Abstract
Background
An organizational approach is proposed as an immediate solution for improving postoperative pain (POP) management. The aim was to evaluate the clinical effectiveness of a quality management system (QMS), based on procedure-specific, multimodal analgesic protocols, modified to meet the individual patients’ requirements.
Methods
Patients from the orthopaedic, gynaecological, visceral, and trauma surgery departments of the university hospital were involved in two prospective surveys. Survey 1 was performed at baseline and survey 2 was performed after the implementation of QMS within an interval of 1 year. The patients were asked to report pain intensity on the visual rating scale, incidence of analgesia-related side-effects, and incidence of pain interference with the items of life quality and their satisfaction with the treatment of POP.
Results
Patients from Survey 2 (n=251) reported 25–30% less pain than those from Survey 1 (n=269) (P<0.0001). Nausea was reported by 40% of the patients from Survey 1 vs 17% from Survey 2, vomiting by 25 vs 11% and fatigue by 76% in Survey 1 vs 30% in Survey 2 (P<0.0001). Life quality and patients’ satisfaction improved in Survey 2 vs Survey 1 (P<0.001).
Conclusions
The implementation of QMS allowed the reduction in POP intensity with a simultaneous decrease in analgesia-related side-effects. This has led to an increased quality of life and patient satisfaction.
Keywords: adverse effects; analgesia; pain, postoperative; quality management
Editor's key points.
Improving postoperative pain control and reducing side-effects are an important clinical challenge.
This study evaluated introduction of a new evidence-based quality management system in the postoperative period.
A variety of approaches were used including individualized, multimodal analgesia, with staff support and education.
Clear clinical benefits were seen with improved pain control, and patient satisfaction and reduced side-effects.
Despite recent advances in understanding the pathophysiology of acute pain and the development of new approaches in treating postoperative pain (POP), the clinical situation regarding POP treatment needs further improvement.1 Postoperative patients still report high levels of acute pain in the aftermath of surgery, while simultaneously experiencing the side-effects of analgesics, used for POP treatment.2,3 In the US hospital environment, 80% of patients reported experiencing pain after surgery, 86% of them complained of moderate, severe, or extreme pain.4 In a Dutch cohort of 1490 patients, who had received POP treatment according to the standard acute pain protocol, 40% reported moderate or severe pain while at rest on the day after surgery.5 In a German collective of 2252 postoperative patients, the median value of maximal pain intensity reached 5/10 and pain intensity whilst moving reached 4/10. These values were associated with unsatisfactory pain treatment.6 At the same time, according to pooled data from prospective studies with more than 100 000 patients, the modern treatment of POP was associated with 30% of patients suffering from nausea, 20% from vomiting, and 24% from excessive sedation.2
Recently, experts proposed a new, organizational approach, based on our understanding of the pathophysiology of acute pain, the pharmacology of analgesics and clinical requirements, as an immediate solution for improving the management of POP.7 This approach applies three principles in routine clinical practice, to achieve an effective POP management: (i) multimodal therapies8 reflected in; (ii) evidence-based procedure-specific protocols9; (iii) adjusted to the patients’ individual analgesic requirements.10
In Germany, several groups of experts in the field of POP, supported by quality assurance methodology, used these three principles to initiate quality management systems (QMSs) aimed at improving POP treatment.11 One of these QMS, entitled ‘Treatment of postoperative and posttraumatic pain’, guided and certified by the German quality and safety monitoring agency, TÜV Rheinland,12 was introduced in 2008 at the University Hospital of Greifswald (Germany). This teaching hospital has 880 beds, 406 of which belong to 11 surgical departments. The QMS has been introduced in all surgical departments.
The aim of our investigation was to evaluate the clinical effectiveness of the QMS, introduced at the university hospital and to study the results of its implementation on patients’ satisfaction with the treatment of POP and its influence on quality of life.
Methods
Design of the investigation and selection criteria
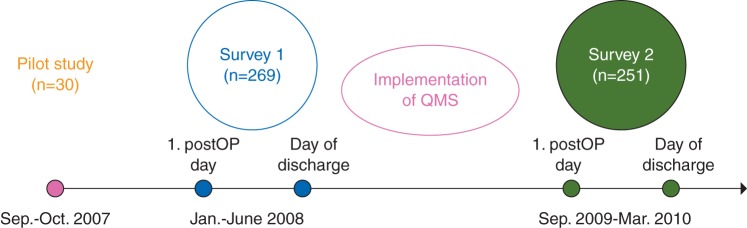
This prospective anonymous pre/post questionnaire investigation was conducted at the university hospital. After approval from the local ethics committee, patients from 4 (175 beds) of the 11 surgical departments such as visceral surgery, gynaecology, orthopaedics, and traumatology were consecutively enrolled, in accordance with selection criteria in two prospective surveys. Patients, who undergo elective surgery with anticipated intensity of POP of ≥3/10 on an 11-point NRS (a priori defined procedure-specific POP intensity, depending on the size and localization of surgical lesion),13 were asked to participate in the survey. The patients had to be able to fill out the study questionnaire on the first postoperative day and on the day of discharge and gave their informed signed consent to participate in the study. Patients, who were younger than 18 yr old, with chronic pain and those with insufficient knowledge of the German language or with cognitive limitations were not included. The patients were informed that they would be participating in a routine quality assurance survey of the hospital, thus the patients remained unaware ‘blinded’ of the purpose of the investigation. Survey 1 was performed at baseline from January to June 2008; Survey 2 was performed after the implementation of QMS from September 2009 to March 2010 (Fig. 1).
Fig 1.
Flow diagram of the study. A pilot pre-test study was performed in 30 patients to test the POP questionnnaire (PPQ) and to generate the data for calculation of the sample size for the study. The first survey was performed in the first half of 2008, where 296 patients received the PPQ on the first postoperative day and on the day of the surgery. The second survey was performed after the implementation of Quality Management Program in treatment of POP in 294 patients. QMS, quality management system.
Quality management system
A QMS for the treatment of POP, including successful methodologies described elsewhere,14–16 was introduced in all 11 surgical departments of the hospital.
QMS included:
structured patient information about POP treatment (including patient flyers with detailed descriptions of pain measurement methods and POP treatment modalities);
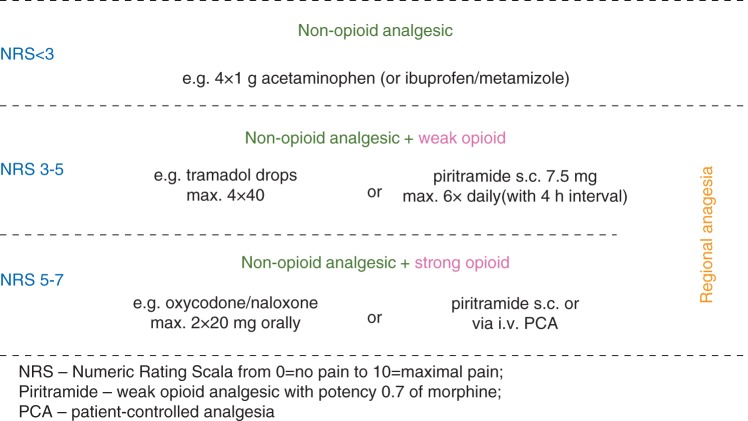
procedure-specific, multimodal analgesic protocols, modified to meet the patients’ individual requirements, based on guidelines for treatment of POP compiled by an international team of experts9 and by experts of the German Society of Anaesthesiology and Intensive Care (DGAI)17 (hospital-internal pathways are given in Figure 2 and Supplementary A and B);
standardized pain measurement (at least once every 8 h), its documentation, and therapeutic consequences of pain level >3 as measured on a numerical rating scale 11 (0=no pain; 10=maximal pain imaginable);
protocols with pathways for the treatment of analgesia-related adverse affects (including guidelines for the prevention and treatment of postoperative nausea and vomiting18 (Supplementary C);
organization of anaesthesia-based 24 h acute pain service10 (Supplementary D);
development of a multidisciplinary task-force in surgical departments (Supplementary D);
definition of the formal responsibilities (of nurses and physicians, within the various departments, Supplementary D);
development of an internal information source on POP management for all departments (Pain Manual) including Standard Operating Procedures on POP therapy and monitoring, accessible online on the university hospital intranet;
continuation of training of the personnel involved in the field of POP treatment;
quality assurance measures including external and internal audits. An external audit is performed once a year by TÜV Rheinland. Internal quality measures include internal audits of each surgical department twice a year and the present project ‘Evaluation of QMS implementation’.
Fig 2.
Scheme of multimodal analgesia adjusted to expected level of postoperative pain13 used for procedure-specific protocols of POP treatment at the surgical departments of the University Hospital Greifswald.
General analgesic regimens
Basic analgesia was provided by the non-opioid analgesics acetaminophen, ibuprofen, and metamizole (Fig. 2). In cases of expected moderate-to-severe pain, opioids were added, including oral tramadol or parenteral piritramide (opioid receptor agonist with 0.7 analgesic potency of morphine), applied via patient-controlled analgesia pumps (Fig. 2, Supplementary A and B). Continuous regional and epidural analgesia (EDA) with ropivacaine were used for POP treatment after orthopaedic, thoracic, and visceral surgery, where appropriate, according to above-mentioned PROSPECT9 and DGAI17 guidelines on POP treatment. No new analgesic drugs were introduced during the investigation.
Survey methodology
A pilot pre-test study was performed on 30 patients to obtain the data for the calculation of the sample size for the present study and to test the POP questionnaire (POP-Q). POP-Q was developed on the basis of a previously validated Brief Pain Inventory19 and included:
Five items to report on the 1st postoperative day (POP-Qs);
Nine items to report on the day of discharge (POP-Qd).
The five items of POP-Qs were: the intensity of (i) maximal pain; (ii) minimal pain after surgery; (iii) pain while at rest and while in movement at that moment; (iv) presence of tiredness, nausea, and vomiting; (v) satisfaction with pain therapy. Pain on movement was registered as the pain intensity during physiotherapeutic mobilization in patients after musculoskeletal surgery; after thoracic and abdominal surgery pain on coughing was the equivalent.
The nine items of POP-Qd included the five POP-Qs items supplemented by: (i) presence of POP at all; (ii) pain intensity while at rest and while in movement immediately after surgery; (iii) whether pain disturbed the following aspects: sleep, mood, mobility, communication with others, and enjoyment of life; (iv) whether the patient had received analgesics for more than 6 months before the surgery.
All items concerning pain intensity were measured using a visual rating scale (VRS-11) from 0=no pain to 10=maximal pain imaginable. Satisfaction with the POP treatment was measured using a VRS-5 from 1=excellent to 5=bad, which, to facilitate the rating, resembles the scale of grades in German schools.
On the day of surgery, consecutive patients were selected according to the eligibility criteria, using the data from intranet software with the surgery plan. On the first postoperative day, these patients received and filled out the POP-Qs. The POP-Qd was left for patients until the day of discharge, whereas the nurses of the department were asked to collect the completed POP-Qd.
Measurement of results and statistics
The intensity of pain taken on VRS-11, side-effects of analgesia, life quality items and patients’ satisfaction with their POP treatment were registered as the measurement of results. Moreover, to compare the severity of surgical procedures between the surveys, each patient received the value of a priori defined POP intensity, using the previously described procedure-specific POP intensity scale.13 An experienced consultant surgeon (J.L.) blindly scaled all surgeries using that a priori POP intensity scale.
To calculate the sample size, we used the data from a pilot pre-test study, assuming a probability of 0.05 and power of 80%. To detect a reduction in POP intensity of at least 30% from baseline, we calculated a sample size of at least 240 patients for each period of measurement pre/post (60 patients from each department). Anticipating a poor rate of returned questionnaires in the survey investigations, we planned to distribute 300 PPQ for each pre/post survey.
The collected data from Survey 1 was compared with that of Survey 2, using the SPSS 19.0 for Mac software and both presented as mean values with standard deviation or median with interquartile range, to give better characteristics of data distribution. Normally, distributed data were analysed using Student's t-test, skewed data were compared using the Mann–Whitney test. Binomially distributed data were analysed using the χ2 test and presented as frequency distribution with absolute numbers and relative distribution in per cent.
Results
Baseline data
The POP questionnaire was returned by 269 (91%) of the patients from Survey 1 (before QMS implementation) and by 251 (85%) from Survey 2 (after QMS implementation). The collective of the Survey 1 patients was comparable with that of the Survey 2, in terms of patient characteristics, ASA health status, and prevalence of analgesic use for more than 6 months before the surgery (Table 1). The patients from both surveys received perioperative catheters for regional and EDA in a comparable number of cases. The anticipated a priori pain intensity scores13 were also similar in patients from both surveys (Table 1). The detailed number of surgical procedures is given in Table 2.
Table 1.
Baseline data (N=520). Data presented as mean (range)*, mean (95% confidence intervals)** or as a number (n) of patients; EDA, epidural analgesia
| Survey 1 | Survey 2 | P | |
|---|---|---|---|
| Questionnaire distributed/returned | |||
| All wards | 296/269 | 294/251 | 0.7 |
| Orthopaedics | 77/75 | 74/64 | 0.9 |
| Traumatology | 78/67 | 70/63 | 0.8 |
| Visceral surgery | 73/68 | 74/59 | 0.9 |
| Gynaecology | 68/59 | 76/64 | 0.7 |
| Age (year)* | 52 (18–88) | 54 (19–89) | 0.08 |
| Gender, f/m (N) | 175/94 | 144/107 | 0.09 |
| ASA I | 54 | 33 | |
| ASA II | 155 | 158 | |
| ASA III | 51 | 50 | |
| ASA IV | 0 | 1 | |
| Patients, taking analgesics last 6 months | 11 | 9 | 0.6 |
| EDA | 18 | 24 | 0.3 |
| Peripheral neural catheters | 12 | 16 | 0.4 |
| Surgery-related a priori pain score** | 5.5 (5.3–5.7) | 5.7 (5.6–5.9) | 0.15 |
Table 2.
Surgical procedures performed in patients. Data presented as a number of patients
| Surgical procedures | Survey 1 | Survey 2 |
|---|---|---|
| Thyroidectomy | 0 | 1 |
| Gastric surgery | 5 | 4 |
| Oesophageal surgery (with thoracotomy) | 1 | 1 |
| Cholecystectomy | 21 | 11 |
| Pancreatic or hepatic resection | 1 | 5 |
| Lung resection | 1 | 0 |
| Laparoscopic appendectomy | 3 | 1 |
| Resection of | ||
| Jejunum | 1 | 3 |
| Colorectal | 10 | 14 |
| Perianal surgery | 4 | 1 |
| Hernia surgery | 21 | 14 |
| Partial resection of rectal abdominal muscle | 0 | 1 |
| Breast surgery | 4 | 4 |
| Lympadenectomy/lymph node dissection | 3 | 1 |
| Laparoscopic sterilization | 4 | 3 |
| Other vaginal procedures | 3 | 0 |
| Explorative laparotomy (palliative) | 1 | 0 |
| Hysterectomy | 44 | 56 |
| Total joint replacement | ||
| Knee | 15 | 20 |
| Hip | 22 | 29 |
| Shoulder | 1 | 0 |
| Arthroscopic knee surgery | 14 | 12 |
| Other surgical procedures on the joints | 7 | 11 |
| Osteosynthesis | ||
| Upper extremity | 20 | 9 |
| Lower extremity | 6 | 23 |
| Removal of metalwork | 17 | 5 |
| Hallux valgus correction | 12 | 4 |
| Arthrodesis | 8 | 2 |
| Spinal surgery | 2 | 5 |
| Amputation/resection of bone parts | 1 | 3 |
| Surgery of skin and skin appendages | 1 | 3 |
| Reconstruction of ligaments and muscles | 15 | 5 |
| Missed data | 1 | 0 |
| Total number | 269 | 251 |
Pain levels
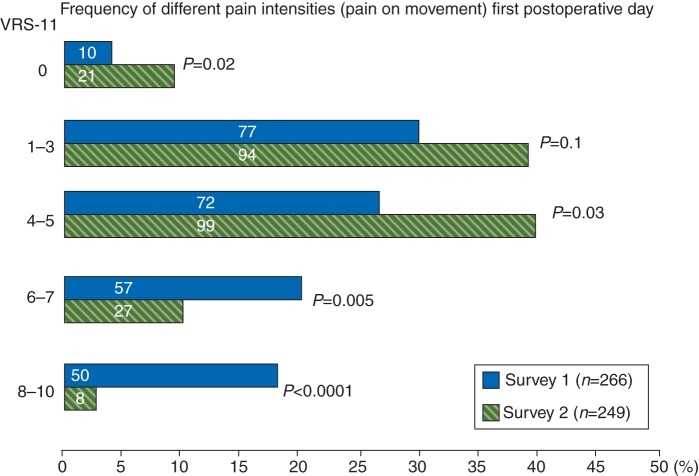
Survey 2 patients reported less pain than their counterparts in Survey 1 in all categories and time-points of measurement (Table 3). Maximal pain intensity after surgery reported by Survey 2 patients was lower than that reported by Survey 1: 4.6 (95% CI: 4.3–4.9) vs 6.0 (95% CI: 5.7–6.3); P<0.0001, as well as Survey 2’s pain while in movement on the first postoperative day being reported as less than Survey 1’s: 3.6 (95% CI: 3.3–3.8) vs 4.9 (95% CI: 4.6–5.2), P<0.0001. Before the implementation of the QMS, the patients were discharged to go home with a pain intensity (in movement) of 2.7 (95% CI: 2.5–2.9), after QMS implementation the patients reported 1.6 (95% CI: 1.5–1.8); P<0.0001, before their being discharged. The analysis of the prevalence for weak, moderate, and severe pain intensity on movement, taken on the first postoperative day, demonstrated that 43% of the Survey 1 patients vs 13% of the patients from Survey 2 had reported severe pain (defined as higher than 6 on the 10-point scale of VRS-11; P<0.0001). Figure 3 gives the prevalence of severe pain, split to levels 6–7 (VRS-11) and 8–10 (extremely high pain). Extremely high pain was reported by 19% of the Survey 1 patients vs 3% of Survey 2 patients (P<0.0001). Four per cent of the Survey 1 patients vs 9% of their Survey 2 counterparts had reported no pain at all after surgery (P=0.02).
Table 3.
Pain levels and satisfaction with pain treatment before (Survey 1) and after (Survey 2) implementation of QMS. Data presented as mean (lower and upper 95% confidence intervals); VRS, visual rating scale
| Intensities of pain (VRS-11) | Survey 1 (n=269) | Survey 2 (n=251) | P |
|---|---|---|---|
| Maximal pain | |||
| Day after surgery | 6.0 (5.7–6.3) | 4.6 (4.3–4.9) | <0.0001 |
| Day of discharge | 5.8 (5.5–6.1) | 4.7 (4.4–4.9) | <0.0001 |
| Minimal pain | |||
| Day after surgery | 1.7 (1.5–1.9) | 1.3 (1.1–1.4) | 0.004 |
| Day of discharge | 1.5 (1.3–1.7) | 0.6 (0.5–0.8) | <0.0001 |
| Pain at rest right now | |||
| Day after surgery | 1.9 (1.7–2.1) | 1.6 (1.4–1.7) | 0.04 |
| Day of discharge | 1.1 (0.9–1.2) | 0.5 (0.4–0.6) | <0.0001 |
| Pain on movement right now | |||
| Day after surgery | 4.9 (4.6–5.2) | 3.6 (3.3–3.8) | <0.0001 |
| Day of discharge | 2.7 (2.5–2.9) | 1.6 (1.5–1.8) | <0.0001 |
| Satisfaction with pain treatment (VRS-5) | |||
| Day after surgery | 1.8 (1.7–1.9) | 1.6 (1.5–1.8) | 0.001 |
| Day of discharge | 1.8 (1.7–1.9) | 1.5 (1.4–1.6) | 0.005 |
Fig 3.
Prevalence of different pain intensities (weak, moderate, severe) on movement taken on the first postoperative day using VRS 0-10 (VRS-11). Data are given as per cent from total number of patients in the survey; numeric values are absolute number of patients; *P<0.0001; χ2-test.
Analgesia-related side-effects
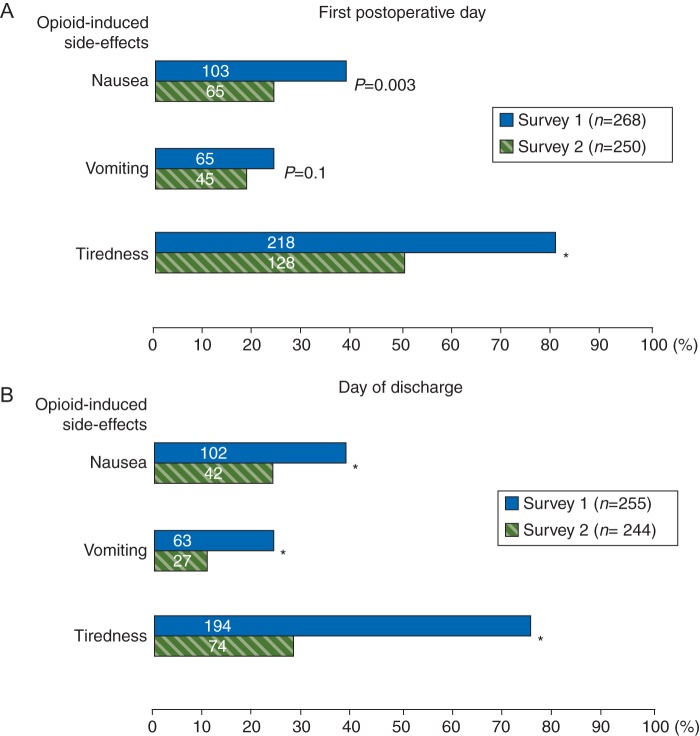
Analgesic side-effects, such as nausea, vomiting, and tiredness, decreased to nearly half after implementation of QMS. Thirty-eight per cent of the Survey 1 patients vs 26% reported nausea on the first postoperative day (P=0.003), on the day of discharge, there were 40% of the Survey 1 patients, who reported nausea vs 17% in Survey 2 (P<0.0001; Fig. 4). Vomiting was reported by 24% of the Survey 1 patients and by 18% in Survey 2 on the first postoperative day (P=0.1); on the day of discharge, 25% of the patients from Survey 1 and 11% from Survey 2 reported vomiting (P<0.0001). Eighty-one per cent of the Survey 1 patients and 51% from Survey 2 mentioned tiredness on the first postoperative day; on the day of discharge, 76% of the Survey 1 patients and 30% of the patients in Survey 2 reported this side-effect of opioid analgesia (P<0.0001).
Fig 4.
Frequency of opioid-induced side-effects on the first postoperative day (a) and on the day of discharge (b) given as per cent from total number of patients in the survey; numeric values are absolute number of patients;*P<0.0001; χ2-test.
Items of life quality and satisfaction with pain therapy
The implementation of QMS was associated with an improvement of measured items of life quality that usually are disturbed by pain in the postoperative period. On the day of discharge, 84% of the Survey 1 patients and 72% of the Survey 2 (P=0.01) reported that POP disturbed general activity. Sixty-five per cent of the patients of Survey 1 vs 47% of Survey 2 (P<0.0001) complained that POP disturbed their sleep. POP disturbed the enjoyment of life in 55% of the Survey 1 patients vs 39% of the Survey 2 patients (P=0.001). Satisfaction with the therapy of POP also improved in Survey 2 vs in Survey 1 (P<0.001; Table 4).
Table 4.
Life-quality items, disturbed by pain and satisfaction with the pain therapy, reported on the day of discharge. Life quality items data presented as per cent (absolute number of patients) and analysed using χ2-test; data of satisfaction with the pain therapy, taken on numeric rating scale (5—very bad; 1—excellent) presented as mean (lower and upper 95% confidence intervals) and analysed using Mann–Whitney test; n.s., not statistically significant
| Survey 1 | Survey 2 | P | |
|---|---|---|---|
| Pain disturbed: | n=254 | n=245 | |
| General activity | 84 (213) | 72 (177) | 0.01 |
| Mood | 32 (81) | 29 (71) | n.s. |
| Sleep | 65 (165) | 47 (116) | <0.0001 |
| Relations with other people | 13 (33) | 12 (30) | n.s. |
| Enjoyment of life | 55 (140) | 39 (96) | 0.001 |
| Satisfaction with pain therapy | n=269 | n=251 | |
| Day after surgery | 1.8 (1.7–1.9) | 1.6 (1.5–1.8) | 0.001 |
| Day of discharge | 1.8 (1.7–1.9) | 1.5 (1.4–1.6) | 0.005 |
Discussion
The introduction of the QMS for treatment of POP, based on clinical implementation of evidence-based procedure-specific protocols of multimodal analgesia, adjusted to individual patients’ requirements has led to an improvement of the POP treatment in surgical patients at the university hospital. The implementation of QMS was associated with lower POP levels and with a simultaneous reduction of analgesia-related side-effects, improved daily activity, sleep, and an overall satisfaction with the treatment of POP.
The values of clinically relevant pain categories (maximal pain and pain on movement) were reduced by 25–30% from the baseline level (Survey 1) after implementation of QMS (Table 2). Baseline levels of pain intensity, measured at 5–6 (median, VRS-11) for maximal pain and at 5 for pain while in movement, are in accordance with previous investigations of the same design, which also described the pain-reducing clinical effect of QMS implementation.20–22 The effect size of POP reduction (pain on movement on the first postoperative day) after QMS implementation from 4.9 (2.5) during Survey 1 to 3.5 (1.9) [mean (sd); VRS-11] during Survey 2 (30% reduction) was comparable with pain relief effect of EDA compared with i.v. patient-controlled analgesia (PCA) with opioids.23 In this meta-analysis, Wu and colleagues23 calculated the pain intensity on movement on the first postoperative day with PCA at 4.9 (1.1) vs EDA 3.4 (1.0) [mean (sd); VAS-11].
The improvement in the mean POP values in our investigation is due to a decrease in patients with severe (6–7; median on VRS-11) and extremely high (8–10 on VRS-11) pain after QMS implementation (Fig. 3). Such a ‘shift’ in patients from the prevalence groups with a high level of POP to lower pain level groups, acceptable as not disturbing the quality of life (<4 on VRS-11) was also demonstrated in previous investigations, studying the implementation of comparable QMS.14,20
Since no new drugs were introduced to the analgesic regimen at the time of the QMS implementation, the observed improvement in pain reduction is likely to be due to the combined effect of a rapid and more differentiated delivery of analgesics (preventive administration according to expected a priori pain intensity, early administration according to evidence-based procedure-specific guidelines, titration according to individual analgesic requirements of patients, etc.) as well as a number of unspecific factors. These factors include preoperative information to patients about the POP treatment, enhanced attention to treatment of pain, and related side-effects of analgesics by medical staff and regular visits by the acute pain service.24
The frequency of the use of regional analgesia for POP treatment was comparable in patients’ collectives from both surveys. However, we observed the dramatic reduction in opioid-related side-effects such as nausea, vomiting (N&V), and tiredness subsequent to the implementation of QMS (Fig. 4). This nearly 50% reduction in the incidence of N&V can be explained by an initially high level (40% for nausea and 25% for vomiting) of these opioid-related side-effects at baseline (Survey 1), on the one hand, and by the implementation of evidence-based guidelines for PONV prevention and treatment,18 as an element of the QMS, on the other hand. The former was probably due to the pre-selection of the patients for this investigation who had received balanced general anaesthesia with volatile anaesthetic and opioid analgesics and who had required i.v. opioid analgesics for the POP treatment with the expected a priori pain level of >5 on NRS-11. Both of these treatments may have produced a high incidence of PONV, as reported by the patients during Survey 1, both on the first postoperative day and on the day of their discharge, but not by Survey 2 patients (Table 3). During Survey 2, the incidence of both N&V decreased during the postoperative period, suggesting the effectiveness of QMS. Variations in tiredness showed a similar tendency: (i) a high level on both days of investigation at baseline (Survey 1); (ii) a decrease in Survey 2 in comparison with Survey 1 after QMS implementation; and (iii) a significant decrease during Survey 2 between the first postoperative day and the day of discharge. Interestingly, none of the previous investigations, which also have statistically demonstrated a significant decrease in pain levels, showed a decrease in analgesic-related side-effects.14–16,20–22
It is likely that the 25–30% decrease in the POP level and the reduction in analgesic-related side-effects led to an improvement in daily activity, sleep, and enjoyment of life. However, the psychological effects of increased attention by medical staff cannot be ruled out. The patients’ satisfaction with pain therapy had also improved after the implementation of the QMS.
These improvements in POP management can be partly attributed to the Hawthorne effect, when the act of measuring induces changes itself. However, the size of the Hawthorne effect is much smaller, than the effects found in our study25 and it usually disappears within 8 weeks since the introduction of ‘something new’.26 Moreover, in order to keep the Hawthorne effect (motivation of personnel, participating in QMS on POP treatment) working, the German quality and safety monitoring agency, TÜV Rheinland, incorporated special monitoring/quality assurance measures, including external and internal audits (see section ‘Quality management system’, statement x).
The inability to apply a multi-centred, parallel-group randomized design was the major limitation of this study. However, it would be complicated to apply this ‘gold standard’ of clinical research because of organizational and ethical reasons. In fact, we do not see the single-centred design as a drawback: on the contrary, our carefully conducted investigation at a single institution had succeeded in demonstrating clinical improvement after QMS implementation. However, the large-scale patient survey, performed in 37 US hospitals after implementation of the Postoperative Pain Management Quality Improvement Project, demonstrated an improvement in structures and processes, critical to improving pain management (such as documented use of pain rating scales, decreased use of i.m. opioids, and the increased use of non-pharmacological strategies), although there was no change in pain results.16 Our investigation was focused on the effectiveness of QMS for POP treatment, suggested previously in numerous publications,6,7,9,11,12,27 and was not aimed at showing the causal links of expected changes. Another limitation was the absence of cost analysis, which might have provided insight into the causal relationships of changes discovered during the study. The next steps may be: (i) cost-effectiveness analysis, including expenses of personnel and analgesics, to evaluate the economic aspects of QMS implementation; (ii) study the influence of QMS on ‘hard’ clinical outcomes—perioperative morbidity and mortality; and (iii) prevent the development of persistent postsurgical pain.28
The implementation of QMS has led to a clinically significant improvement of POP treatment, accompanied by a simultaneous decrease in analgesia-related side-effects, which have led to an increased quality of life and the satisfaction of the patients.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Acknowledgements
The authors thank the staff who took part in the implementation of the QMS and the patients who took part in this study.
Declaration of interest
None declared.
Funding
The work was supported by internal funding of the Department of Anaesthesiology and Intensive Care Medicine, University Medicine of Greifswald.
References
- 1.Dahl JB, Mathiesen O, Kehlet H. An expert opinion on postoperative pain management, with special reference to new developments. Expert Opin Pharmacother. 2010;11:2459–70. doi: 10.1517/14656566.2010.499124. doi:10.1517/14656566.2010.499124. [DOI] [PubMed] [Google Scholar]
- 2.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–23. [PubMed] [Google Scholar]
- 3.Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584–91. doi: 10.1093/bja/aei227. [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. doi:10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 5.Sommer M, de Rijke JM, van Kleef M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol. 2008;25:267–74. doi: 10.1017/S0265021507003031. doi:10.1017/S0265021507003031. [DOI] [PubMed] [Google Scholar]
- 6.Maier C, Nestler N, Richter H, et al. The quality of pain management in German hospitals. Dtsch Arztebl Int. 2010;107:607–14. doi: 10.3238/arztebl.2010.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010;112:220–5. doi: 10.1097/ALN.0b013e3181c6316e. doi:10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]
- 8.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107((Suppl 1)):i27–40. doi: 10.1093/bja/aer358. doi:10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 9. http://www.postoppain.org/ (accessed 13 October 2011)
- 10.Rawal N, Berggren. Organization of acute pain services: a low-cost model. Pain. 1994;57:117–23. doi: 10.1016/0304-3959(94)90115-5. doi:10.1016/0304-3959(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 11. http://www.schmerzfreies-krankenhaus.de/ (accessed 13 October 2011)
- 12. http://www.tuv.com/de/deutschland/gk/managementsysteme/medizin_gesundheitswesen/initiative_schmerzfreie_klinik/initiative_schmerzfreie_klinik.jsp. (accessed 13 October 2011) [Google Scholar]
- 13.Jaffe RA. Anesthesiologist's Manual of Surgical Procedures. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 14.Meissner W, Ullrich K, Zwacka S, Schreiber T, Reinhart K. Quality management in postoperative pain therapy. Anaesthesist. 2001;50:661–70. doi: 10.1007/s001010100194. doi:10.1007/s001010100194. [DOI] [PubMed] [Google Scholar]
- 15.Bardiau FM, Taviaux NF, Albert A, Boogaerts JG, Stadler M. An intervention study to enhance postoperative pain management. Anesth Analg. 2003;96:179–85. doi: 10.1097/00000539-200301000-00038. [DOI] [PubMed] [Google Scholar]
- 16.Dahl JL, Gordon D, Ward S, Skemp M, Wochos S, Schurr M. Institutionalizing pain management: the Post-Operative Pain Management Quality Improvement Project. J Pain. 2003;4:361–71. doi: 10.1016/s1526-5900(03)00640-0. doi:10.1016/S1526-5900(03)00640-0. [DOI] [PubMed] [Google Scholar]
- 17. http://www.awmf.org/uploads/tx_szleitlinien/041-001_S3_Behandlung_akuter_perioperativer_und_posttraumatischer_Schmerzen_aktualisierte_Fassung_04-2009_05-2011.pdf. (accessed 3 October 2011)
- 18.Gan TJ, Meyer TA, Apfel CC, et al. Society for Ambulatory Anesthesia . Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. doi:10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 20.Diby M, Romand JA, Frick S, Heidegger CP, Walder B. Reducing pain in patients undergoing cardiac surgery after implementation of a quality improvement postoperative pain treatment program. J Crit Care. 2008;23:359–71. doi: 10.1016/j.jcrc.2007.11.005. doi:10.1016/j.jcrc.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Lehmkuhl D, Meissner W, Neugebauer EA. Evaluation of the ‘initiative pain-free clinic’ for quality improvement in postoperative pain management. A prospective controlled study. Schmerz. 2011;25:508–15. doi: 10.1007/s00482-011-1054-z. doi:10.1007/s00482-011-1054-z. [DOI] [PubMed] [Google Scholar]
- 22.Klammer F, Gehling M, Klammer A, Fass J, Tryba M. An algorithm for postoperative pain management in visceral and thoracic surgery: an observational study. Zentralbl Chir. 2011 doi: 10.1055/s-0031-1271430. doi: 10.1055/s-0031-1271430. [DOI] [PubMed] [Google Scholar]
- 23.Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–88. doi: 10.1097/00000542-200511000-00023. doi:10.1097/00000542-200511000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Capuzzo M, Gilli G, Paparella L, et al. Factors predictive of patient satisfaction with anesthesia. Anesth Analg. 2007;105:435–42. doi: 10.1213/01.ane.0000270208.99982.88. doi:10.1213/01.ane.0000270208.99982.88. [DOI] [PubMed] [Google Scholar]
- 25.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. doi:10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark RE, Surgue BM. In: Instructional Technology: Past, Present and Future. Anglin GJ, editor. Englewood, CO: Libraries Unlimited Inc.; 1991. pp. 327–43. [Google Scholar]
- 27.Powell AE, Davies HT, Bannister J, Macrae WA. Challenge of improving postoperative pain management: case studies of three acute pain services in the UK National Health Service. Br J Anaesth. 2009;102:824–31. doi: 10.1093/bja/aep066. doi:10.1093/bja/aep066. [DOI] [PubMed] [Google Scholar]
- 28.Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth. 2011;107:25–9. doi: 10.1093/bja/aer116. doi:10.1093/bja/aer116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.