Abstract
The gut flora plays an important role in the pathogenesis of the complications of cirrhosis. Hepatic encephalopathy (HE) represents a broad continuum of neuropsychological dysfunction in patients with acute or chronic liver disease and/or porto-systemic shunting of blood flow and it manifests with progressive deterioration of the superior neurological functions. The pathophysiology of this disease is complex, as it involves overproduction and reduced metabolism of various neurotoxins, particularly ammonia. Management of HE is diversified and requires several steps: elimination of precipitating factors, removal of toxins, proper nutritional support, modulation of resident fecal flora and downregulation of systemic and gut-derived inflammation. This review will provide an overview of gut barrier function and the influence of gut-derived factors on HE, focusing on the role of gut microbiota in the pathogenesis of HE and the recent literature findings on its therapeutic manipulation.
Keywords: Hepatic encephalopathy, Gut microbiota, Cirrhosis, Non-absorbable disaccharides, Rifaximin
INTRODUCTION
Humans have been proposed to be a “meta-organisms” consisting of a huge number of bacterial cells that are metabolically and immunologically integrated with somatic cells[1]. This interaction is especially important in the gastrointestinal tract, where the commensal bacteria, known as intestinal microbiota, are an integral component of human gut physiology and, together with the intestinal mucosa, form an important barrier against pathogens. Gut homeostasis and physiology are closely linked to the liver since it receives the intestinal blood content through the portal system and influences intestinal functions through bile secretion into the lumen[2]. Thus, alterations of gut barrier seem to play an important role in the pathogenesis and progression of liver damage[3]. Understanding of both partners in this normal gut-liver interaction is critical to the development of new therapeutic modalities to treat or prevent liver disease and its complications. This review will provide an overview of gut barrier function and the influence of gut-derived factors on hepatic encephalopathy (HE), focusing on the role of gut microbiota in the pathogenesis of HE and the recent literature findings on its therapeutic manipulation.
HOST-MICROBIOTA CROSS-TALK
The human intestine provides residence to more than 1014 bacteria, a number which is 10 times the number of somatic cells in the human body[4,5]. Microorganisms start colonizing the gut immediately after birth and are characterized by a succession of different population until a stable, adult microbiota has been established. In this bacterial community anaerobes are more abundant than aerobes and the majority of the species are from the genera Bacteroidetes and Firmicutes[6]. Bacterial density and types differ substantially from lower small intestine to distal colon, and are regulated by physiological conditions. The specific populations also vary among individuals and in the same individual during periods of illness or dietary changes[7]. Microarray analysis of intestinal transcriptional responses and molecular taxonomic methodologies have greatly increased our understanding of the gut microbiota composition, activities and functions[1,8,9]. In a healthy individual the host/microbiota relationship is characterized by a homeostatic symbiosis, in which the host provides nutrients and a stable environment and, in turn, the microbiota ensures optimal epithelial functioning.
GUT-LIVER AXIS
Receiving most of its blood supply from the intestine through the portal circulation, the liver is exposed to gut-derived toxins, including bacteria and bacterial products, and must be prepared to react against these potential systemic pathogens. For this purpose it contains a large number of resident immune cells including macrophages, dendritic cells, lymphocytes, natural killer cells. These cells act together with other non-parenchymal cells like endothelial and stellate cells to produce an organized response to these potentially highly inflammatory factors[10,11]. The role of immune cells during inflammatory response or chronic liver injury and the potential impact of gut-derived toxins on these processes have been extensively studied[2]. In particular bacterial overgrowth and altered intestinal permeability result in high plasmatic levels of bacterial endotoxins, such as lipopolysaccharide (LPS), peptidoglycan, and various lipopeptides also termed pathogen-associated molecular patterns. Endotoxemia could be responsible for initiation of the liver damage, through its interaction with specific recognition receptors, the toll like receptors on the surface of immune cells. These receptors contribute to adaptive immune response and regulation of inflammation and represent a link between intestinal flora changes, endotoxemia, and liver damage[12]. Liver cirrhosis is characterized by several abnormalities of both the systemic and local immune systems and in particular by reduced phagocytic activity of Kupffer cells[13]. A great deal of evidence indicates that patients with cirrhosis could present increased intestinal permeability[14]. This is related to structural changes that occur in the presence of portal hypertension and hypertensive enteropathy: an altered oxide-reductive state with consequent oxidative damage of the brush border membrane and the overproduction of nitric oxide resulting in tight junctions expansion and cytoskeleton destruction[15]. Altered intestinal permeability, together with bacterial overgrowth and immune dysfunction are associated with the migration of bacteria or their products from the gut to mesenteric lymph nodes or to other organs, a process known as bacterial translocation (BT). This phenomenon, in association with the presence of vascular shunts, is responsible for increased circulating levels of LPS. Blood concentration of bacterial endotoxin directly correlates with the severity of liver disease and participates in the initiation of a complex series of mechanisms that lead to the development of cirrhosis complications[16]. In particular, the main consequences of portal hypertension and BT are the occurrence of infections and HE.
Bacterial infections are present in about 15%-47% of patients with liver cirrhosis and are especially related to Gram-negative bacteria. The most frequent are spontaneous bacterial peritonitis (SBP), urinary tract infections, pneumonia, pleural empyema and sepsis. It has been shown that patients with SBP have a higher prevalence of small intestinal bacterial overgrowth (SIBO)[17] and altered intestinal permeability[18] than patients without SBP. Ammonia and other toxic substances derived from the gut, in the presence of portal and systemic shunts as well as of reduced liver clearance capability, represent the pathogenic mechanisms of HE as described in the following paragraph.
GUT MICROBIOTA AND HE
HE is a reversible neuropsychiatric disorder associated with liver dysfunction after exclusion of other potential causes of brain disease[19] and is characterized by poor survival[20]. Main features are disturbances in cognitive function, personality and behaviour with a wide spectrum of neuropsychiatric abnormalities that range from mild impairment of cognitive function and consciousness to coma. There are two types of HE named overt and minimal HE (OHE, MHE). The first is present in 30%-45% of patients with cirrhosis and in 10%-50% of patients with transjugular intrahepatic portosystemic shunt[21]. OHE can be diagnosed clinically through a constellation of signs and symptoms by several scoring systems[22]. The most widely used are the West-Haven criteria (Table 1) which are based on neurological examination and specific questionnaires to detect mental status changes. In addition, OHE can be further divided into episodic or persistent depending on time course and clinical behaviour: episodic HE remains below the clinical detection level among different episodes, whereas persistent HE is always clinically evident[23]. Prevalence for MHE ranges from 30% up to 80%, probably because there is still no accepted gold standard for diagnosis of MHE and the majority of the screening tests are time-consuming and cumbersome to perform[23,24]. It is characterized by alterations of psychometric and neurophysiological tests in otherwise asymptomatic patients[25]. This condition is considered a preclinical stage of OHE and is associated with poor quality of life, high risk of traffic violations and accidents and increased progression to OHE[26-28]. The diagnosis of MHE aims to detect attention deficits and processing speed and is based on different batteries of tests. Among them, the portal systemic encephalopathy (PSE) syndrome test, that includes number connection test A (NCT A), number connection test B (NCT B), line tracing test errors and time, serial dotting test and digit symbol test, has been validated in different countries and is recommended by the Working Group on HE[29]. Although the pathogenesis of this disorder is not well understood, a key role is played by circulating gut derived toxins of the nitrogenous compounds with a resultant neuroglial injury in the setting of a systemic inflammatory milieu (Figure 1). In particular, the currently accepted hypothesis is that endogenous neurotoxic substances escape from catabolism by the liver, due both to the impaired function of the cirrhotic liver and to the presence of portal-systemic shunts. These substances circulate in the systemic blood flow, reach the brain through the blood-brain barrier and results in different severity of cerebral impairment[30]. Several factors have been shown to precipitate HE, including gastrointestinal bleeding, electrolyte imbalances, infection, and medications such as sedatives and diuretics. They work by increasing the underlying inflammatory milieu, increasing toxins production or reducing the threshold for mental status decline, or a combination of the above. Ammonia, mercaptans, phenols, short and medium-chain fatty acids and benzodiazepine-like compounds have all been found to be elevated in cirrhotic patients with HE. The majority of these toxic substances are produced in the intestine by the bacterial flora, and are absorbed into the portal venous flow. Thus, gut microflora contribute to the pro-inflammatory state of cirrhosis even in the absence of overt infection[31]. In addition, cirrhotic patients have a substantial alteration of the gut microecology with a high prevalence of SIBO and delayed oro-cecal transit time (OCTT)[17,32-36]. The latter has a multifactorial ethiology. First, it could be due to the presence of autonomic neuropathy that has been demonstrated as an independent predictor of reduced intestinal motility in this kind of patients[37]. Moreover, patients with autonomic dysfunction were found to have a higher incidence of new onset of HE[38]. Second, a delayed OCTT may be associated with metabolic alterations that occur in patients with portal hypertension and portal-systemic venous shunting. Finally, SIBO itself may leads to delayed OCTT since it has been shown a significant improvement of intestinal motility after antibiotic therapy[36]. There might be also a link between the cognitive impairment in cirrhosis with inflammation and specific bacterial taxa. For example, recent evidence showed how interleukin (IL)-23 system and innate immune response were highly correlated with several bacterial families in patients with cirrhosis and HE, and how there was a direct correlation between cognition, Porphyromonadaceae and Alcaligeneceae families[39]. Based on these considerations, the manipulation of gut flora could be useful in the treatment of HE.
Table 1.
West-Haven criteria for hepatic encephalopathy
| Stage | Consciousness | Intellect and behaviour | Neurological findings |
| 0 | Normal | Normal | Normal examination1 |
| 1 | Trivial lack of awareness | Impaired attention span; altered sleep; euphoria | Mild asterixis |
| 2 | Lethargic | Disoriented; inappropriate behaviour depression | Asterixis; slurred speech |
| 3 | Somnolent but arousable | Gross disorientation; bizarre behaviour | Muscular rigidity/clonus hyper-reflexia |
| 4 | Coma | Coma | Decerebrate posturing |
If impaired psychomotor testing, then minimal hepatic encephalopathy.
Figure 1.
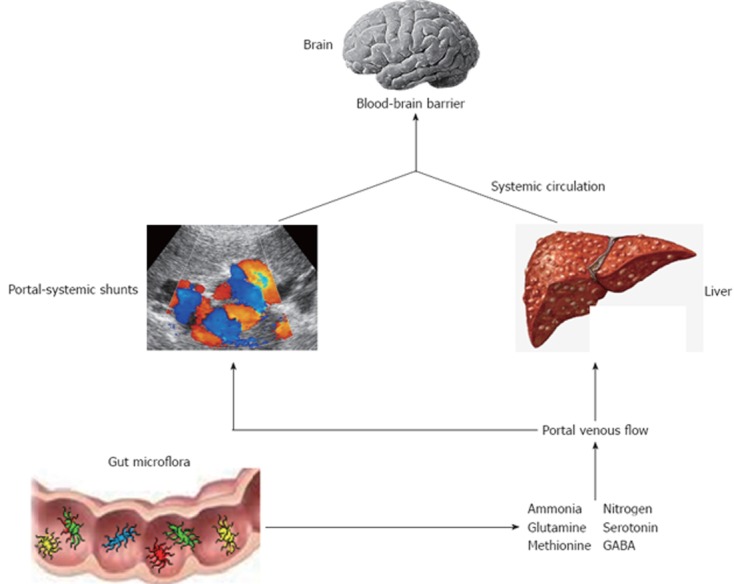
Pathophysiology of hepatic encephalopathy in liver cirrhosis. GABA: γ-aminobutyric acid.
MODULATION OF GUT MICROBIOTA FOR THE MANAGEMENT OF HE
Treatment goals and options are dependent on the stage and acuity of HE[23]. Once the diagnosis of OHE is confirmed, an extensive search for potential precipitating factors should be instituted along with treatment of OHE. As previously described, the leading causes are gastrointestinal bleeding, sepsis, dehydration resulting from diuretics, diarrhoea or vomiting, transjugular intra hepatic porto systemic shunting, constipation and the use of sedative and narcotic drugs. Their treatment can reverse OHE in most cases. However, when it is not possible to identify a precipitating factor despite an exhaustive search, specific treatment for OHE should be instituted. Most therapies for HE focus on treating episodes as they occur and are directed at reducing the nitrogenous load in the gut, an approach that is consistent with the hypothesis that this disorder results from the systemic accumulation of gut-derived neurotoxins in patients with impaired liver function and portosystemic shunting[40]. Therefore, the majority of therapeutic options currently in use are directed towards the gut. Prebiotics are non-digestible food ingredients that act by directly stimulating the growth of bacterial strains potentially beneficial to the host like Bifidobacteria and Lactobacilli, thereby indirectly reducing the influence of potentially more harmful resident flora (i.e. urease-producing species). They come in the form of indigestible fibers and have shown benefit for the management of HE, particularly MHE, both as prebiotics and when used in combination with probiotics (in which case they are termed synbiotics)[41,42]. Probiotics are living non pathogenic microorganisms that are thought to exert an effect in HE by reducing intestinal ammonia production by enterocyte glutaminase and reduce BT, modulate gut permeability and modulate pro-inflammatory responses. Furthermore, probiotics bypass the small bowel and get fermented by colonic bacteria to form lactic, acetic, and butyric acids, and gas (mainly hydrogen); any resultant prokinetic effect may increase the expulsion of ammoniagenic bacteria[43]. Probiotics have been studied for the treatment of HE and have shown some benefit, mostly in the setting of minimal disease[24,44,45]. The bacterial species that appear to be most successful include Lactobacilli and Bifidobacteria. Probiotics may also improve overall liver function, perhaps by reducing translocation and subsequent endotoxemia and by ameliorating the hyperdynamic circulation[41]. To quantify unambiguously the beneficial and harmful effects of any probiotics at any dosage, a recent meta-analysis identified seven randomized trials for the treatment of acute or chronic HE. The authors of this Cochrane review assessed a range of outcomes including death, recovery, adverse events, and quality of life. There was no benefit of probiotics shown for any of the primary outcomes including mortality. On the other hand, there was a significant difference in secondary outcomes such as lowering of plasma ammonia concentration compared with no treatment. The authors concluded that this finding is of questionable importance, not recommending the use of probiotics for patients with HE until further randomized clinical trials are undertaken[46]. Non-absorbable disaccharides, such as lactulose and lactilol, have traditionally been considered the first-line drug therapy for lowering the production and absorption of ammonia[47]. These substances are metabolized by the intestinal bacteria to acetic and lactic acid. The consequent acidification of the colonic contents creates a hostile environment for the survival of intestinal bacteria involved in the production of ammonia and facilitates the conversion of NH3 to non-absorbable NH4+. Moreover, their cathartic effects cause an increased in faecal nitrogen excretion[48]. The non-absorbable disaccharides have been used for decades with anecdotal and clinical trial experience[49]. However, side effects of lactulose therapy, including cramping, diarrhoea and flatulence, result in frequent noncompliance[40]. Overdosage may also result in severe diarrhoea, electrolyte disturbances and hypovolaemia that, if severe enough, may itself precipitate encephalopathy symptoms. In spite of their anedoctal usefulness, in the last years the true efficacy of the disaccharides for this indication has been questioned. As the use of lactulose pre dated randomized controlled trials, a comprehensive meta-analysis endorsed by the Cochrane Collaboration did not find any significant difference in outcomes in patients treated with and without lactulose[50]. Based on a critical analysis of available published literature, Als-Nielsen et al[50] concluded that the evidence in favour of utilizing non-absorbable disaccharides in HE did not meet the current minimum criteria for adequacy. In fact, although in some cases the administration of lactulose was associated with improvement in mental status, it is difficult to assess the reason for improvement since precipitating factors were simultaneously being corrected. On the other hand newer clinical studies suggest benefits with lactulose conferring improved neuropsychometric and quality of life scores[47]. Also, two recently published meta-analysis on the clinical efficacy and safety of lactulose in patients with MHE, provide substantial evidence for the beneficial effects of non-absorbable disaccharides[51,52]. Therefore, at the present time, there is a lack of sufficient evidence to completely dismiss the use of non-absorbable disaccharides for the treatment of HE, while compliance and cost effectiveness should be carefully balanced against clinical outcomes. Antimicrobial agents have long been utilized as an alternative treatment option for patients intolerant or unresponsive to non-absorbable disaccharides due to their ability to inhibit ammonia production by intestinal bacteria. Neomycin is the most commonly used antimicrobial for HE, but despite the legitimate theoretical rationale for its use, there is a paucity of clinical data to support this practice[48,53]. Moreover, the occurrence of serious adverse effects such as nephrotoxicity and ototoxicity limit their use to relatively short periods of time. Other antimicrobials, including metronidazole and vancomycin, have been studied to a more limited extent than neomycin[48]. However, long-term use of metronidazole has been associated with neurotoxicity in patients with cirrhosis, whereas the risk for enteric bacteria resistance preclude the routine use of vancomycin for HE. Thus, with the potential for serious adverse effects and the lack of demonstrated clinical benefit, the routine management of HE with conventional antibiotics should be questioned. Rifaximin is a poorly absorbed synthetic antibiotic with a broad spectrum of antibacterial activity, against aerobic and anaerobic Gram positive and Gram-negative organisms[54]. As a derivative of rifamycin, it similarly works by blocking bacterial RNA synthesis. However, rifaximin has an additional pyridoimidazole ring that allows for high concentrations in the gastrointestinal tract and minimal systemic drug absorption.
Due to its low rate of systemic bioavailability, the safety profile of rifaximin appears to be superior to that of systemic antibiotics, particularly for patients with liver disease, making it suitable for long-term use. Moreover, the risk of bacterial resistance appears to be lower with rifaximin than with systemic antibiotics because bacteria outside the gastrointestinal tract are not exposed to appreciable selective pressure[55]. The safety and efficacy profiles of rifaximin as treatment of overt HE have been extensively explored in several clinical trials[48]. The results of controlled double-bind studies demonstrated that rifaximin was more effective than non-absorbable disaccharides in the treatment of acute HE: mental state, electroencephalogram irregularities and PSE-index were all significantly improved in the rifaximin group[56-59]. While rifaximin was well tolerated, adverse events, including flatulence, diarrhoea, nausea and anorexia, were reported by patients in the disaccharides group. A recent study found a reduced hospitalization rate during rifaximin therapy compared with that of lactulose[60]. Once again, the HE grade was significantly lower and patients compliance was significantly higher in the rifaximin group. Further data suggest that rifaximin is at least as effective as neomycin in decreasing plasma ammonia levels and in improving the clinical symptoms related to HE with fewer clinically significant adverse events during a 21 d treatment period[61]. Based on these findings, the Cochrane review recommends the use of rifaximin in the treatment of acute HE[50]. Until recently, there has not been any conclusive evidence to support routine use of pharmacological prophylaxis to prevent future recurrence in patients who have recovered from an acute episode of HE. However, the prevention of episodes of HE is an important goal in the treatment of patients with liver disease, especially since symptoms of overt encephalopathy are debilitating and decrease the ability for self-care, leading to frequent hospitalizations, and a poor quality of life. A recent clinical trial has been conducted to evaluate the efficacy of rifaximin as secondary prophylaxis of overt HE[62]. This double blind, placebo controlled, multicentre trial randomized 299 patients with a recent history of recurrent, overt HE to receive either rifaximin or placebo for a period of 6 months. The majority of the patients in both groups were also maintained on concomitant lactulose therapy. During the study period, an acute episode of HE occurred in a significantly lower percentage of patients in the rifaximin group (22.1%) than in the placebo group (45.9%), with a hazard ratio (HR) of 0.42.
Furthermore, there was a significantly reduced risk of hospitalization in the rifaximin group when compared with placebo: 13.6% vs 22.6% of patients respectively with a corresponding HR of 0.50. No significant difference in the incidence of adverse events was found between the two groups. The authors concluded that the addition of rifaximin to a standard lactulose regimen may offer advantages in terms of decreasing risk of both acute HE episodes as well as hospitalizations when compared with lactulose alone. Overall, this pivotal study expands previously reported findings of the efficacy of rifaximin in the treatment of overt HE and demonstrates a clinically relevant benefit of rifaximin as pharmacological prophylaxis of HE. The protective effect of rifaximin was confirmed by an extension trial performed on the same patients to assess the efficacy of rifaximin in maintaining remission over time[63]. The results of this preliminary study support a possible long-term protective pharmacological effect. In addition, an ancillary analysis of data from the same trial to assess the effect of rifaximin on health-related quality of life (measured via the Chronic Liver Disease Questionnaire) provided evidence that rifaximin improves perception of daily well being and quality of life outcomes in all domains adversely affected by underlying liver diseases[64]. Therefore at this time, both lactulose and rifaximin can be used to prevent recurrent episodes of OHE. Ambivalence remains as concerning the treatment of MHE. It is clear that MHE has a major impact on quality of life and should be promptly diagnosed and treated. However, for many years, various treatment modalities including lactulose, probiotics and dietary manipulation, have been studied with unconvincing evidence. Limits on the use of antibiotics have been exceeded by the results of the randomised ischaemic mitral evaluation trial[65], which showed unequivocally that rifaximin improves psychometric test performance scores as early as after 2 wk of treatment. The same patients experienced, also, a significant improvement in health related quality of life, which was strongly correlated with the improvement in cognitive functions. The authors speculated that there are two possible mechanisms by which rifaximin could lead to an improvement in MHE: first by reducing the ammonia-producing bacteria in the gut[61], second by decreasing BT and inflammation.
The results of the RIME trial represent an important step in the establishment of rifaximin as an effective and safe treatment for MHE and are in agreement with the findings of a more recent study which demonstrates improved driving skills following rifaximin treatment in patients with MHE[66]. In this double-blind placebo controlled trial, patients randomized to rifaximin had a significant reduction in the number of total driving errors, and specifically speeding tickets and illegal turns, on a driving simulator, compared with those on placebo. Driving requires balance and integration of different cognitive functions, such as attention, adequate reaction time, visuo-motor coordination and can be considered a practical interpretation of the cognitive domains affected in MHE[67]. Since patients on rifaximin presented increased plasmatic levels of the anti-inflammatory cytokine IL-10, the authors speculated that rifaximin could act by regulating local intestinal immunity and inflammation. The same authors provide in another paper a cost effective analysis based on a Markov model of progression from cirrhosis without MHE, to MHE and to OHE focused on motor vehicle crashes as an objective endpoint. The results of this analysis indicate that diagnosis of MHE followed by lactulose therapy could result in substantial societal cost savings by preventing major motor vehicle crashes among MHE patients. In contrast, because of its high monthly cost, treatment with rifaximin is unlikely to generate overall cost savings unless the rifaximin monthly cost is substantially reduced[68].
CONCLUSION
Different chronic liver diseases are associated with alterations of the intestinal microbiota, gut barrier dysfunction and translocation of bacteria or bacteria-derived antigens into the systemic circulation. The same processes, in turn, exacerbate liver disease leading to enhanced tissue damage and development of complications such as systemic infections, phosphate buffered saline, hepato renal syndrome and portal-systemic encephalopathy. Modulation of the gut microbiota may represent a good therapeutic target for the prevention and treatment of different complications associated with liver cirrhosis. In particular, due to its low rate of systemic bioavailability and a good tolerability profile, the non-absorbable antibiotic rifaximin could be an ‘ideal’ antimicrobial for selective targeting at the gastrointestinal tract in this kind of patients. As reviewed, at present this antibiotic is recommended for the treatment of acute HE. Furthermore, recent literature appears to support a favourable benefit-risk ratio for rifaximin both in the prevention of overt HE and in the treatment of minimal HE.
However, additional high-quality clinical trials are needed to more clearly define the effectiveness of long-term or periodic treatment with rifaximin on gut microbiota modulation in cirrhotic patients with HE.
Footnotes
Peer reviewers: Peter Ferenci, Professor, Department of Internal Medicine IV, Division of Gastroenterology and Hepatology, Waehringer Guertel 18-20, Vienna A-1090, Austria; Philip Rosenthal, MD, Professor of Pediatrics and Surgery, UCSF, 500 Parnassus Avenue, Box 0136, MU 4-East, San Francisco, CA 94143-0136, United States
S-Editor Shi ZF L-Editor A E-Editor Xiong L
References
- 1.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norman K, Pirlich M. Gastrointestinal tract in liver disease: which organ is sick? Curr Opin Clin Nutr Metab Care. 2008;11:613–619. doi: 10.1097/MCO.0b013e32830a70bc. [DOI] [PubMed] [Google Scholar]
- 3.Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract. 2010;2010:453563. doi: 10.1155/2010/453563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–58. doi: 10.1097/MOG.0b013e3282f323f3. [DOI] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 10.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 12.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamura T, Miura S, Tsuzuki Y, Hara Y, Hokari R, Ogawa T, Teramoto K, Watanabe C, Kobayashi H, Nagata H, et al. Alteration of intestinal intraepithelial lymphocytes and increased bacterial translocation in a murine model of cirrhosis. Immunol Lett. 2003;90:3–11. doi: 10.1016/j.imlet.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 15.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 16.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 17.Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362–1370. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 18.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
- 19.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 20.Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030–1040. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Nolte W, Wiltfang J, Schindler C, Münke H, Unterberg K, Zumhasch U, Figulla HR, Werner G, Hartmann H, Ramadori G. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology. 1998;28:1215–1225. doi: 10.1002/hep.510280508. [DOI] [PubMed] [Google Scholar]
- 22.Hassanein T, Blei AT, Perry W, Hilsabeck R, Stange J, Larsen FS, Brown RS, Caldwell S, McGuire B, Nevens F, et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104:1392–1400. doi: 10.1038/ajg.2009.160. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Sharma BC, Puri V, Sarin SK. An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2008;20:506–511. doi: 10.1097/MEG.0b013e3282f3e6f5. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, Hammeke TA, Pinkerton SD, Saeian K. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600.e1. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Hafeezullah M, Zadvornova Y, Martin E, Schubert CM, Gibson DP, Hoffmann RG, Sanyal AJ, Heuman DM, Hammeke TA, et al. The effect of fatigue on driving skills in patients with hepatic encephalopathy. Am J Gastroenterol. 2009;104:898–905. doi: 10.1038/ajg.2009.7. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 29.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 30.Moriwaki H, Shiraki M, Iwasa J, Terakura Y. Hepatic encephalopathy as a complication of liver cirrhosis: an Asian perspective. J Gastroenterol Hepatol. 2010;25:858863. doi: 10.1111/j.1440-1746.2010.06242.x. [DOI] [PubMed] [Google Scholar]
- 31.Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, Hamilton JA, Bengmark S, Williams R, Visvanathan K. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–1164. doi: 10.1053/jhep.2003.50180. [DOI] [PubMed] [Google Scholar]
- 32.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 33.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Sadik R, Abrahamsson H, Björnsson E, Gunnarsdottir A, Stotzer PO. Etiology of portal hypertension may influence gastrointestinal transit. Scand J Gastroenterol. 2003;38:1039–1044. doi: 10.1080/00365520310004939. [DOI] [PubMed] [Google Scholar]
- 36.Van Thiel DH, Fagiuoli S, Wright HI, Chien MC, Gavaler JS. Gastrointestinal transit in cirrhotic patients: effect of hepatic encephalopathy and its treatment. Hepatology. 1994;19:67–71. [PubMed] [Google Scholar]
- 37.Maheshwari A, Thuluvath PJ. Autonomic neuropathy may be associated with delayed orocaecal transit time in patients with cirrhosis. Auton Neurosci. 2005;118:135–139. doi: 10.1016/j.autneu.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari A, Thomas A, Thuluvath PJ. Patients with autonomic neuropathy are more likely to develop hepatic encephalopathy. Dig Dis Sci. 2004;49:1584–1588. doi: 10.1023/b:ddas.0000043368.73042.b0. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bass NM. Review article: the current pharmacological therapies for hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25:23–31. doi: 10.1111/j.1746-6342.2006.03218.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 42.Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010;22:199–206. doi: 10.1097/MEG.0b013e328330a8d3. [DOI] [PubMed] [Google Scholar]
- 43.Wright G, Chattree A, Jalan R. Management of hepatic encephalopathy. Int J Hepatol. 2011;2011:841407. doi: 10.4061/2011/841407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007;52:3259–3265. doi: 10.1007/s10620-006-9687-y. [DOI] [PubMed] [Google Scholar]
- 45.Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, Pleuss JA, Krakower G, Hoffmann RG, Binion DG. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 46.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;(11):CD008716. doi: 10.1002/14651858.CD008716.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 48.Phongsamran PV, Kim JW, Cupo Abbott J, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs. 2010;70:1131–1148. doi: 10.2165/10898630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 50.Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044. doi: 10.1002/14651858.CD003044.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Luo M, Li L, Lu CZ, Cao WK. Clinical eficacy and safety of lactulose for minimal hepatic encephalopathy: a metaanalysis. Eur J Gastroenterol Hepatol. 2011;23:1250–1257. doi: 10.1097/MEG.0b013e32834d1938. [DOI] [PubMed] [Google Scholar]
- 52.Shukla S, Shukla A, Mehboob S, Guha S. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2011;33:662–671. doi: 10.1111/j.1365-2036.2010.04574.x. [DOI] [PubMed] [Google Scholar]
- 53.Strauss E, Tramote R, Silva EP, Caly WR, Honain NZ, Maffei RA, de Sá MF. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542–545. [PubMed] [Google Scholar]
- 54.Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36–66. doi: 10.1159/000081990. [DOI] [PubMed] [Google Scholar]
- 55.Debbia EA, Maioli E, Roveta S, Marchese A. Effects of rifaximin on bacterial virulence mechanisms at supra- and sub-inhibitory concentrations. J Chemother. 2008;20:186–194. doi: 10.1179/joc.2008.20.2.186. [DOI] [PubMed] [Google Scholar]
- 56.Alcorn J. Review: rifaximin is equally or more effective than other antibiotics and lactulose for hepatic encephalopathy. ACP J Club. 2008;149:11. [PubMed] [Google Scholar]
- 57.Fera G, Agostinacchio F, Nigro M. Rifaximin in the treatment of hepatic encephalopathy. Eur J Clin Res. 1993;4:57–66. [Google Scholar]
- 58.Mas A, Rods J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodrguez-Martnez D, Fernndez-Rodrguez C, Coll I, Pardo A; Spanish Association for the Study of the Liver Hepatic Encephalopathy Cooperative Group. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, doubledummy, controlled clinical trial. J Hepatol. 2003;38:518. doi: 10.1016/s0168-8278(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019–1032. doi: 10.1592/phco.28.8.1019. [DOI] [PubMed] [Google Scholar]
- 60.Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737–741. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- 61.Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991;23:175–178. [PubMed] [Google Scholar]
- 62.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 63.Poordad F, Bass N, Sanyal AJ, Sheikh MY, Mullen KD, Sigal S, Frederick T, Brown RS, Joshi S, Merchant K, et al. The protective effect of rifaximin (1100 mg daily) from hepatic encephalopathy observed in a double-blind placebo controlled study is substantiated and durable over the long term. Hepatology. 2009;50:448A–449A. [Google Scholar]
- 64.Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, Vemuru RP, Mazen Jamal M, Huang S, Merchant K, et al. Randomised clinical trial: rifaximin improves HRQL in cirrhotic patients with hepatic encephalopathy – a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853–861. doi: 10.1111/j.1365-2036.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- 65.Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial) Am J Gastroenterol. 2011;106:307–316. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- 66.Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478–487.e1. doi: 10.1053/j.gastro.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, Hoffmann RG, Stravitz RT, Heuman DM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175–1183. doi: 10.1002/hep.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55:1164–1171. doi: 10.1002/hep.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]


