Abstract
Ameloblastic carcinoma is a very rare malignant odontogenic tumour with characteristic histopathological and clinical features, which requires aggressive surgical treatment and surveillance and, therefore, differs from ameloblastoma. Metastasis typically occurs in the lung. Only one patient with metastasis to the skull has previously been described and no prior case reports have presented MRI and positron emission tomography-CT (PET-CT) imaging findings. We describe a case of ameloblastic carcinoma with metastasis to the skull and lung with emphasis on imaging features including MRI and PET-CT.
Keywords: ameloblastic carcinoma, odontogenic tumour, magnetic resonance imaging, computed tomography, positron emission tomography computed tomography
Introduction
Ameloblastoma is an odontogenic tumour of the jaw, which arises from dental embryonic remnants and represents 1% of all jaw tumours. It is generally considered a prototypical benign neoplasm; however, local recurrence commonly occurs.1 Metastases are known to occur in roughly 2–5% of cases and 80% involve the lung.2 Ameloblastic carcinoma is a term given to tumours that display histologically malignant features in both primary and metastatic sites with a pattern otherwise resembling ameloblastoma.2
A review of the literature from 1927 to 2006 revealed 66 cases of ameloblastic carcinoma: 20 cases reported metastases, the majority to the lung;3 with only 1 case of ameloblastic carcinoma arising from the anterior skull base;4 1 case of direct extension to the skull base; and 2 case reports, on the same patient, of metastasis to the skull.5 Review of the literature reveals there is very little written regarding the MRI and 18-fluorodeoxyglucose positron emission tomography-CT (PET-CT) features of ameloblastic carcinoma. Here we report a case of ameloblastic carcinoma with metastasis to the skull, 4 year status post-resection and radiation therapy, with emphasis on the imaging features including CT, MRI and PET-CT.
Case report
A previously healthy 16-year-old boy presented with a progressively growing non-tender right mandibular mass, which inhibited the patient's bite mechanism without airway obstruction or speech inhibition. Physical examination demonstrated a firm, indurated mass measuring 8 cm in diameter replacing the right mandibular body, extending from the right canine tooth to the ramus and floor of the mouth. Mild trismus was noted, without cranial nerve deficits, dental caries or lymphadenopathy.
Non-contrast CT revealed a large expansile lesion in the posterior body and angle of the right mandible with cortical disruption (Figure 1). The lesion contained an unerupted third molar, and displaced second and first molar teeth. MRI demonstrated a solid lesion with focal regions of increased T2 signal, which correlates with the cystic degeneration noted on pathological specimens and heterogeneous enhancement on post-contrast T1 weighted images (Figure 2). The lesion exerted significant mass effect on the adjacent structures without evidence of vascular or neural invasion. No lymphadenopathy was noted. Subsequent CT of the chest, abdomen and pelvis demonstrated no evidence of metastases.
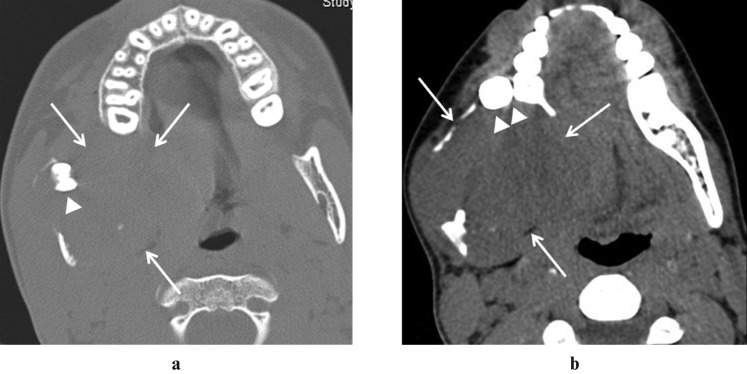
Figure 1.
Axial non-contrast CT in bone (a) and soft tissue (b); an algorithm demonstrates a large soft-tissue mass (arrows) with bone destruction. An unerupted third molar (arrowhead) is seen within the lesion, and the second molar (double arrowheads) is displaced anteriorly
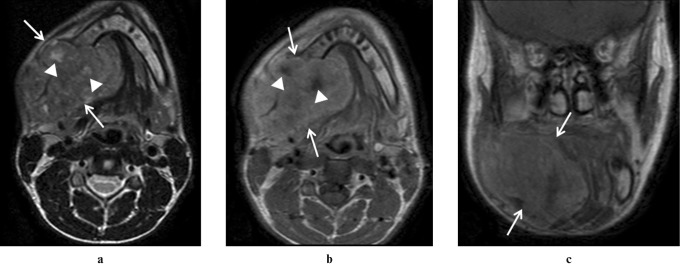
Figure 2.
(a) Axial turbo spin echo T2 weighted MRI shows a large heterogeneous signal lesion (arrows) with focal regions of increased T2 signal (arrowheads) in the posterior body and angle of the right mandible. (b) Axial and (c) coronal post-contrast spin echo T1 weighted images demonstrate heterogeneous enhancement with foci of no enhancement corresponding to T2 high signal (arrowheads) consistent with cystic degeneration noted pathologically
Incisional biopsy revealed ameloblastic carcinoma. The patient underwent partial mandibulectomy with a wide margin excision, fibular free flap reconstruction and level I–V lymph node dissection.
Gross pathological review demonstrated a multilobulated firm shiny white mass measuring 8 × 6 × 5 cm involving the medial and posterior mandible (Figure 3). Histologically, the specimen demonstrated sheets of atypical spindled epithelial cells exhibiting nuclear pleomorphism separated by areas of hyalinized fibrous stroma. Some areas of odontogenic epithelium were remarkable for columnar cells with reverse nuclear polarity surrounding central areas of hyperchomatic spindle cells and stellate reticulum-like change. Focal regions of cystic degeneration were noted. No vascular invasion or nodal involvement was noted. Immunohistochemistry demonstrated positive staining for cytokeratins (AE1/3 and CAM5.2), with negative staining for vimentin. The pathological diagnosis was ameloblastic carcinoma.
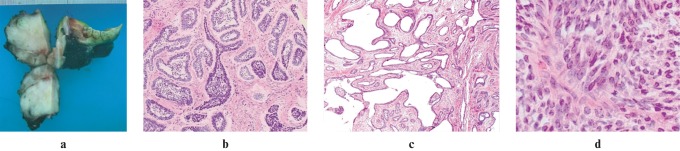
Figure 3.
(a) Gross specimen demonstrates a multilobulated firm white mass. Cut sections reveal a single white, firm, shiny mass involving the medial to posterior mandible. (b) Low power microscopic image of haematoxylin and eosin (H&E) stain demonstrates an ameloblastoma-like finding. (c) Low power microscopic image H&E stain demonstrates multiple regions of cystic degeneration. (d) High power microscopic image of H&E stain demonstrates an area of densely packed blue spindle cells. Sheets of atypical spindled epithelial cells exhibit nuclear pleomorphism separated by areas of hyalinized fibrous stroma
Post-operatively the patient underwent radiation therapy and routine clinical and radiological surveillance. The patient presented 4 years post-diagnosis with a rapidly growing, non-tender right forehead mass. Non-contrast CT showed an expansile osteolytic lesion measuring 2.6 × 4.5 × 2.3 cm involving the right frontal bone, eroding the anterior and posterior tables with adjacent dural thickening, without definite evidence of intracranial extension (Figure 4a). PET-CT showed increased metabolic activity with maximum standardized uptake value (SUVmax) of 6.0 within the frontal bone lesion (Figure 4b). Further, mild increased metabolic activity, maximum SUV of 2.0, was identified within a 12 × 9 mm nodule in the lower lobe of the left lung (Figure 4c).
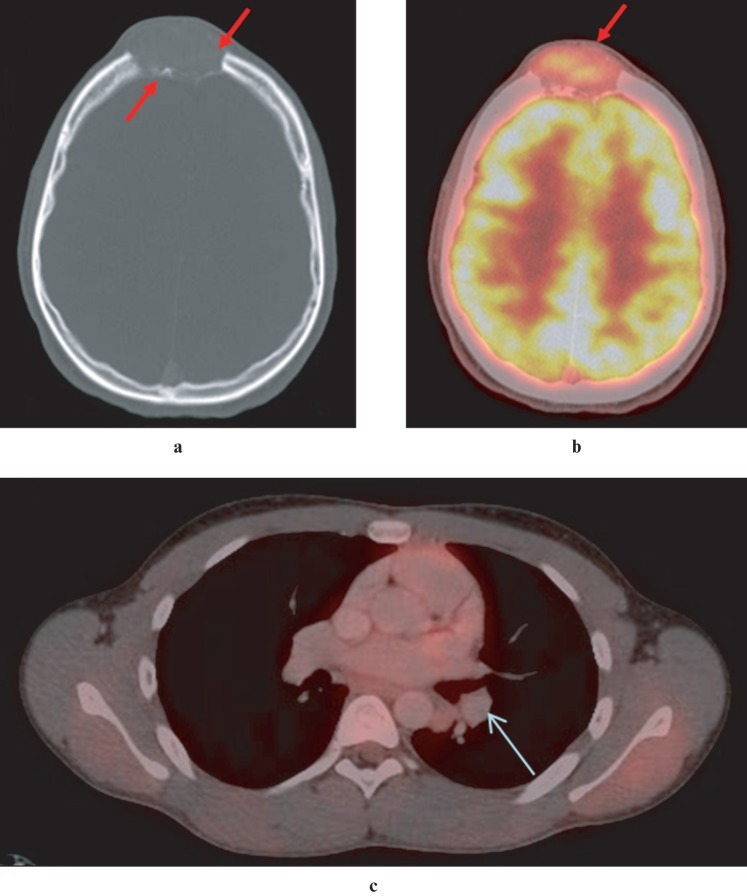
Figure 4.
(a) Axial CT image demonstrates an expansile, osteolytic lesion, eroding the outer and inner tables of the frontal bones in the midline. (b) Axial positron emission tomography (PET)-CT image demonstrates moderate 18-fluorodeoxyglucose (FDG) uptake (maximum standardized uptake value (SUVmax) 6.0) in frontal soft tissue mass, suggestive of malignancy. Intense FDG uptake in the grey matter of the brain is physiological. (c) Axial PET-CT image of the thorax demonstrates moderate FDG uptake (SUVmax 2.0) in a 12 mm × 9 mm nodule in the lower lobe of the left lung, suggestive of pulmonary metastasis
Needle aspiration of the right frontal bone lesion revealed cellular changes and an immunohistochemical profile consistent with metastasis from ameloblastic carcinoma (Figure 5). Immunohistochemistry studies demonstrated positive staining for cytokeratins (AE1/3 and CAM5.2), with negative staining for vimentin. Subsequent left lower lobe superior segmentectomy also confirmed metastatic ameloblastic carcinoma.
Figure 5.
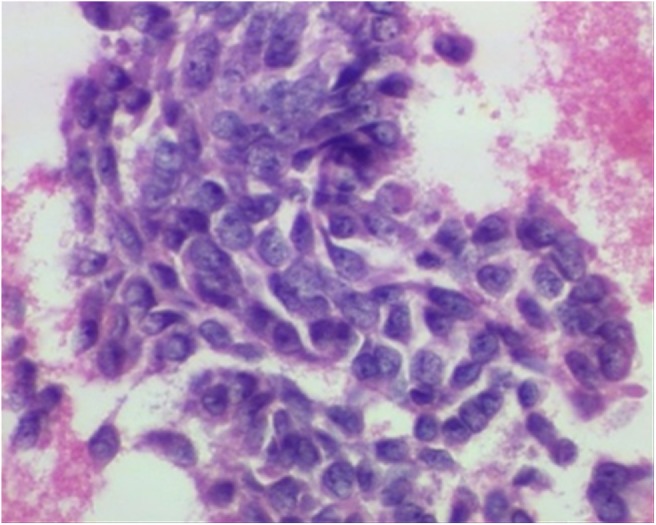
High-power microscopic image of haematoxylin and eosin stain of a specimen from a fine needle aspiration of the skull lesion demonstrates densely packed blue spindle cells consistent with metastatic ameloblastic carcinoma
Discussion
According to the Slootweg and Müller6 classification system, odontogenic carcinomas include five subclassifications: malignant (metastasizing) ameloblastoma, ameloblastic carcinoma, primary intraosseous squamous cell carcinoma, clear cell odontogenic carcinoma, malignant epithelial odontogenic ghost cell tumour. The World Health Organization defines ameloblastoma as a locally aggressive benign odontogenic tumour, and metastasizing (malignant) ameloblastoma and primary and secondary ameloblastic carcinoma as malignant tumours.7
Ameloblastic carcinoma is thought to originate from dental embryonic remnants, possibly from the rests of dental lamina, a developing enamel organ or the epithelial lining of an odontogenic cyst. There is much debate within the literature regarding the classification and distinction of ameloblastic carcinoma and malignant ameloblastoma.8
In the literature, “malignant ameloblastoma” and “ameloblastic carcinoma” have been differentiated. Malignant ameloblastoma refers to metastatic benign ameloblastomas, whereas ameloblastic carcinoma portrays histologically malignant features in both primary and metastatic sites with a pattern otherwise resembling ameloblastoma.3
The differential diagnosis includes benign odontogenic tumours, including ameloblastoma; odontogenic cysts; non-odontogenic cysts; benign non-odontogenic solid lesions, including central giant cell granuloma; malignant odontogenic tumours, including clear cell odontogenic carcinoma; ameloblastic fibrosarcoma; odontogenic carcinoma; odontogenic carcinosarcoma and primary intraosseous carcinoma; and malignant non-odontogenic tumours, including squamous cell carcinoma and metastasis from other primary carcinoma most commonly lung, breast and gastrointestinal tract.9
Ameloblastic carcinoma and ameloblastoma can have a similar radiographical appearance; however, certain imaging features may aid the diagnostic distinction. Radiographically both lesions can be radiolucent, either unilocular or multilocular, which generally has a honeycomb appearance with tooth root resorption. Both lesions often have distinct borders, slight marginal sclerosis without periosteal new bone formation, loss of lamina dura, resorption of the tooth apex and tooth displacement. A review of the literature shows there is limited discussion about the MRI features of ameloblastic carcinoma, and no prior case of PET-CT. MRI can provide additional details regarding the location; solid component of lesion; extension within the bone marrow, which is important for surgical resection or reconstruction planning; involvement of adjacent extraosseous structures; and highlight aggressive features outside the diagnostic capability of CT. Aggressive features, previously described for ameloblastomas, include a large solid component, osseous and/or tooth root destruction and extraosseous extension. Such features support the malignant nature of the tumour and increased recurrence and/or metastatic incidence.10 Gadolinium-enhanced images can demonstrate strong enhancement in solid components of the tumour, including papillary projections, walls and septa. Ameloblastic carcinoma generally exhibits more aggressive features such as dystrophic calcifications, mixed solid and cystic regions, which have led some authors to recommend MRI as the best imaging modality.11,12
Although rarely employed, PET-CT has been used in patients with ameloblastoma to demonstrate metastatic disease not reliably identified on other imaging modalities.13 Two recent articles discuss the use of PET-CT in the diagnosis, characterization and metastatic spread of recurrent ameloblastoma.13,14 A literature review demonstrated no reference to PET-CT in the setting of ameloblastic carcinoma.
Advanced imaging techniques including MRI and PET-CT aided in the diagnosis, characterization, pre-operative planning and post-operative restaging. CT demonstrated the aggressive nature of the tumour including the presence of significant cortical and tooth root destruction. MRI images reinforced the aggressive nature of the tumour, demonstrating large solid components, heterogeneous enhancement, cystic degeneration and extraosseous extension. MRI was essential to evaluate intraosseous and extraosseous tumour extension and for perineural invasion, which would have complicated the surgical resection and reconstruction and changed the medical and surgical management. In this case, pre-operative CT and MRI imaging features suggested a malignant tumour as opposed to ameloblastoma, important information that guided patient counselling regarding operative expectations and risks as well as likelihood of post-operative chemotherapy, radiotherapy and possibility of recurrence. Resection of mandibular masses is an extensive and potentially disfiguring surgery; therefore pre-operative understanding of the extent and malignant potential of the tumour is essential to achieve a curative and cosmetically acceptable result. Although PET-CT was not readily available when this patient was initially diagnosed, it proved essential for restaging when tumour recurrence was suspected. PET-CT distinguished between isolated local recurrence and distant metastatic spread and identified two pulmonary metastases in this case, which was essential to guide surgical and oncological treatment.
Although only pathological analysis can conclusively differentiate ameloblastic carcinoma from ameloblastoma, MRI can provide useful information regarding aggressiveness and extent of the lesion and guide the surgical approach and surveillance schedule. Pre-operative PET-CT is useful for initial staging and pre-operative planning including primary mass and lymph node resection, although it was not available in our case. Post-operative MRI and PET-CT can aid in the differentiation of post-operative fibrosis and tumour recurrence as well as restaging for potential local nodal and distant metastatic disease.12
In conclusion, we have reported CT, MRI and PET-CT findings in a case of an ameloblastic carcinoma of the mandible with metastasis to the skull and lung 4 years after the initial treatment, unlike typically reported cases of ameloblastic carcinoma with isolated metastases to the lung. Advanced imaging techniques played an essential role in the diagnosis, surgical planning, treatment and surveillance.
Acknowledgments
The authors would like to thanks Dr Robert Pistey, Department of Pathology, Boston Medical Center, Boston, MA, and Dr Shveta Arya. Department of Pathology, Boston Medical Center, Boston, MA.
References
- 1.Inoue N, Shimojyo M, Iwai H, Ohtsuki H, Yasumizu R, Shintaku M, et al. Malignant ameloblastoma with pulmonary metastasis and hypercalcemia. Report of an autopsy case and review of the literature. Am J Clin Pathol 1988;90:474–481 [DOI] [PubMed] [Google Scholar]
- 2.Newman L, Howells GL, Coghlan KM, DiBiase A, Williams DM. Malignant ameloblastoma revisited. Br J Oral Maxillofac Surg 1995;33:47–50 [DOI] [PubMed] [Google Scholar]
- 3.Benlyazid A, Lacroix-Triki M, Aziza R, Gomez-Brouchet A, Guichard M, Sarini J. Ameloblastic carcinoma of the maxilla: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:e17–e24 [DOI] [PubMed] [Google Scholar]
- 4.Ozlugedik S, Ozcan M, Basturk O, Deren O, Kaptanoglu E, Adanali G, et al. Ameloblastic carcinoma arising from anterior skull base. Skull Base 2005;15:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azumi T, Nakajima T, Takeuchi S, Fukushima M, Ishiki T. Malignant ameloblastoma with metastasis to the skull: report of case. J Oral Surg 1981;39:690–696 [PubMed] [Google Scholar]
- 6.Slootweg PJ. Malignant odontogenic tumours: an overview. Mund Kiefer Gesichtschir 2002;6:295–302 [DOI] [PubMed] [Google Scholar]
- 7.Barnes L, Eveson JW, Reichert P, Sidransky D. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press: 2005, pp 284. [Google Scholar]
- 8.Avon SL, McComb J, Clokie C. Ameloblastic carcinoma: case report and literature review. J Canad Dent Assoc 2003;69:573–576 [PubMed] [Google Scholar]
- 9.Dunfee BL, Sakai O, Pistey R, Gohel A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics 2006;26:1751–1768 [DOI] [PubMed] [Google Scholar]
- 10.Minami M, Kaneda T, Yamamoto H, Ozawa K, Itai Y, Yoshikawa K, et al. Ameloblastoma in the maxillomandibular region: MR imaging. Radiology 1992;184:389–393 [DOI] [PubMed] [Google Scholar]
- 11.Asaumi J, Hisatorni M, Yanagi Y, Matsuzaki H, Choi YS, Kawai N, et al. Assesment of ameloblastomas using MRI and dynamic contrast-enhanced MRI. Eur J Radiol 2005;56:25–30 [DOI] [PubMed] [Google Scholar]
- 12.Minami M, Kaneda T, Ozawa K, Yamamoto H, Itai Y, Ozawa M, et al. Cystic lesions of the maxillomandibular region: MR imaging distinction of odontogenic keratocysts and ameloblastomas from other cysts. AJR Am J Roentgenol 1996;166:943–949 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen BD. Malignant ameloblastoma with thoracic vertebral metastasis: PET/CT and MR imaging. Clin Nucl Med 2005;30:450–452 [DOI] [PubMed] [Google Scholar]
- 14.Auluck A, Shetty S, Desai R, Mupparapu M. Recurrent ameloblastoma of the infratemporal fossa: diagnosis implications and a review of the literature. Dentomaxillofac Radiol 2007;36:416–419 [DOI] [PubMed] [Google Scholar]