbenzimide (right). Horizontal field width of each single frame = 20 microns.
Figure 3.
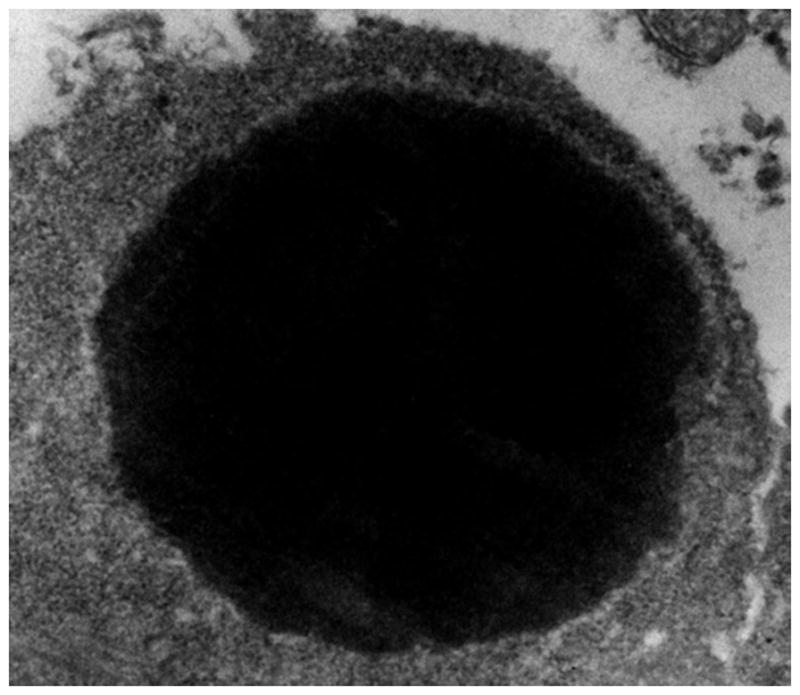
Condensation of collapsed chromatin in an apoptotic ovarian cancer cell. Cell membranes are retained, while some of them form surface blebs (upper left portion).
Figure 1 is the representative result of the first group of assays. Rapid cryoimmobilization of cells secured retention of life-like cellular ultrastructure at nanometer resolution. This cellular ultrastructure was revealed with field emission scanning electron microscopy (FESEM) on the freeze-dried samples. Controls containing identical samples of ovarian cancer cells were transfected with constructs coding the non-specific intracellular scFv antibodies. A few hours after receptor-targeted delivery the transgenes for the scFv antibody against antioxidative enzymes, ovarian cancer cells formed abundant cell membrane blebs (Figure 1). These blebs are characteristic and early signs of apoptosis, while reflecting molecular compartmentalization of the cell. The identical ovarian cancer cells treated with non-specific antibody carrying transgenes were entirely healthy-looking and free from any effects upon the cells (not shown).
The panel in Figure 2 illustrates the representative results of the second group of assays, which were detected with laser scanning confocal fluorescence microscopy (LSCFM). General view of the cells is shown with transmitted detector (Figure 2, left section). Apoptotic alterations in cellular chemistry are manifested by two phenomena: translocation (flip) of phasphatidylserine (PS) and reorganization of chromatin. In both cases cells retain their viability and membranes remain intact. This phenomenon is demonstrated by propidium iodide exclusion (Figure 2, middle section). At the onset of apoptosis, PS relocates from the internal leaflet to the cell membrane exterior as demonstrated with annexin or with the genetically engineered scFv antibodies against PS in LSCFM (not shown). More specifically, chromatin suffers definite collapse and fragmentation (Figure 2, right section). The ovarian cancer cells transfected with the intracellular antibodies against the main groups of blood did not exhibit these effects (not shown).
Figure 3 illustrates the hallmark of apoptosis - chromatin collapse and condensation with disintegration of the genome. These phenomena were captured with the rapid cryo-immobilization technique. Therefore, they represent the life-like appearance of the occurring phenomena of apoptosis. Apoptotic disintegration of the genome was revealed on two ways: pulse gel electrophoresis of the genomic DNA (not shown here) and genome disintegration with EFTEM (Figure 3). The latter method offered a significant advantage over electrophoresis as it assured the direct insight into structural changes which occur in the entire nucleus upon activation of the apoptotic signaling pathway. The image illustrates the compact cluster of genomic DNA characteristic of apoptotic degradation of the genome.
DISCUSSION
Herein, we report the proof of concept for a new gene therapy by means of intracellular expression of transgenes for the single chain variable fragment (scFv) antibodies against antioxidative enzymes (AOE). The main anti-oxidative enzymes that were the targets for the genetically engineered scFv antibodies were manganese superoxide dismutase (SODMn), copper-zinc superoxide dismutase (SODCuZn), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPX). Their disabling leads to accumulation of reactive oxidative species (ROS), thus oxidative stress beyond capabilities of the specifically targeted cancer cells for managing it. Therefore, apoptotic signaling pathways become activated and start driving the ovarian cancer cells into apoptosis.
Success of this experimental therapy approach can be attributed primarily to the high specificity and affinity of the genetically engineered scFv antibodies. The small size of the scFv antibodies allowed us to map individual domains on macromolecules. With this high precision, it also allowed us to target these antibodies to the active or neutral domains. Such a precise targeting efficiently allowed us to disable the functions of anti-oxidative enzymes.
Apoptosis of cancer cells induced by the increased levels of reactive oxygen species is a mechanism well described in radiation therapy. Exposure to the high levels of ionizing radiation leads to the rapid increase of free radicals beyond those, which are manageable by AOE. Excessive levels of ROS cause induction of apoptotic signaling pathways. However, sustained therapeutic effects on the cancer cells via ROS require multiple sessions of exposures to radiation. Moreover, all the cells within the radiation beam are destined for destruction. This is also the case with the ova in proximity of the targeted ovarian cancer cells. Moreover, radiation causes mutations within the DNA of the ova, which may lead to the congenital disorders in offspring. These problems are avoided in the protocol described here. This protocol will be the subject of forthcoming studies in which molecules will be directly and specifically affected by the generated ROS to trigger apoptotic signaling pathways activation.
Chemotherapy and immunotherapy require multiple rounds of applications. These cause repetitive side effects, immunizations, and hardships on the patient. These problems are greatly reduced in our experimental therapy described herein. A single session administration of a therapeutic is a clear advantage of the proposed therapy. The antibody guided, ovarian cancer receptor targeted delivery of transgenes expressed from the targeted cell specific promoters, may be sufficient for a long term, continuous expression of intracellular antibodies. Therefore, it has obvious advantages for the patient. Compared to immuno- and pharmaco-therapeutics, one dose of the scFv antibody has a much lower potential for immunizing the patient, than applications of multiple rounds of monoclonal antibodies or radio-immunotheraputics.
Above all, the first and foremost advantage of this experimental therapy is the protection of oocytes. Retaining the unharmed ova is equivalent to retention of women’s fertility, thus ability for procreation. Although, in classical therapies, the chances for retaining fertility exist, the oocytes subjected to chemotherapy and radiation therapies may suffer serious mutations with hard to foresee consequences to the children.
Streamlining this experimental therapy into the clinics faces at least three main challenges: increased production, specificity of delivery in vivo, and innate response. Lab scale production and affinity purification are very expensive already. Therefore, the immediate attempts lead to reduction of the costs of production by expressing these scFv antibodies in plants. In sillico simulations and in vitro works, supplemented with tests on endothelial and a variety of other non-cancerous cells, offer only limited numbers of molecules and molecular clusters which may exhibit homology with those targeted by the scFv antibodies engineered in this project. Therefore, their streamlining to the in vivo environment will be the next step. Finally, the innate response triggered by the toll-like receptors and involving reticulo-endothelial cells may create problems for efficient delivery of the transgene complexes to their destinations. These will require development of means to bypass such responses by modifications of therapeutic complexes; e.g., by PEGylation. Expanding this therapy to treatment of other neoplasms will be contingent primarily upon defining unique molecules displayed on their cell surfaces.
To summarize, this cancer suicide gene therapy opens up new routes for the personalized and targeted therapy of the ovarian cancers.
Acknowledgments
We acknowledge with thanks access to the NIH National Facility for Nuclear Magnetic Resonance at Madison and the National Biotechnology Resource, the SDSU Functional Genomic Center and support from the RR 570 grant from the NIH, RR 2301 grant from the NIH, SGER 0096014 from the NSF, and the funds from the PBMEF. We appreciate thediscussions with Dr. M. Anderson, Dr. T. Cheesbrough, Dr. C. Dwivedi, Dr. M. Hildreth, Dr. J. Markley, Dr. J. McCarthy, Dr. J. Nickles, and Dr. J. Ruffolo.
LITERATURE CITED
- Bardwell D, Bast RC., Jr Early detection of ovarian cancer. Disease Markers. 2007;23:397–410. doi: 10.1155/2007/309382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Research. 1990;50:4087–4091. [PubMed] [Google Scholar]
- Cannistra SA, Moutons UA, Pension RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, Mc-Guire W. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. Journal of Clinical Oncology. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- Chang CL, Tsai YC, He L, Wu TC, Hung CF. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Research. 2007;67:10047–57. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep AL, Palmer C, McCormick F, Rauen KA. Mutation Analysis of BRAF, MEK1 and MEK2 in 15 ovarian cancer cell lines: Implications for therapy. Public Library of Science Online. 2007;12:e1279, 1–7. doi: 10.1371/journal.pone.0001279. www.plosone.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kaye SB. Bevacizumab for the treatment of epithelial ovarian cancer: will this be its finest hour? Journal of Clinical Oncology. 2007;25:5150–152. doi: 10.1200/JCO.2007.13.6150. [DOI] [PubMed] [Google Scholar]
- Kesic V. Fertility after the treatment of gynecologic tumors. Recent Results Cancer Res. 2008;178:79–95. doi: 10.1007/978-3-540-71274-9_9. [DOI] [PubMed] [Google Scholar]
- King BL, Carter D, Foellmer HG, Kacinski BM. Neu proto-oncogene amplification and expression in ovarian adenocarcinoma cell lines. American Journal of Pathology. 1992;140:23–31. [PMC free article] [PubMed] [Google Scholar]
- Malecki M. Preparation of plasmid DNA in transfection complexes for fluorescence and electron spectroscopic imaging. Scanning Microscopy -Supplements. 1996;10:1–16. [PubMed] [Google Scholar]
- Malecki M, Hsu A, Truong L, Sanchez S. Molecular immunolabeling with recombinant single-chain variable fragment (scFv) antibodies designed with metal-binding domains. Proceedings of the National Academy of Sciences USA. 2002;99:213–218. doi: 10.1073/pnas.261567298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki M. HER2/Neu gene expression products evaluated with superparamagnetic, genetically engineered antibodies. Proceedings SD Academy of Sciences. 2007;86:297–308. [PMC free article] [PubMed] [Google Scholar]
- Milam RA, Milam MR, Ayer RB. Detection of early-stage ovarian cancer by FDG-PET-CT in a patient with BRCA2-positive breast cancer. Journal of Clinical Oncology. 2007;25:5657–5658. doi: 10.1200/JCO.2007.14.3412. [DOI] [PubMed] [Google Scholar]
- Numnum TM, Makhija S, Lu B, Wang M, Rivera A, Stoff-Khalili M, Alvarez RD, Zhu ZB, Curiel DT. 2007 Improved anti-tumor therapy based upon infectivity-enhanced adenoviral delivery of RNA interference in ovarian carcinoma cell lines. Gynecol Oncol. 2008;108(1):34–41. doi: 10.1016/j.ygyno.2007.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palayekar MJ, Herzog TJ. The emerging role of epidermal growth factor receptor inhibitors in ovarian cancer. Int J Gynecol Cancer. 2008;18(5):879–90. doi: 10.1111/j.1525-1438.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- Pfeiler G, Horn F, Lattrich C, Klappenberger S, Ortmann O, Treeck O. Apoptotic effects of signal transduction inhibitors on human tumor cells with different PTEN expression. Oncology Reports. 2007;17:1305–1309. [PubMed] [Google Scholar]
- Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GK, Mullan PB, Johnston PG, Wilson RH, Harkin DP. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clinical Cancer Research. 2007;13:7413–20. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- Shin SJ, Chen B, Hyjek E, Vazquez M. Immunocytochemistry and fluorescence in situ hybridization in HER-2/neu status in cell block preparations. Acta Cytologica. 2007;51:552–7. doi: 10.1159/000325793. [DOI] [PubMed] [Google Scholar]
- Silva E, Gatalica Z, Snyder C, Vranic S, Lynch JF, Lynch HT. Hereditary Breast Cancer: Part II. Management of Hereditary Breast Cancer: Implications of Molecular Genetics and Pathology. Breast J. 2008;14(1):14–24. doi: 10.1111/j.1524-4741.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Tamada Y, Shigirahara K, Suzuki A, Susumu N, Ishida I, Aoki D. Human monoclonal antibody for ovarian clear cell carcinoma-2, a human monoclonal antibody with antitumor activity against ovarian cancer cells that recognizes CA125-like antigen. Int J Gynecol Cancer. 2008;18(5):996–1006. doi: 10.1111/j.1525-1438.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- Tuma RS. Inconsistency of HER2 test raises questions. Journal of the National Cancer Institute. 2007;99:1064–1065. doi: 10.1093/jnci/djm075. [DOI] [PubMed] [Google Scholar]


