Abstract
The name astroglia unifies many non-excitable neural cells that act as primary homeostatic cells in the nervous system. Neuronal activity triggers multiple homeostatic responses of astroglia that include increase in metabolic activity and synthesis of neuronal preferred energy substrate lactate, clearance of neurotransmitters and buffering of extracellular K+ ions to name but a few. Many (if not all) of astroglial homeostatic responses are controlled by dynamic changes in the cytoplasmic concentration of two cations, Ca2+ and Na+. Intracellular concentration of these ions is tightly controlled by several transporters and can be rapidly affected by the activation of respective fluxes through ionic channels or ion exchangers. Here, we provide a comprehensive review of astroglial Ca2+ and Na+ signalling.
Keywords: Astrocyte, Homeostasis, Excitability, Ca2+ signalling, Na+ signalling
1. Astroglia - the homeostatic cells of the brain
The nervous system in mammals represents complex network formed by several distinct cell types of neural crest and non-neural crest origin. In the central nervous system (CNS) the neural cells are neurones, astrocytes, NG2 glia and myelinating oligodendrocytes, whereas the non-neural cells are the microglia. In the peripheral nervous system the neural elements include sensory, sympathetic and parasympathetic as well as enteric neurones and highly diversified peripheral glia represented by satellite glial cells, enteric glia, and myelinating, non-myelinating and perisynaptic Schwann cells. All these diverse cells are unified through several levels of intercellular signalling accomplished by inter- and intracellular diffusion of various molecules that either bind and stimulate the plasmalemmal receptors or penetrate cellular membranes through specific ion channels, thus initiating electrical excitation (by virtue of charges carried by ions) or triggering local cytoplasmic responses by interacting with variety of intracellular targets.
Among many neural cells forming the CNS astroglial cells have a specific role of a main homeostatic element. Astrocytes (which means star-like cells, the name invented by Michael von Lenhossek [1,2]) represent a highly heterogeneous cell population, which include protoplasmic astrocytes of grey matter of the brain and the spinal cord, fibrous astrocytes localised in the white matter, classical radial glia that acts as a pluripotent neural precursor cell during development, radial Müller retinal glial cells, pseudo-radial cerebellar Bergmann glial cells, velate astrocytes of cerebellum, tanycytes that connect ventricular walls with parts of hypothalamus and spinal cord, pituicytes in the neuro-hypophysis, and perivascular and marginal astrocytes. The brain of higher primates also contains interlaminar, polarised and varicose projection astrocytes, with all these types being particularly developed in the human brain (see [3–14] for details and relevant references). In addition, astroglia cover several types of specialised cells such as ependymocytes, choroid plexus cells and retinal pigment epithelial cells.
Astrocytes appeared early in evolution, first as supporting cells and then gained their functional importance in the course of specialisation of the nervous system. Functions of astrocytes are many: i) they define the brain microanatomy and provide structural support for other cellular elements of the CNS; ii) they synthesise and store glycogen and produce lactate, the latter being preferred metabolic substrate for neurones; iii) they control homeostasis and turnover of several key neurotransmitters and neuromodulators (for example, glutamate and adenosine); and iv) they regulate synaptic connectivity through supporting synaptogenesis, controlling ion/transmitter concentrations in the synaptic cleft and regulating synaptic plasticity via secreting various neuroactive factors [14–16].
2. S pecific nature of astroglial excitability
Excitability of neurones and neuroglia are fundamentally different. Signalling in neuronal networks is mainly accomplished through propagating waves of transient opening of the plasmalemmal ion channels that provide fluxes of ions underlying electrical excitability. These propagating waves, manifested by action potentials, can rapidly (up to 100 m/s) convey excitation through neuronal axons. Electrical signals, when reaching neuronal terminals, activate local Ca2+ entry that initiates exocytotic release of neurotransmitters. The latter diffuse through the synaptic cleft and transfer excitation to the postsynaptic neuronal structures through the activation of specific receptors [17–22].
Glial cells, in contrast to neurones, cannot generate plasmalemmal action potentials, because of low densities of voltage-operated ion channels and high expression of K+ channels that prevent substantial depolarisations of glial membranes. Instead, glial cells use intracellular signalling routes where local gradients of ions interact with intracellular targets and trigger physiological reactions. In addition, glial cells, and astrocytes in particular, are physically connected into syncytia by means of intercellular contacts represented by gap junctions. The gap junctions are made by closely apposing plasmalemma of two neighbouring cells with a narrow (~ 2 – 2.5 nm) intercellular cleft. Here, specialised intercellular channels, which span both membranes and establish direct intercellular contacts, are concentrated. These junctional channels are composed of two aligned “hemichannels” or connexons each made up from 6 subunits or connexins [23,24]. Out of about 20 known connexins [25,26] astroglial cells express mainly connexins Cx43, and to a lesser extent Cx30 and Cx26; as minor constituents of their gap junctions, astrocytes can also express Cxs 45, 40 and 46 [27]. These gap junctional channels have a large pore (with a diameter of ~ 1.5 nm), which allows intercellular passage of ions and various active substances such as second messengers [e.g., inositol 1,4,5 trisphosphate (InsP3)], nucleotides (ATP, ADP) or metabolic substrates (glucose). As a result, the gap junctions provide a specific route for intercellular and long-range signalling, which is manifested in propagating waves of ionic (Ca2+ or Na+) signals, or metabolic waves. The communication through gap junctions is of course much slower when compared with the spread of action potentials (on average, for example, Ca2+ waves propagate through astroglial syncytia with a speed of ~ 20 – 40 μm/s); however, it is more diversified and may provide a substrate for integrating intercellular signalling. It should be noted, however, that, although the above described gap junctional communication contributes to intercellular Ca2+ waves, it is ATP, which gets released from astrocytes, that serves as an extracellular signal to majorly support the intercellular Ca2+ waves [28–31].
Fast signalling in astroglial cells, which can be initiated by neuronal activity or many different neurotransmitters and neuromodulators, is mediated by intracellular ion gradients. In this essay, we shall concentrate on two ions, Ca2+ and Na+, which mediate signalling in astroglia and discuss in detail glial signalling mediated by them. It should be noted that the regulation of the Ca2+ dynamics (and possibly also of Na+) could differ in various subcellular locations of astrocytes, which could result in local signaling, rather than the above long-range intercellular waves. For instance, astrocyte perisynaptic processes are the sites where synapses can evoke local Ca2+ elevations that could result in a local feedback signalling via the gliotransmitter release [32–34].
3. Ca2+ signalling in astroglia
3.1. Molecular machinery of Ca2+ signalling
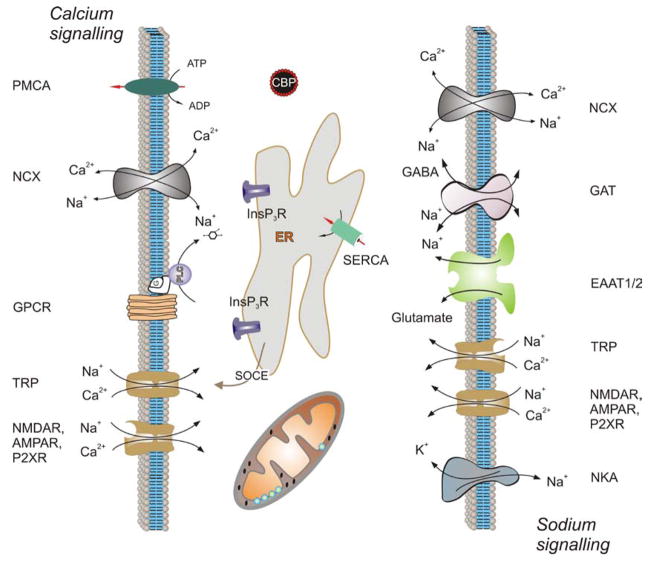
Evolutionary, Ca2+ has emerged as one of the most universal intracellular second messengers, due to its unique qualities (flexible coordination chemistry, high affinity for carboxylate oxygen, which is the most frequent motif in amino acids, and rapid binding kinetics) and by its availability in the primordial ocean [35,36]. At high concentrations, Ca2+ ions cause numerous anti-life effects such as protein and nucleic acid aggregations or precipitation of phosphates; in addition ATP-based energetics require low levels of Ca2+ in the cytosol. These factors stipulated the general principle of Ca2+ signalling, which is based around steep concentration gradients for Ca2+ between the cytosol and the extracellular environment as well as various intracellular compartments. These concentration gradients create electro-driving force for Ca2+ aimed at the cytosol where resting Ca2+ concentration is kept at level between 50 and 100 nM. Ca2+ movements across cellular membranes occur either via diffusion through Ca2+ permeable channels or by transport with ATP-consuming pumps or ion-dependent exchangers; the former underlie downhill Ca2+ translocation (i.e. in the direction of electro-chemical gradient) whereas the latter provides for up-hill (i.e. against electro-chemical gradient) Ca2+ flux. Ca2+-permeable ion channels are represented by several families, which include highly Ca2+ selective voltage-gated Ca2+ channels, intracellular Ca2+ channels (InsP3 receptors or InsP3Rs and ryanodine receptors or RyRs), and plasmalemmal Ca2+-release activated Ca2+ channels (CRAC channels, that on a molecular level represent activity of Orai proteins) and cationic channels with various degrees of Ca2+ permeability (Figure 1). The latter cationic channels are represented by ligand-gated channels (or ionotropic neurotransmitter receptors such as, for example, glutamate, ATP or nicotinic acetylcholine receptors), by extended family of transient receptor potential (TRP) channels and some other types of cationic channels. Ca2+ transport against concentration gradients is mainly accomplished by plasmalemmal Ca2+ ATPases (PMCA or plasmalemmal Ca2+ pumps), by SarcoEndoplasmic reticulum ATPases (SERCA or endoplasmic reticulum Ca2+ pumps) and by ion exchangers of which the Na+/Ca2+ exchanger (NCX) is by far the most important. Inside the cell, Ca2+ is buffered by Ca2+ binding proteins (CBPs), affinity of which to Ca2+ differs in different cellular compartments. For example, Ca2+ affinity of cytosolic CBPs lies in a low nM range, whereas endoplasmic reticulum (ER) CBPs have KD for Ca2+ at ~ 0.5 mM. These different affinities determine the range of diffusion of Ca2+ ions. In the cytosol, CBPs limit diffusion and favour development of local high-Ca2+ concentration microdomains, whereas, in the ER, CBPs allow almost free and long-distance Ca2+ diffusion that being instrumental for making ER Ca2+ tunnels [37,38]. Cellular Ca2+ homeostasis is also regulated by mitochondria which are able to accumulate Ca2+ (via electrochemically driven diffusion through Ca2+ selective channel generally referred to as Ca2+ uniporter) and to release Ca2+ through mitochondrial Na+/Ca2+ exchanger as well as transient openings of mitochondrial permeability transition pore [39,40].
Figure 1.
Molecular cascades of Ca2+ and Na+ signalling in astroglia (see text for details). Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CBP, Ca2+ binding protein; EAAT, excitatory amino acid transporter; ER, endoplasmic reticulum; G, G-protein; GABA, γ-aminobutyric acid; GAT, GABA transporter; GPCR, G-protein coupled receptor; InsP3R, inositol 1,4,5 trisphosphate-gated Ca2+ channel/receptor; NCX, Na+/Ca2+ exchanger; NKA, Na+/K+ ATPase; NMDAR, N-methyl D-aspartate receptor; PLC, phospholipase C; PMCA, plasmalemmal Ca2+-ATPase; P2XR, purinergic 2X receptor, SERCA, sarco(endoplasmic) reticulum Ca2+ ATPase; SOCE, store-operate Ca2+ entry; TRP, transient receptor potential.
Effectors of Ca2+ signals are Ca2+ regulated enzymes (also known as “Ca2+ sensors”), binding of Ca2+ to which affects functional activity. These Ca2+ sensors are many; they have different affinities to Ca2+ and are heterogeneously distributed between cellular compartments. These specificities of Ca2+ sensors sensitivity to Ca2+ and their cellular distribution underlie amplitude and spatial coding of Ca2+ signals.
The shape and spatio-temporal organisation of Ca2+ signals are defined by the interplay between Ca2+ diffusional fluxes and Ca2+ transport (Figure 1). Combinations of these are multiple and labile; as was conceptualised by Michael Berridge, cells can create and rapidly modify “Ca2+ signalling toolkits” that adapt Ca2+ signalling to the environmental requirements [41,42]. Another important feature of Ca2+ homeostatic/signalling system is its autoregulation by Ca2+ ions themselves, as transient changes in Ca2+ concentration establish multiple feedback mechanisms that modify the handling of itself. As a rule, most of Ca2+ permeable channels are subject to Ca2+-dependent inactivation, which develops either through direct binding of Ca2+ ions to the channel or Ca2+-dependent channel phosphorylation. Similarly, Ca2+ pumping by SERCA is regulated by Ca2+ concentration within the ER lumen; this intraluminal Ca2+ concentration also controls the availability of intracellular Ca2+ channels for activation. Conceptually, lowering Ca2+ concentration in the ER facilitates Ca2+ uptake and reduces channels activation, whereas increase in intra-ER Ca2+ concentration facilitates channels opening and reduces SERCA activity (see [43,44] for detailed discussion). Finally Ca2+ fluxes are modulated by mitochondria which, by providing ATP and dynamic Ca2+ buffering, regulate plasmalemmal Ca2+ entry and ER Ca2+ uptake [45,46].
3.2. Endoplasmic reticulum as a main source of astroglial Ca2+ signalling
Astroglial cells respond with intracellular Ca2+ elevation to a broad variety of external stimuli from direct mechanical stimulation to a multitude of neurotransmitters, neuromodulators, hormones and other biologically active substances. The ability of astroglia to react with [Ca2+]i elevation to almost every neuroligands it encounters was firmly established in experiments in cell cultures [47–52]. These early experiments were fundamental for glial research because, they demonstrated that astrocytes are potentially capable of expressing virtually every receptor modality and that most of these receptors are coupled to ER through the phospholipase C (PLC)/InsP3 signalling cascade. These experiments also highlighted remarkable plasticity of astroglial cells in vitro, as indeed, these cells were able to rapidly modify receptor expression pattern. First studies of astrocytes in situ, in brain slices, confirmed the primary importance of InsP3-ER link in generation of astroglial Ca2+ signals [53–56]. At the same time, these experiments also found that receptor expression in astroglia in neural tissue is restricted to match that of neurons, i.e. immediate neurotransmitter environment [13,16,52,57].
The ER is one of the largest intracellular organelles, which is involved in a variety of fundamental cellular processes such as protein synthesis, protein folding and trafficking haulage of secretory products etc. [58–61]. The ER is also a key organelle of Ca2+ signalling, being arguably the largest dynamic Ca2+ store able to accumulate, store and release Ca2+ ions in response to (patho)physiological stimulation. Ca2+ accumulation into the ER lumen is accomplished by SERCA pumping; the Ca2+ concentration in the ER at rest (also known as intraluminal Ca2+ concentration, [Ca2+]L) is maintained at 0.2 – 1.0 mM range [62–66]. Release of Ca2+ from ER in astroglia is primarily mediated by InsP3 receptors; and their inhibition by pharmacological agents (e.g., heparin) or by genetic deletion often prevents development of Ca2+ signals in astrocytes [55,67]. Functional role of second type of ER Ca2+ release channel, the RyR, a Ca2+-gated Ca2+ channel, in astroglial Ca2+ dynamics remains controversial, although astrocytes express RyR both in vitro and in situ [68–70] and RyRs contribute to Ca2+ signals necessary for glutamate release via regulated exocytotic pathway [71]. The InsP3Rs are simultaneously controlled by InsP3 and Ca2+ ions and therefore local increase in [Ca2+]i facilitates receptor opening and promotes Ca2+-induced Ca2+ release through InsP3R. This feature underlies the occurrence of propagating Ca2+ waves which, in essence, represent a wave of ER membrane excitation manifested by propagating recruitment of InsP3 receptors and coordinated with the extracellular spread of ATP. These Ca2+ waves are important for astrocyte physiology; astroglial stimulation usually occurs at the level of distal processes, and Ca2+ waves convey this excitation to the soma. Furthermore, astroglial Ca2+ wave travels through astroglial syncytia, being therefore a substrate for astroglial long-range signalling [72,73]. In addition to InsP3, astrocytes can also utilize other ER Ca2+-mobilizing second messengers in response to external stimuli, most notably cyclic adenosine diphosphoribose (cyclic ADP-ribose) [74,75] and nicotinic acid adenine dinucleotide phosphate, (NAADP) [76–78].
Ca2+ signals, produced by activation of ER Ca2+ release, control many functions of astroglia. In particular, ER-originated Ca2+ signals are critical for inducing exocytotic release of neurotransmitters (such as, for example, ATP, glutamate or D-serine) from astrocytes (see [79,80] for review and references). Inhibition of Ca2+ accumulation into the ER by specific blockade of SERCA pumps with thapsigargin, that leads to exhaustion of the ER Ca2+ content due to an unopposed leak through the endomembrane, effectively eliminated Ca2+-dependent release of glutamate from cultured astrocytes [71]. The same effect was achieved after inhibition of InsP3 receptors by membrane-permeable antagonist diphenylboric acid 2-aminoethyl ester (2-APB), which can also affect the store-operated Ca2+ entry discussed next. The role for ER Ca2+ signalling cascade in controlling astroglial gliotransmission was subsequently corroborated in experiments in acute slices (e.g., [81–83]].
3.3. Plasmalemmal Ca2+ influx in astrocytes: role of TRP channels and ionotropic receptors
Despite the fact that ER Ca2+ store acts as a main source for astroglial Ca2+ signalling, astrocytes also possess several mechanisms for Ca2+ entry that produce physiologically relevant Ca2+ signals (Figure 1). There is little evidence that astrocytes in situ can express functional voltage-gated Ca2+ channels, although these channels have been detected in several in vitro experiments (see [80] for detailed review). Two major pathways controlling plasmalemmal Ca2+ entry in astroglial cells are represented by store-operated and ligand-operated ion channels.
The store-operated Ca2+ entry is generally present in a majority of electrically non-excitable cells. This Ca2+ influx pathway (initially described as a “capacitative” Ca2+ entry) [84,85] is controlled by the Ca2+ content in the ER lumen, where decrease in [Ca2+]L results in the opening of plasmalemmal Ca2+-permeable channels [86]. Activation of the store-operated Ca2+ entry fulfils two functions: first, it provides Ca2+ for replenishment of the ER store (the capacitative function), and second, it is important for producing the sustained (“plateau”) phase of the Ca2+ signal that often outlasts the period of cell stimulation. There are several molecular determinants of the store-operated Ca2+ entry. Many types of cells express specific (ICRAC) store-operated channels characterised by extremely high Ca2+ selectivity and very low single channel conductance. Activation of these channels reflects interaction of stromal interaction molecule (STIM) proteins (that detect ER Ca2+ concentration) with Orai (named after Greek gate-keeping goddesses [87]) proteins that form the plasmalemmal channel [88]. Alternatively store-operated Ca2+ influx may involve activation of TRPC channels [89].
The store-operated Ca2+ entry is functionally expressed in astroglia [90,91]. Initial experimental evidence indicates the role for TRPC channels. They are expressed in astrocytes at both mRNA and protein levels, and TRPC activity is involved in shaping astroglial Ca2+ signals [92–94]. Further analysis revealed that in astrocytes the TRPC channels are assembles of brain native heteromultimers [92,95] containing obligatory TRPC1 (channel forming subunit) and TRPC4 and/or TRPC5 (auxiliary subunits). Inhibition of TRPC1 channel expression by antisense mRNA or its occlusion with blocking antibody directed at an epitope in the pore forming region of the TRPC1 protein significantly decreased store-operated Ca2+ influx in cultured astrocytes [92,95] and reduced plateau phase of ATP-activated [Ca2+]i transients [95]. Likewise, immunological inhibition of TRPC1 protein substantially decreased mechanically-induced Ca2+ signalling in astrocytes and suppressed Ca2+-dependent glutamate release [95].
It has been recently demonstrated that astrocytes possess functional STIM1 and Orai1 molecules which play a role in thrombin-induced cytosolic Ca2+ dynamics [96]. This was demonstrated in cultured astrocytes, investigated using immunocytochemisty and Ca2+ imaging. Overexpression and silencing (using short interfering RNA) of STIM1/Orai1 led to enhanced or muted Ca2+ dynamics in astrocytes, respectively.
The second pathway for plasmalemmal Ca2+ entry in astrocytes is associated with ionotropic receptors (ligand-gated Ca2+-permeable channels). Several types of ionotropic receptors are present in astrocytes in vitro, in situ and in vivo (see [97–99]. The most important astroglial ionotropic receptors are α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl D-aspartate (NMDA) glutamate receptors and P2X purinoceptors. Often, astroglial AMPA receptors do not express GluR-B (GluR2) subunit, which makes these receptors moderately Ca2+ permeable [100,101]. The NMDA receptors identified in cortical astrocytes [102–105] differ in their biophysics and pharmacology from the neuronal ones. In particular, astroglial NMDA receptors are weakly (if at all) sensitive to Mg2+ block at physiological resting potential, and their Ca2+ permeability is ~ 2 times lower as compared to neurones (PCa/Pmonovalent ~ 3 vs. ~10 in neurones [106]. Nonetheless, synaptic activation of astroglial NMDA receptors in cortical slices results in substantial Ca2+ signals [106].
Astrocytes express P2X1/5 and P2X7 purinoceptors, which may create Ca2+ fluxes [102,105,107]. The P2X1/5 have moderate Ca2+ permeability (PCa/Pmonovalent ~ 2), which is sufficient to produce physiologically relevant Ca2+ signals upon appropriate stimulation [106]. The P2X7 receptors activation may result in massive Ca2+ influx, although this signalling is most likely present only in pathology [108].
Astrocytes also express TRPA1 channels, which generate frequent occurrence of local, punctate Ca2+ influx. This activity of TRPA1 contributes to the resting Ca2+ levels in astrocytes [34]. This ion channel is best known as a chemosensor for various environmental noxious stimuli causing pain [109–111], albeit it can also be activated by cold [112] or heat [113].
4. Na+ signalling in astroglia
4.1. Dynamic changes in cytoplasmic Na+ concentration in astrocytes
At rest, astrocytes have relatively high cytosolic Na+ concentration ([Na+]i); in various astroglial preparations (i.e. in culture and in acute slices) it was determined at ~ 15 – 20 mM (cultured hippocampal astrocytes, 15 – 16 mM [114]; cultured astrocytes from visual cortex, 17 mM [115]; astrocytes in cortical slices, 17 – 20 mM [116]; see also [117] for comprehensive review). These levels of resting [Na+]i in astrocytes are almost twice higher when compared to neurones (~ 4 – 10 mM; see e.g., [114,118–120]); high cytosolic Na+ in astrocytes also has functional consequences because it sets reversal potential for many Na+-dependent transporters/exchangers, which shall be discussed below.
Stimulation of astrocytes (either mechanical or chemical) induces transient and complex changes in [Na+]i. For example, application of glutamate to astrocytes in vitro evoked local [Na+]i transients and propagating Na+ waves spreading though astroglial syncytium [121–123]. Similarly, both single-cell [Na+]i transients and astroglial Na+ waves were observed in astroglial preparations in situ. In cerebellar Bergmann glia, glutamate induced [Na+]i increase by 10 – 25 mM above the resting level [124,125]; in hippocampus, glutamate induced [Na+]i rise and astroglial Na+ waves [126]. Astroglial [Na+]i in hippocampus was also reported to rise by ~ 7 mM following stimulation with γ-aminobutyric acid (GABA) [116]. Finally, astroglial [Na+]i increases are induced by stimulation of synaptic inputs which has been detected in both cerebellum and hippocampus [125,127,128].
4.2. Molecular mechanisms controlling [Na+]i in astroglia
The cytosolic Na+ concentration in astrocytes is regulated by Na+ diffusion through plasmalemmal channels, by Na+ transport through ATP-dependent pumps and by Na+ translocation by multiple ion exchangers (Figure 1). Main route for plasmalemmal diffusion of Na+ across the plasmalemma is associated with ionotropic glutamate and purinoceptors, which produce substantial Na+ fluxes upon activation. In Bergmann glia, for example, stimulation of AMPA receptors with kainate increases [Na+]i by ~ 20–25 mM [124]. Na+ can also enter astrocytes through TRP channels, non-specific mechanosensitive cationic channels and possibly through Epithelial Na+ Channel (ENaC)/Degenerin family 21 channels or proton-activated Acid Sensing Ion Channels (ASICs); for review see [117]. Astrocytes in subfornical organ express specific type of Na+ channels sensitive to fluctuations in extracellular Na+ concentration. These channels (classified as Nax channels) are involved in astroglial chemosensing and regulation of body Na+ homeostasis [129]. All in all, physiological stimulation of astrocytes trigger substantial Na+ influx, which is mainly mediated by ionotropic receptors and possibly by TRPC channels activated following depletion of ER Ca2+ stores.
The Na+- K+ pump or Na+/K+ ATPase (NKA) is the main energy-dependent astroglial Na+ transporter. Astrocytes throughout the CNS express the NKA a1/a2 subunits. The Na+/K+ ATPase is activated following an increase in [Na+]i and hence every transient [Na+]i elevation promotes Na+ efflux in exchange for K+ influx, which may represent a link between cytosolic Na+ fluctuations and K+ buffering. Astrocytes are also in possession of multiple ion exchangers or solute carriers (SLC; of which more than 50 families embracing ~ 380 members are known [130,131]) that utilise the energy stored in pre-existing ion concentration gradients.
Arguably, the most physiologically important ion exchanger is the Na+/Ca2+ exchanger or NCX, which belongs to the SLC8 family [132]. All 3 main isoforms, NCX1, NCX2 and NCX3 are expressed in astroglia. Importantly, the NCX proteins are often concentrated in astroglial perisynaptic processes where they co-localise with NKA and plasma membrane glutamate transporters [133]. The NCX can mediate the transport of both Na+ and Ca2+ in both directions; generally NCX may operate either in the forward mode (Ca2+ extrusion associated with Na+ influx) or in the reverse mode (Ca2+ entry associated with Na+ extrusion). This is determined by (i) stoichiometry of the exchanger, which is 3Na+:1Ca2+, (ii) transmembrane concentration gradients for Na+ and Ca2+, and (iii) the level of membrane potential. High resting [Na+]i in astrocytes sets the reversal potential of the NCX ~ −80 mV (see [117] for calculations and further details), which is very close to the resting membrane potential. Consequently, the NCX in astrocytes dynamically fluctuates between forward/reverse modes and mediates both Ca2+ entry and [Ca2+]i clearance as well as Na+ influx/efflux [115,124,134,135]. The reverse mode of the NCX is triggered by mild depolarisation and by Na+ influx through either ionotropic receptors or neurotransmitter transporters discussed below.
The Na+-dependent neurotransmitter transporters in astrocytes are mainly represented by plasma membrane transporters for glutamate and GABA. The glutamate transporters, generally classified as the excitatory amino acid transporters 1 to 5 (EAAT1 to EAAT5 belonging to SLC1 family), are fundamental for glutamate homeostasis. Astrocytes, which specifically express EAAT1 and EAAT2 (homologues of which in rodents are known as glutamate transporter 1, or GLT1, and glutamate-aspartate transporter or GLAST) act as the main sink for glutamate in the CNS accumulating ~80% of glutamate released in the course of synaptic transmission [136]. Glutamate accumulated into astrocytes is rapidly converted (by another astroglia-specific enzyme glutamine synthetase [137,138]) into glutamine; the latter is either transported to neurones, where it acts as the major precursor for glutamate and GABA and thus is indispensable for sustained synaptic activity (the glutamate-glutamine or GABA-glutamine shuttles), or is utilised for astroglial energetics [137]. The stoichiometry of EAAT1/2 is 1 Glu−:3 Na+:1K+:1H+, of which Na+, proton and glutamate enter the cell in exchange to K+ efflux. As a result of this stoichiometry and transmembrane gradients of relevant ions, the reversal potential for glutamate transporters is more positive than 50 mV [117]. This makes the reversal of glutamate transport impossible in physiological conditions; only during strong pathological insults accompanied by massive [Na+]i overload and very high extracellular K+ accumulation can the glutamate transport change direction and provide additional glutamate, which may exacerbate excitotoxicity [139]. In physiological conditions, activation of glutamate transport in astrocytes triggers inward Na+ current which can elevate [Na+]i by 10 – 20 mM [117,125]. Astrocytes also express GABA transporters of GAT1 and GAT3 types (SLC6 family), which are localised predominantly in astroglial processes surrounding inhibitory synapses. GABA transport via GAT3 can be affected by TRPA1 activity (decreased TRPA1 function leads to reduction in GABA uptake), which subsequently affects nearby GABA-ergic synaptic transmission [34]. GABA transporters provide for a transmembrane symport of 1 GABA molecule (uncharged in physiological conditions), 2 Na+ ions and 1 Cl− anion. Activation of GABA transporters also result in Na+ influx that can elevate [Na+]i by ~ 7 mM [116]. Importantly, the reversal potential for GABA transporters lies very close to astrocytic resting membrane potential and therefore even small elevation in [Na+]i can switch the transporter into reverse mode and hence facilitate GABA release from astrocytes; this release which can inhibit neuronal excitability was indeed detected in cortical slices [116].
Cellular Na+ homeostasis is also regulated by mitochondria which are able to accumulate Na+ through mitochondrial Na+/Ca2+ exchanger [40]. The NCLX, the solute carrier SLC24A6, is essential molecular component of this exchanger [140].
4.3. Functional role of astroglial Na+ signalling
Dynamic fluctuations in cytoplasmic Na+ concentration can affect surprisingly wide array of molecular targets and cascades that are critical for the homeostatic function of astroglia. First of all, [Na+]i modulates homeostasis of several neurotransmitters, that include principal excitatory transmitter glutamate and inhibitory transmitters GABA and glycine. Glutamate uptake is critical for termination of excitatory transmission and as the first step in glutamate-glutamine/GABA-glutamine shuttle. Increase in [Na+]i decreases the efficacy of glutamate transport; as it were glutamatergic transmission activates Na+ influx into astrocytes via ionotropic receptors and EAATs. Thus increased [Na+]i, which coincides with the peak of glutamatergic synaptic transmission event, temporarily decreases glutamate uptake, thus transiently increasing the effective glutamate concentration in the synaptic cleft. Levels of [Na+]i also influence glutamine synthetase as well as export of glutamine from astrocytes to neurones. The latter is mediated by Na+-coupled neutral amino acid transporter SNAT3/SLC38A3 and is directly controlled by [Na+]i [141].
Astroglial [Na+]i also regulates GABA-ergic transmission through (i) controlling astroglial GABA uptake via GAT1/3 pathway and (ii) by maintaining GABA synthesis in neuronal terminals by supplying glutamine. Astroglial GABA transport system is easy to reverse, because (as mentioned before) its reversal potential is set close to the resting potential of astrocyte. Thus, mild depolarisation and even small increases in [Na+]i may reverse the GAT-dependent transport making astrocytes a source of GABA. Additionally, GABA-ergic transmission turned out very sensitive to astroglial glutamine supply, and inhibition of glutamine synthetase substantially suppresses GABA-ergic inhibitory transmission [142]. Similarly astroglial [Na+]i regulates the efficacy of glycine clearance from the relevant synapses.
Dynamic changes in astroglial [Na+]i modulate Ca2+ signalling by defining the mode of operation of NCX. Increase in [Na+]i were shown to induce additional Ca2+ influx that contributed to neurotransmitter-evoked [Ca2+]i transients [124]. Such Ca2+ entry through NCX was even demonstrated to induce exocytotic release of neurotransmitters from astroglia [115,134,143].
Astroglial Na+ signals are coupled to several important homeostatic pathways. In particular [Na+]i levels directly control the activity of NKA and Na+/K+/Cl− co-transporter NKCC1, thus regulating K+ buffering. The [Na+]i controls the activity of Na+-proton exchanger and Na+-bicarbonate transporter, both being critical for pH homeostasis (see [117] for further discussion).
Finally, [Na+]i controls one of the most fundamental astroglial functions - that is the metabolic support of neurones. The latter occurs in the form of astrocyte-neurone lactate shuttle, when astrocytes supply active neurones with their preferred energy substrate lactate [144–146]. Neuronal activity-induced elevation of astroglial [Na+]i triggers lactate synthesis mediated through NKA; and therefore astroglial Na+ signalling is fundamental for neuronal metabolic support.
5. Concluding remarks
Rapid astroglial signalling, that is fundamental for neuronal-glial communications, is mediated through fluctuations of cytoplasmic concentrations of two principal cations Ca2+ and Na+. Neuronal activity can trigger complex spatio-temporal changes of [Ca2+]i and [Na+]i in astrocytes, which in turn regulate multiple effector pathways that control homeostatic function of these glial cells.
Acknowledgments
We thank Manoj K. Gottipati for comments on a previous version of this manuscript. Authors research is supported by Alzheimer’s Research Trust (UK) Programme Grant (ART/PG2004A/1) to AV; by National Science Foundation (CBET 0943343) grant to VP,; and by The Spanish Government, Plan Nacional de I+D+I 2008–2011 and ISCIII- Subdirección General de Evaluación y Fomento de la Investigación (PI10/02738) to AV.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- [Ca2+]i
cytoplasmic free Ca2+ concentration
- [Ca2+]L
intra-ER (or intraluminal) free Ca2+ concentration
- CNS
central nervous system
- CRAC
Ca2+-release activated Ca2+
- ER
endoplasmic reticulum
- GABA
γ-aminobutyric acid
- InsP3R
inositol 1,4,5 trisphosphate (InsP3)-gated Ca2+ channel/receptor
- [Na+]i
cytoplasmic Na+ concentration
- NCX
Na+/Ca2+ exchanger
- NKA
Na+/K+ ATPase
- NMDA
N-methyl D-aspartate
- PLC
phospholipase C
- PMCA
plasmalemmal Ca2+ ATPase
- RyR
ryanodine receptor
- SERCA
sarco(endoplasmic) reticulum Ca2+ ATPase
- SLC
solute carrier
- TRP
transient receptor potential
Footnotes
Authors Contributions
VP and AV conceptualized and wrote the manuscript; both authors approve the submitted version.
Competing financial interests
The authors declare that they have no competing financial interests.
References
- 1.Lenhossek Mv. Zur Kenntnis der Neuroglia des menschlichen Ruckenmarkes. Verh Anat Ges. 1891;5:193–221. [Google Scholar]
- 2.Lenhossek Mv. Der feinere Bau des Nervensystems im Lichte neuester Forschung. 2. Fischer’s Medicinische Buchhandlung H. Kornfield; Berlin: 1895. [Google Scholar]
- 3.Kimelberg HK. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 4.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Verkhratsky A. Physiology of neuronal-glial networking. Neurochem Int. 2010;57:332–343. doi: 10.1016/j.neuint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Verkhratsky A, Parpura V, Rodriguez JJ. Where the thoughts dwell: the physiology of neuronal-glial “diffuse neural net”. Brain Res Rev. 2011;66:133–151. doi: 10.1016/j.brainresrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Verkhratsky A, Butt A. A textbook. John Wiley & Sons; Chichester: 2007. Glial Neurobiology. [Google Scholar]
- 12.Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10:826–836. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353:45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Parpura V, Verkhratsky A. Neuroglia at the crossroads of homoeostasis, metabolism and signalling: evolution of the concept. ASN Neuro. 2012:4. doi: 10.1042/AN20120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, et al. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parpura V, Verkhratsky A. The astrocyte excitability brief: From receptors to gliotransmission. Neurochem Int. 2012;61:610–621. doi: 10.1016/j.neuint.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz B, Miledi R. Propagation of electric activity in motor nerve terminals. Proc R Soc Lond B Biol Sci. 1965;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- 20.Katz B, Miledi R. The effect of calcium on acetylcholine release from motor nerve terminals. Proc R Soc Lond B Biol Sci. 1965;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- 21.Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 22.Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. doi: 10.1016/j.ceca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Dermietzel R. Gap junction wiring: a ‘new’ principle in cell-to-cell communication in the nervous system? Brain Res Rev. 1998;26:176–183. doi: 10.1016/s0165-0173(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 24.Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- 25.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, et al. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie PB, Segal M, Kater SB. Independent regulation of calcium revealed by imaging dendritic spines. Nature. 1991;354:76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- 29.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 30.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, et al. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 33.Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium. 2005;38:161–169. doi: 10.1016/j.ceca.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pflugers Arch. 2003;446:447–454. doi: 10.1007/s00424-003-1094-z. [DOI] [PubMed] [Google Scholar]
- 38.Petersen OH, Verkhratsky A. Endoplasmic reticulum calcium tunnels integrate signalling in polarised cells. Cell Calcium. 2007;42:373–378. doi: 10.1016/j.ceca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol. 1992;262:H1699–1704. doi: 10.1152/ajpheart.1992.262.6.H1699. [DOI] [PubMed] [Google Scholar]
- 40.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 43.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero-Hernandez A, Dagnino-Acosta A, Verkhratsky A. An intelligent sarco-endoplasmic reticulum Ca2+ store: release and leak channels have differential access to a concealed Ca2+ pool. Cell Calcium. 2010;48:143–149. doi: 10.1016/j.ceca.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Kopach O, Kruglikov I, Pivneva T, Voitenko N, Verkhratsky A, Fedirko N. Mitochondria adjust Ca(2+) signaling regime to a pattern of stimulation in salivary acinar cells. Biochim Biophys Acta. 2011;1813:1740–1748. doi: 10.1016/j.bbamcr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44:6–13. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993;7:134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- 48.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 49.Cornell Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 50.Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- 51.Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 52.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 53.Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci. 1995;15:7861–7871. doi: 10.1523/JNEUROSCI.15-12-07861.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by a1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci. 1996;8:1198–1208. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 55.Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. Glutamate-triggered calcium signalling in mouse bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience. 1999;92:1051–1059. doi: 10.1016/s0306-4522(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 56.Porter JT, McCarthy KD. Adenosine receptors modulate [Ca2+]i in hippocampal astrocytes in situ. J Neurochem. 1995;65:1515–1523. doi: 10.1046/j.1471-4159.1995.65041515.x. [DOI] [PubMed] [Google Scholar]
- 57.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4:e00080. doi: 10.1042/AN20110061. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 59.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 60.Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 61.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 62.Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, et al. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mogami H, Tepikin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solovyova N, Verkhratsky A. Monitoring of free calcium in the neuronal endoplasmic reticulum: an overview of modern approaches. J Neurosci Methods. 2002;122:1–12. doi: 10.1016/s0165-0270(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 65.Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. EMBO J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- 67.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, et al. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H. Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J. 2002;16:84–86. doi: 10.1096/fj.01-0380fje. [DOI] [PubMed] [Google Scholar]
- 69.Verkhratsky A, Solovyova N, Toescu EC. Calcium excitability of glial cells. In: Volterra A, Haydon P, Magistretti P, editors. Glia in synaptic transmission. OUP; Oxford: 2002. [Google Scholar]
- 70.Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 71.Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- 72.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 73.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verderio C, Bruzzone S, Zocchi E, Fedele E, Schenk U, De Flora A, et al. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 75.Bruzzone S, Verderio C, Schenk U, Fedele E, Zocchi E, Matteoli M, et al. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J Neurochem. 2004;89:264–272. doi: 10.1111/j.1471-4159.2003.02326.x. [DOI] [PubMed] [Google Scholar]
- 76.Heidemann AC, Schipke CG, Kettenmann H. Extracellular application of nicotinic acid adenine dinucleotide phosphate induces Ca2+ signaling in astrocytes in situ. J Biol Chem. 2005;280:35630–35640. doi: 10.1074/jbc.M507338200. [DOI] [PubMed] [Google Scholar]
- 77.Singaravelu K, Deitmer JW. Calcium mobilization by nicotinic acid adenine dinucleotide phosphate (NAADP) in rat astrocytes. Cell Calcium. 2006;39:143–153. doi: 10.1016/j.ceca.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Barcelo-Torns M, Lewis AM, Gubern A, Barneda D, Bloor-Young D, Picatoste F, et al. NAADP mediates ATP-induced Ca2+ signals in astrocytes. FEBS Lett. 2011;585:2300–2306. doi: 10.1016/j.febslet.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 79.Malarkey EB, Parpura V. Mechanisms of transmitter release from astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho) physiology of the nervous system. Springer; New York: 2009. [Google Scholar]
- 80.Parpura V, Grubisic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta. 2011;1813:984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 81.D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 85.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 86.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 87.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 88.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 90.Pivneva T, Haas B, Reyes-Haro D, Laube G, Veh RW, Nolte C, et al. Store-operated Ca2+ entry in astrocytes: different spatial arrangement of endoplasmic reticulum explains functional diversity in vitro and in situ. Cell Calcium. 2008;43:591–601. doi: 10.1016/j.ceca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, et al. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;21:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- 92.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca 2+]I oscillations and is not involved in capacitative Ca 2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 95.Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- 96.Moreno C, Sampieri A, Vivas O, Pena-Segura C, Vaca L. STIM1 and Orai1 mediate thrombin-induced Ca(2+) influx in rat cortical astrocytes. Cell Calcium. 2012 doi: 10.1016/j.ceca.2012.08.004. in press,dx.doi.org/10.1016/j.ceca.2012.1008.1004. [DOI] [PubMed] [Google Scholar]
- 97.Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: What is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta. 2011;1813:992–1002. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 99.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 100.Muller T, Moller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- 101.Seifert G, Steinhauser C. Ionotropic glutamate receptors in astrocytes. Prog Brain Res. 2001;132:287–299. doi: 10.1016/S0079-6123(01)32083-6. [DOI] [PubMed] [Google Scholar]
- 102.Lalo U, Palygin O, North RA, Verkhratsky A, Pankratov Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell. 2011;10:392–402. doi: 10.1111/j.1474-9726.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 103.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 105.Oliveira JF, Riedel T, Leichsenring A, Heine C, Franke H, Krugel U, et al. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex. 2011;21:806–820. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- 106.Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium. 2010;48:225–231. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, et al. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tai C, Zhu S, Zhou N. TRPA1: the central molecule for chemical sensing in pain pathway? J Neurosci. 2008;28:1019–1021. doi: 10.1523/JNEUROSCI.5237-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 113.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol. 1996;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012;4:e00075. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Unichenko P, Myakhar O, Kirischuk S. Intracellular Na+ concentration influences short-term plasticity of glutamate transporter-mediated currents in neocortical astrocytes. Glia. 2012;60:605–614. doi: 10.1002/glia.22294. [DOI] [PubMed] [Google Scholar]
- 117.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 118.Kiedrowski L, Wroblewski JT, Costa E. Intracellular sodium concentration in cultured cerebellar granule cells challenged with glutamate. Mol Pharmacol. 1994;45:1050–1054. [PubMed] [Google Scholar]
- 119.Knopfel T, Guatteo E, Bernardi G, Mercuri NB. Hyperpolarization induces a rise in intracellular sodium concentration in dopamine cells of the substantia nigra pars compacta. Eur J Neurosci. 1998;10:1926–1929. doi: 10.1046/j.1460-9568.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 120.Pisani A, Calabresi P, Tozzi A, Bernardi G, Knopfel T. Early sodium elevations induced by combined oxygen and glucose deprivation in pyramidal cortical neurons. Eur J Neurosci. 1998;10:3572–3574. doi: 10.1046/j.1460-9568.1998.00398.x. [DOI] [PubMed] [Google Scholar]
- 121.Kimelberg HK, Pang S, Treble DH. Excitatory amino acid-stimulated uptake of 22Na+ in primary astrocyte cultures. J Neurosci. 1989;9:1141–1149. doi: 10.1523/JNEUROSCI.09-04-01141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 124.Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- 125.Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch. 2007;454:245–252. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- 126.Langer J, Stephan J, Theis M, Rose CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia. 2012;60:239–252. doi: 10.1002/glia.21259. [DOI] [PubMed] [Google Scholar]
- 127.Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia. 2008;56:1138–1149. doi: 10.1002/glia.20685. [DOI] [PubMed] [Google Scholar]
- 128.Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol. 2009;587:5859–5877. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, et al. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 130.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins Introduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 131.Ren Q, Chen K, Paulsen IT. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res. 2007;35:D274–279. doi: 10.1093/nar/gkl925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J. 2007;406:365–382. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- 133.Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–234. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, et al. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- 135.Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, et al. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Cai 2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- 136.Danbolt NC. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 137.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 138.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- 140.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 142.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 144.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 145.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2011.149. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]