Abstract
Objectives
The purpose of this study was to determine sonographically, in parotid glands of human immunodeficiency virus-positive patients, the condition of glands with or without enlargement, and propose a classification system for the patterns observed using diagnostic ultrasound imaging.
Methods
In this prospective clinical study, ultrasound scans were performed on 200 patients aged 4–62 years at Mulago Hospital, Uganda.
Results
There were four main distinct ultrasound pathological patterns in the parotids, i.e. lymphocytic aggregations (LAs), lymphoepithelial cysts (LECs), fatty infiltration (FI) and lymphadenopathy only. There were additional subdivisions depending on the presence of echogenic foci and intraparotid lymphadenopathy. Of those patients (n = 64) without parotid enlargement, only 8% showed normal ultrasound features, whereas 34% showed LECs and 31% showed LAs. Of those (n = 136) with parotid enlargement, 46% showed LECs, 23% showed FI and 15% showed LAs. The overall prevalence of LECs in the study sample was 42%. LECs were multiple, mainly between 7 mm and 12 mm in diameter and 26% showed internal echogenic foci either mobile or stationary. In contrast, LAs tended to be ill-defined, less than 5 mm and were not associated with posterior acoustic enhancement. Features differentiating LAs from LECs have not been previously described. Parotid FI (lipodystrophy) was noted in patients on highly active antiretroviral therapy, who showed lesser prevalence of LECs after 12 months of treatment.
Conclusions
Our study of 200 patients is probably the largest such study in the English language literature. The wide spectrum of diagnostic ultrasound patterns was categorized into four main groups (ten subgroups).
Keywords: parotid gland, human immunodeficiency virus infection, ultrasonography, lymphoepithelial cysts, lymphocytic aggregations
Introduction
Human immunodeficiency virus (HIV) infection was first recognized in Uganda in 1982. By the end of 2003, 70% of the world's affected population were living in the sub-Saharan region of Africa,1 and in Uganda alone 78 000 people died in 2003. Even though HIV prevalence in Uganda has reportedly fallen from 30% in 1987 to 6.1% in 2007,2 it still remains very high, and acquired immunodeficiency syndrome (AIDS) is still claiming tens of thousands of lives each year. The number of people living with AIDS in Uganda is estimated at one million. Uganda has been hailed as a rare success story in the fight against HIV and AIDS, widely viewed as having the most effective national response in sub-Saharan Africa. There has always been political openness and honesty about the epidemic, the risks and how they might best be avoided. The approach used in Uganda has been named the ABC approach (abstinence, be faithful, condoms).
HIV manifests as a myriad of signs and symptoms. Enlargement of the parotid glands is a frequent occurrence, estimated at 6–10% overall incidence3,4 and up to 30% in paediatric cases.5 Occasionally, parotid swelling is the first sign of HIV infection in the patient. The parotid swellings can be very large and disfiguring (Figures 1 and 2), and are often due to lymphoepithelial cysts (LECs). LECs with cervical lymphadenopathy are considered to be pathognomonic for HIV infection. It has also been documented that parotid disease in HIV-1 infection has increased from 6–10%3,4 to 51% in AIDS.6 HIV-related parotid swellings are often bilateral, soft, painless, asymmetrical and slow growing and are associated with persistent generalized cervical lymphadenopathy. Patients have a classical presentation of decreased CD4 cell counts, whereas the CD8 cell counts are often raised.7
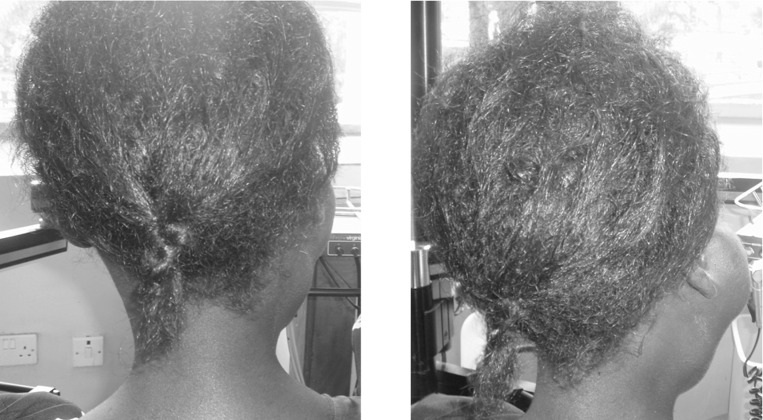
Figure 1.
Extremely large unilateral parotid swelling in a young woman. Note distortion of right ear lobe and undulating contour of parotid
Figure 2.

Patient with bilateral asymmetrical parotid swellings
In Uganda, parotid swellings, as per hospital protocol for all other swellings, are routinely subjected to blind aspiration biopsy and open excision without any diagnostic imaging investigations. HIV patients with large parotid swellings also tend to request surgical reduction for aesthetic reasons. However, surgery in such cases is difficult owing to the presence of multiple cysts, and recurrence is common. The cosmetic results of surgery are often poor, sometimes even worse than the initial presenting cosmetic complaint. Thus it is necessary to find a reliable non-invasive method of diagnosing HIV-related parotid lesions, and to avoid surgery where possible. With the recent availability of local expertise and equipment, ultrasound investigation provides non-invasive diagnosis and obviates surgical intervention. Ultrasound imaging is already widely accepted worldwide for diagnosis of soft tissue swellings of the head and neck region and is used by some researchers for imaging the parotid glands in HIV patients.4,8–12 The relative low cost of ultrasound imaging is an important consideration for resource-constrained African countries.
Aims and objectives
This prospective clinical study, using ultrasound imaging, was performed on HIV-positive patients in Mulago, Uganda, to:
determine the condition of the parotid glands, with and without enlargement
observe and categorize the diagnostic ultrasound patterns of parotid glands
determine the prevalence of the main ultrasound pattern groups.
Materials and methods
Ethics approval was obtained from the Research Committee at the Infectious Disease Institute, the Mulago Hospital Ethical Committee and the Research Ethics Committee at King's College London, UK (ref. no. CREC/06/07/180).
Patients were recruited from two centres. One was the Infectious Disease Institute in Mulago, a major referral outpatient clinic for HIV-positive patients, with daily patient numbers ranging from 200 to 300 patients. The other centre was the Oral Surgery Outpatient Clinic in the new Mulago Hospital, a major national referral hospital for patients with diverse oral pathology mainly for trauma and tumours, with approximately 50 outpatients and 30 inpatients seen daily.
200 patients aged 4–62 years (mean = 34) were recruited; all were HIV positive as demonstrated by blood tests. The patients may or may not have had parotid swelling, past or present (unilateral or bilateral), or no swelling but with or without complaint of pain or discomfort in the parotid region. Both swelling and pain were self-reported.
The recruitment process took place every working day over a period of 4 months. All potential patients were addressed in the main reception area as they arrived at the hospitals. They were addressed again in smaller groups of about 15 while in the waiting room awaiting review by the general practitioner. The purpose of the study was explained to the whole group, and all were invited to join the study. Those patients who agreed to participate were then individually interviewed by the first author and answered a questionnaire about their HIV manifestations and treatment. They were asked to give written informed consent for ultrasound imaging to be carried out. A slightly different consent form was used for minors, which explained the study in simpler terms, and their parents or guardians gave written consent. An appointment was then given for ultrasound scan after a period of 4–24 h.
Exclusion criteria were applied to those too weak to walk to the ultrasound department, which was approximately 150 m away; all critically ill patients; those with draining abscesses in the parotid area; those who had had any surgical intervention in the parotid glands, facial nerve palsy, meal related swellings or any signs of rheumatoid arthritis. Some patients who were already involved in other research studies did not volunteer for this study.
Out of the study group of 200 patients, 136 presented with parotid swelling and the remaining 64 did not. There were 140 females and 60 males. In terms of drug treatment, 78 patients were taking highly active antiretroviral therapy (HAART) and Septrin, 120 were on Septrin or Dapsone, and 2 patients were not on drug treatment. The range (interquartile) of CD4 cell counts was 6–902 cells per mm3, with 309 as median.
Ultrasound scanning was performed using a broadband linear probe at a frequency between 5 MHz and 12 MHz (Medison, Korea Doechi-Dong, Kangnum-Ku, Korea). Scanning was performed by the first author and always in the presence of one of two other experienced ultrasound radiologists. All three had agreed at the beginning of the study the terminology to describe various ultrasound features (Table 1), and interpretation of images was standardized by the three radiologists together scanning more than 10 non-study patients in order to ensure consensus and consistency.
Table 1. Sonographic features in the parotid gland, how they are described, what they look like and how to interpret them.
| Feature | Descriptors of individual feature | Appearance | Interpretation |
| Echogenicity | Isoechoic | Mid-grey, medium echoes | Normal parotid gland |
| Anechoic | Black, no echoes | May be cyst, neoplasm or other | |
| Hypoechoic | Dark grey, weak echoes | May be fatty degeneration | |
| Hyperechoic | Light grey or white, strong echoes | ||
| Echotexture | Homogeneous | Even texture | Normal parotid gland |
| Heterogeneous | Mixture of high and low echogenicity, coarse texture | ||
| Heterogeneous echotexture | Hypoechoic or anechoic areas or nodules | Dark grey or black | May be cyst, lymph node, neoplasm or other |
| Size | Measure using electronic cursors | ||
| Shape | Round or other | ||
| Margins | Distinct or not | ||
| Number | Single or multiple | ||
| Distribution | Local or whole gland | ||
| Posterior acoustic enhancement | Increased echo strength in the area beyond (“behind”) a nodule | Associated with cystic lesion (because fluid inside the cyst allows better transmission of ultrasound) | |
| Echogenic foci | Small white spots, mobile or stationary, scattered or clumped | May be debris or particles (clumps of cells, protein or other) suspended in fluid; if strong, echoes may be microcalcifications | |
| Posterior acoustic shadow | Black (vertical) stripe typically beyond echogenic foci, may be narrow or wide | Associated with microcalcifications or possibly gas bubbles (narrow); associated with mandible (wide). Caused by ultrasound being unable to penetrate calcified structures | |
| Hyperechoic areas | Light grey or white | May be salivary stone or mucus plug | |
| Size | Measure using electronic cursors | ||
| Shape | Round or other | ||
| Margins | Distinct or not | ||
| Number | Single or multiple | ||
| Hyperechoic lines | Light grey or white lines | Salivary ducts, fatty septae or nerve (rarely seen) | |
| Hyperechoic lines with posterior attenuation | White lines, beyond which echogenicity is much reduced | Gland probably replaced by fatty tissue (thus attenuates ultrasound) | |
| Lymph nodes | Hypoechoic with echogenic hilum which may be vascular | Black or dark grey, round or oval shape, distinct margins, may be single or multiple | Normal hilum has linear shape. Reactive node has larger more rounded hilum which is vascular |
| Location/position | Intraparotid or extraparotid | ||
| Vascularity (colour Doppler) | Present | Colour line(s), may pulsate | Differentiates blood vessels from other anechoic linear structures |
| Present, site central | Colour line(s) inside a nodule | Associated with reactive lymph node (vascular hilum), neoplasm or other | |
| Present, site peripheral | Colour line(s) at edge of nodule | Associated with neoplasm | |
| Absent | May be normal. May indicate necrosis if inside a heterogeneous nodule |
For each patient in the study, at the time of the ultrasound scan, a detailed report was written by the first author in consultation with the other radiologist present. In the parotid glands, all relevant features were carefully recorded, i.e. echotexture of the whole gland, echogenicity, size and margins of each nodule, detection of lymph nodes as well as their size and position, whether intraparotid or peri/extraparotid, the presence or absence of echogenic foci and any associated vascularity as determined using colour Doppler ultrasound. For each patient, a diagnosis was made based on the pattern of sonographic features (Table 2). Where the left and right sides were asymmetrical, diagnosis was made based on the more severely affected side.
Table 2. Categorization of sonographic patterns that form the main diagnoses in parotid glands; proposal for a new classification system.
| Diagnosis | Pattern of features |
| Normal parotid | Homogeneous and isoechoic |
| Occasional normal intraparotid lymph nodes | |
| Lymphocytic aggregations | Heterogeneous appearance |
| “Coarse” echotexture | |
| Diffuse mainly small hypoechoic or anechoic areas interspersed within normal isoechoic areas | |
| Moderate to ill-defined margins | |
| Size usually less than 5 mm | |
| Not associated with posterior acoustic enhancement | |
| Subgroups | |
| Internal echogenic foci | |
| May be microcalcifications | |
| Intraparotid lymphadenopathy | |
| Lymphoepithelial cysts | Heterogeneous appearance |
| Prominent round hypoechoic area | |
| Well-circumscribed margins | |
| Size usually > 5 mm, up to several cm | |
| Internal septa | |
| Posterior acoustic enhancement | |
| Subgroups | |
| Internal echogenic foci | |
| May be microcalcifications | |
| Intraparotid lymphadenopathy | |
| Fatty infiltration | Whole gland hypoechoic, with posterior attenuation |
| Subgroup | |
| Intraparotid lymphadenopathy | |
| Lymphadenopathy | Oval-shaped hypoechoic areas or nodules |
| Echogenic hilum with hilar blood flow seen on colour Doppler |
In addition to the parotid glands, ultrasound scanning was carried out of the submandibular regions, for salivary glands and lymph nodes, and of the deep cervical chains, posterior triangles and thyroid gland. Both axial and coronal planes were viewed for all structures, and also oblique planes where necessary. Several patients including those with mobile echoes within LECs were selected to undergo ultrasound-guided fine needle aspiration cytology (FNAC) using a 22 gauge needle. Owing to financial constraints, FNAC could not be performed on all patients.
Results
Within the study population, there were four distinct pathological patterns of ultrasound features within the parotid glands, with three patterns showing further subdivisions depending on the presence of echogenic foci and intraparotid lymphadenopathy (Table 3). In total there were 10 recognizable patterns, including normal. The four main patterns were lymphocytic aggregations (Figure 3a–c), lymphoepithelial cysts (Figure 4a–d), fatty infiltration (Figure 5a,b) and lymphadenopathy only (Figure 6). The main ultrasound patterns, subdivisions and their relation to the patients' clinical presentation are summarized in Table 3. Of the main ultrasound patterns, lymphocytic aggregations (LAs) and LECs shared some similarities and also had certain differences, which are summarized in Tables 2 and 4. For those patients on antiretroviral drug treatment, the relationship between the ultrasound patterns and the length of treatment is summarized in Table 5.
Table 3. Types of intraparotid sonographic patterns, their prevalence and relation to clinical presentation.
| Ultrasound pattern | Totals | Ranking order of frequency | No swelling | Unilateral swelling | Bilateral swellings | |
| Normal | 5 | 9 | 5 | 0 | 0 | |
| Lymphocytic aggregations | Alone | 6 | 8 | 4 | 1 | 1 |
| + echogenic foci | 6 | 8 | 4 | 1 | 1 | |
| + lymphadenopathy | 28 | 3 | 12 | 1 | 15 | |
| Lymphoepithelial cysts | Alone | 14 | 7 | 1 | 4 | 9 |
| + echogenic foci | 22 | 4 | 2 | 6 | 14 | |
| + lymphadenopathy | 48 | 1 | 19 | 10 | 19 | |
| Fatty infiltration | Alone | 20 | 5 | 5 | 0 | 15 |
| + lymphadenopathy | 19 | 6 | 3 | 2 | 14 | |
| Lymphadenopathy only | 32 | 2 | 9 | 1 | 22 | |
| Total | 200 | 64 | 26 | 110 | ||
Lymphoepithelial cysts with lymphadenopathy is the most common category and is ranked number 1 in the “ranking order of frequency” column. The other patterns are ranked accordingly
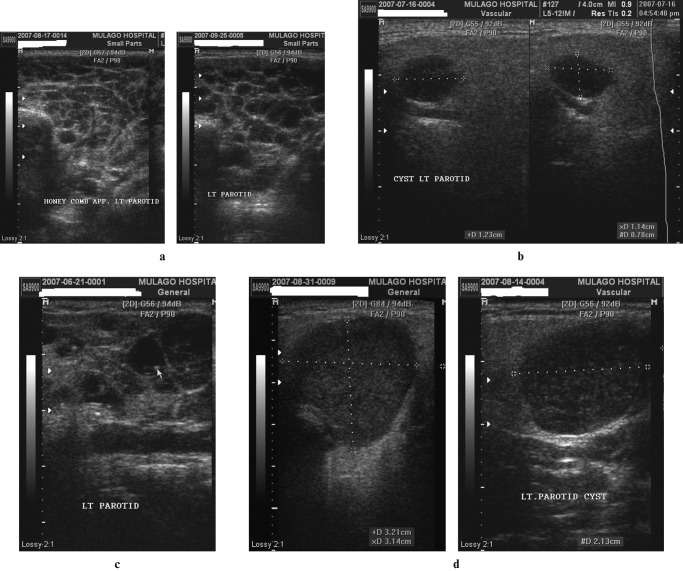
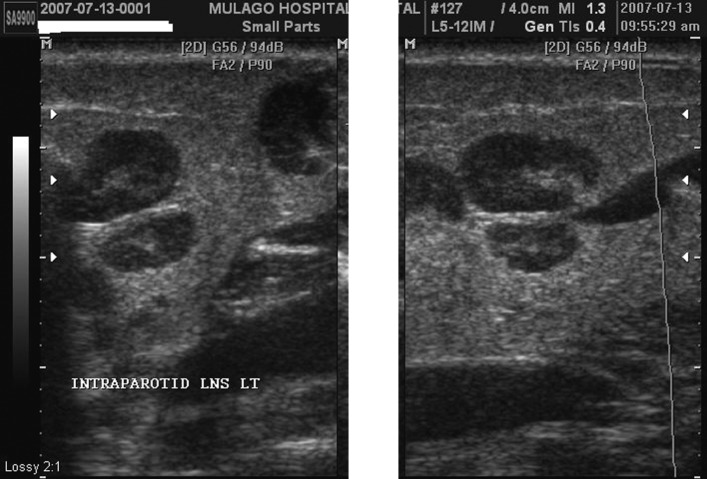
Figure 3.
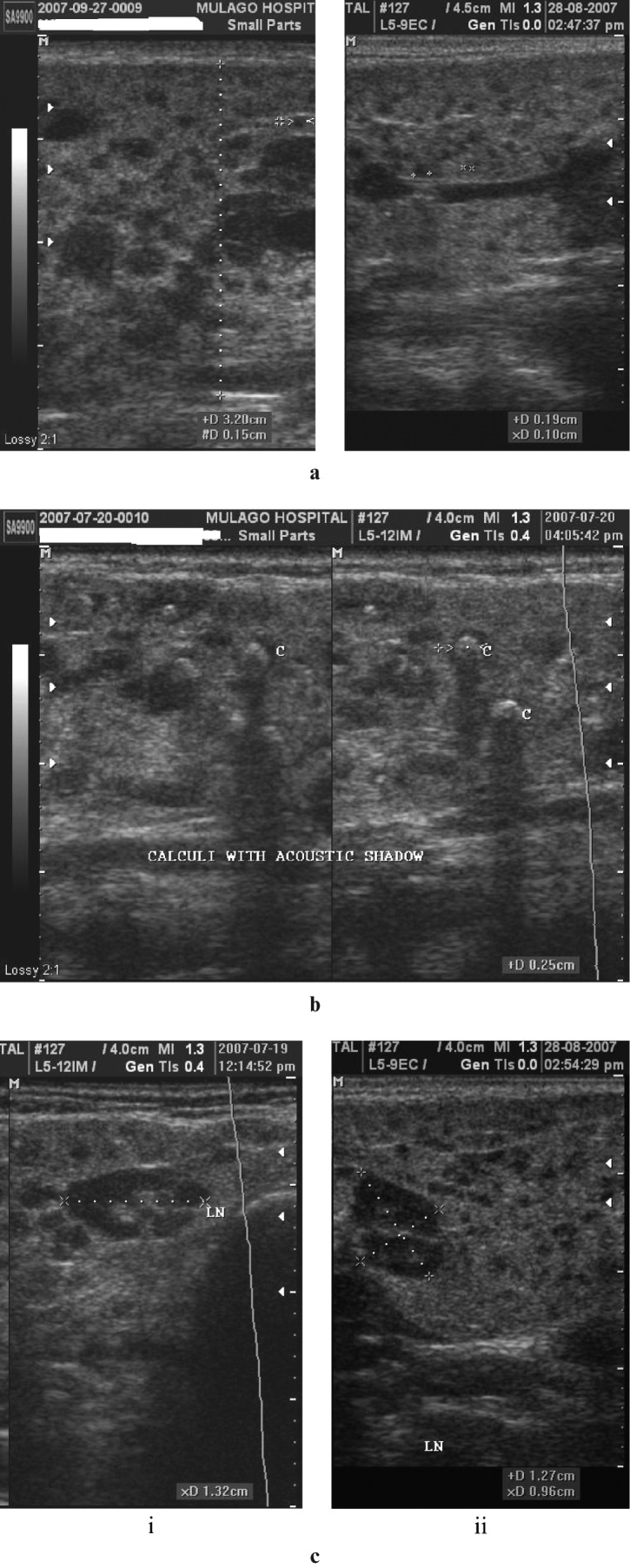
(a) Lymphocytic aggregations. Coronal scans of the parotids demonstrating a heterogeneous appearance, with diffuse, mainly small, hypoechoic or anechoic areas interspersed within apparently normal echogenic gland substance. (b) Lymphocytic aggregations with internal echogenic foci (two images juxtaposed). The coronal scans of the right parotid gland appear heterogeneous. Within hypoechoic areas are numerous echogenic foci resembling microcalcifications (short hyperechoic lines with dark posterior acoustic shadows). (c) Lymphocytic aggregations with lymphadenopathy. Axial (i) and coronal (ii) scans of the right parotid gland, demonstrating asymmetrical distribution of multiple hypoechoic areas, together with intraparotid lymph nodes (LN). Electronic cursors show the size of one of the nodes in three dimensions, approximately 13 × 13 × 10 mm
Figure 4.
(a) Lymphoepithelial cysts: honeycomb multicystic pattern. Transverse sections of the left parotid in a patient with bilateral parotid enlargement. There is a prominent honeycomb pattern formed by lymphoepithelial cysts of approximately the same size occupying almost all of the parotid gland substance. (b) Solitary lymphoepithelial cysts without internal mobile echoes (two images juxtaposed). Coronal sections of the left parotid in a patient with bilateral parotid enlargement. There are scattered solitary hypoechoic nodules seen with posterior acoustic enhancement. The largest cyst measures 11 × 12 × 8 mm, as shown by the electronic cursors. (c) Lymphoepithelial cysts with internal stationary echogenic foci. Coronal scan of a parotid demonstrating glandular tissue being replaced by cystic areas of varying sizes. Some of the cysts have septations whereas others have echogenic foci with posterior acoustic shadows. (d) Lymphoepithelial cysts with internal mobile echoes. Axial and coronal scans of the left parotid in a patient with bilateral parotid enlargement. There are multiple hypoechoic and heterogeneous cysts that exhibit posterior acoustic enhancement and multiple minute internal mobile echoes
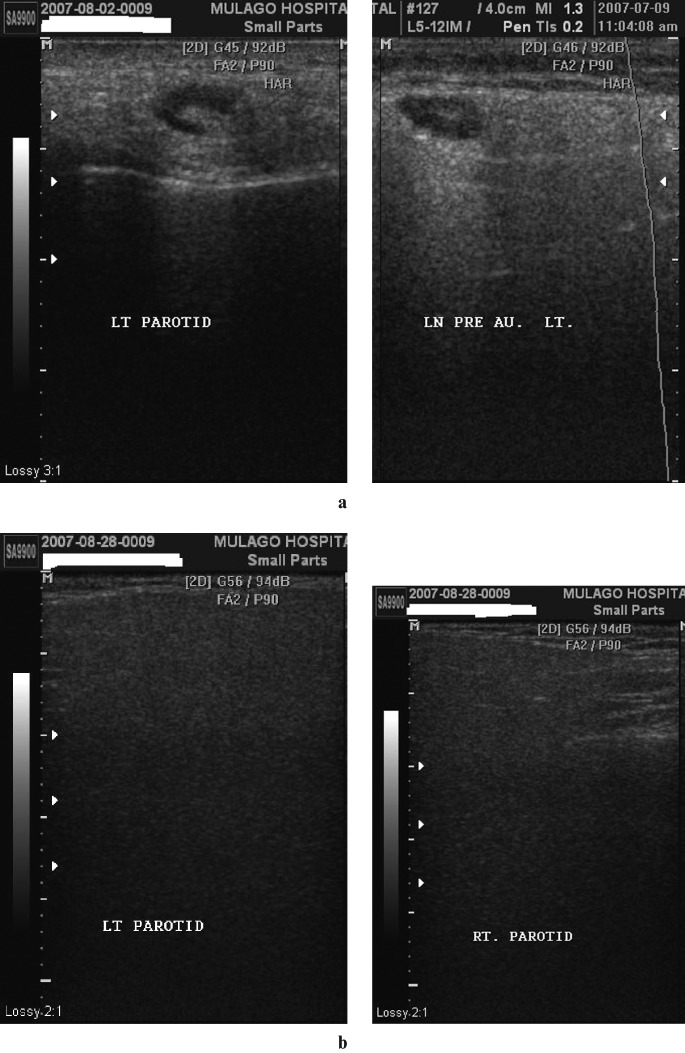
Figure 5.
(a) Fatty infiltration with lymphadenopathy. Coronal sections of a parotid showing an enlarged reactive lymph node within the gland. Note the prominent echogenic hilum and modest posterior acoustic enhancement. (b) Fatty infiltration without lymphadenopathy. Coronal sections of left and right parotids which appear very hypoechoic and homogeneous with posterior attenuation, rendering the images very dark. There are no cysts or lymph nodes
Figure 6.
Lymphadenopathy only. Coronal sections of the parotid glands of a patient with post-auricular lymphadenopathy. There are multiple intra- and extraparotid lymph nodes appearing as hypoechoic elliptical areas with a central, hyperechoic, fatty hilum. No intraparotid cysts are seen
Table 4. Characteristics of lymphoepithelial cysts and lymphocytic aggregations.
| Lymphoepithelial cysts (n = 84) | Lymphocytic aggregations (n = 40) | ||
| Prevalence (%) (out of 200) | 42 | 20 | |
| Number of lesions in each affected gland | < 10 | 17 (20%) | 1 (2%) |
| ≥ 10 | 67 (80%) | 39 (98%) | |
| Size of lesions (mm) | Mean (range) | 10 (2–75) | 3 (1–7) |
| < 5 mm | 28 (33%) | 38 (95%) | |
| 5–10 mm | 44 (52%) | 2 (5%) | |
| > 10 mm | 12 (14%) | 0 | |
| Mobile echoes/echogenic foci (%) | 22 (26%) | 6 (15%) | |
Table 5. Relationship between the diagnostic ultrasound patterns and patients' length of antiretroviral drug treatment to date (n = 78).
| Ultrasound pattern | Length of drug treatment |
|||
| <6 months | 6–12 months | >12 months | Total | |
| Normal | 0 | 0 | 0 | 0 |
| LA | 2 | 0 | 2 | 4 |
| LA + echogenic foci | 0 | 0 | 0 | 0 |
| LA + lymphadenopathy | 5 | 2 | 4 | 11 |
| LEC | 1 | 1 | 0 | 2 |
| LEC + echogenic foci | 3 | 1 | 2 | 6 |
| LEC + lymphadenopathy | 5 | 1 | 3 | 9 |
| FI | 2 | 4 | 14 | 20 |
| FI + lymphadenopathy | 2 | 4 | 13 | 19 |
| Lymphadenopathy only | 0 | 5 | 2 | 7 |
| Total | 20 | 18 | 40 | 78 |
FI, fatty infiltration; LA, lymphocytic aggregations; LEC, lymphoepithelial cysts
Out of the study group of 200 patients, 136 (68%) patients presented with parotid enlargement. Of those without parotid enlargement, only 8% (5) showed normal parotid features on ultrasound, and 92% (59) showed various abnormal parotid features including 34% (22) who showed LECs and 31% (20) who showed LAs (Table 3). Among those with parotid swellings, the main diagnostic ultrasound patterns were LECs at 46% and fatty infiltration (FI) at 23%. Other patterns included LAs and plain lymphadenopathy at 15% and 17% respectively. No patient with parotid enlargement showed normal ultrasound appearance (Table 3). The prevalence of LECs in all patients, with or without parotid enlargement, was 42% (84 patients out of the total 200).
Discussion
Compared with other studies4,8–12 that use ultrasound imaging to investigate parotid enlargement in HIV-positive patients, our study involved the fairly large number of 200 patients. We also used a relatively modern, high-resolution ultrasound machine. Therefore, our study was able to demonstrate a very high degree of sonographic detail, and to categorize ultrasound diagnostic features into four main groups and ten subgroups of patterns. There are very few published reports that attempt to classify the appearance of HIV parotid disease on diagnostic imaging. Dave et al13 based their three-tiered classification system on four paediatric cases that were investigated with CT scans. Mandel10 recognized 3 patterns on the ultrasound scans of 13 patients. Vona et al9 performed ultrasound scans on 64 patients and described the presence of lymphocytic cysts and lesions and the persistence of glandular parenchyma, but they did not put forward a classification system. We are proposing a new classification system (Table 2), based on some suggestions by the above but mostly on the findings of our own study.
Prevalence of lymphoepithelial cysts
In our study, of those patients (n = 136) with parotid enlargement, 46% showed LECs. Of those patients (n = 64) with no obvious parotid swelling, 34% showed LECs. The overall prevalence of LECs in the study sample was 42%.
Comparison with other studies is difficult because most of them performed diagnostic imaging whether ultrasound, CT or MRI for patients only with visible swelling, whereas in our study we carried out ultrasound imaging on a large group of HIV patients with and without parotid swellings. In other studies, there is less distinction between LECs and other similar-looking lesions and some authors refer to LECs by different names. Nevertheless, it is interesting to note some past reports using ultrasonography as the diagnostic imaging modality. Mandel's study10 showed LECs in all 13 cases (100%). Similarly, Gooding et al12 showed LEC in all three cases (100%). In Vona et al's study9 of 64 cases, the prevalence of “anechoic and non-homogeneous areas” (similar appearance to our LECs) was 60%. In Goddart et al's study11 4 out of 24 HIV-positive children had parotid enlargement and these 4 children showed “acinar enlargement” on ultrasound (100%). In Martinoli et al's study,8 from 9 patients 14 “parotid nodules” were sampled by ultrasound-guided FNAC and 10 were shown to be cysts. Soberman et al4 found that 10 out of 100 HIV-positive children had parotid enlargement; 3 of the 10 showed LECs on ultrasound and the other 7 “lymphoid infiltration”.
Some published reports seem to confuse LECs with parotid enlargement: they quote the same low (6–10%) prevalence rates for both. However, as we and others have found, prevalence of LECs is high. In addition, not all parotid enlargement can be attributed to LECs, and not all LECs manifest as clinically evident parotid enlargement.
Clinical presentation
In the study population, 68% (136) had visible parotid swelling and in fact the gross cosmetic impediment was their main presenting complaint. Out of the 64 patients who had no obvious swellings, 59 (92%) patients did actually have abnormalities in their parotid glands upon ultrasound scanning (Table 3). This was an unexpected finding and highlights the need to monitor the parotid glands from the outset. It also emphasizes the usefulness of ultrasound imaging to monitor the glands. When the swellings occurred they were more likely to be bilateral although asymmetrical, unlike Shugar et al's14 study, which reported the majority of patients as having unilateral swellings. Most of our patients reported swellings being unilateral initially but later becoming bilateral. This is similar to the results of Owotade et al,15 and suggests the progressive involvement of salivary gland tissue by the HIV infection. It is also possible that patients in developed countries, such as those in Shugar et al's study,14 tend to present at an earlier stage of the disease, whereas patients in developing countries, such as those in Owotade et al's study,15 are inclined to present at a later stage.
Lymphoepithelial cysts vs lymphocytic aggregations
Lymphoepithelial cysts are said to be pathognomonic of HIV infection. However, there is a similar-looking entity, known by some authors as, lymphoepithelial lesions or lymphocytic aggregations. These lesions have characteristics that do not completely fit those for LECs, and may well have different aetiology, although some authors do not always make a distinction, perhaps because of lack of high-resolution imaging. In this paper, the ultrasound characteristics of LECs and LAs are described in detail in Tables 2 and 4 and Figures 3 and 4. In our study, we have found that there appears to be a difference in size between these two entities. Individual lesions of LECs tend to be greater than 5 mm in diameter (mainly 7–12 mm) and are associated with posterior acoustic enhancement. On the other hand, individual lesions of LAs tend to be smaller than 5 mm and, in this study, are never associated with posterior acoustic enhancement. These potentially differentiating features do not appear to have been described previously.
Fatty infiltration
39 (19.5%) patients showed FI (lipodystrophy) in the parotid glands. Drug treatment using HAART is effective at reducing salivary gland enlargement.16 However, the protease inhibitors component of HAART has been shown to cause side-effects of parotid FI,17 paradoxically manifesting as parotid swelling. The length of time before this side-effect becomes clinically evident is not known. Our study suggested that after 12 months of drug treatment, FI was more prevalent. The lesser prevalence of LECs at this time explains the reduction in gland swelling, the intended purpose of HAART, and may be an objective measure of the success of drug treatment.
Echogenic foci
Of the 200 patients, 28 presented with echogenic foci within LECs and LAs. In 12 patients these were mobile echoes and in 16 they were stationary. Echogenic foci were each less than 1 mm and most probably were tiny particles suspended in fluids of variable viscosity. Some echogenic foci were accompanied by posterior acoustic shadows (Figure 3b), which would suggest the former to be microcalcifications or even possibly gas bubbles. Only a few reports have mentioned similar such entities. Dave et al13 identified multiple tiny radiopaque dots on non-contrast-enhanced CT scans in the parotids of one patient with bilateral parotid enlargement and interpreted the dots as microcalcifications. Vona et al9 showed sonograms of lymphoepithelial cysts which contained “high level echoes in suspension”. These proved to be crystals of calcium oxalate upon FNAC. In Dave et al's article13 microcalcifications were found in only one child out of the four in their study of patients who had benign LECs and were HIV positive. In our study, echogenic foci were common in the paediatric population.
In two of our patients in whom echogenic foci were found within large cysts, FNAC showed lymphocytes, neutrophils, macrophages and epithelial squames. We were able to neither prove nor disprove microcalcifications.
Surgery
Patients identified by ultrasound imaging as having HIV-related parotid enlargement were considered to be unsuitable for surgery, especially if they were seeking cosmetic improvement, and they were treated with drugs. Ultrasound also helped to reassure those who had feared the presence of a parotid neoplasm by effectively demonstrating the cause of the facial swelling.
Unsuspected disease
The ultrasound scan showed unsuspected other disease in a small number of patients. Eight patients had matted lymph nodes in the neck and were referred for investigations to rule out tuberculosis. Two patients had solid mass lesions in the neck and were referred for aspirational biopsy and treatment. The other patient died a week later from unknown cause.
Suggestions for future studies include ultrasound-guided FNAC to differentiate lymphocytic aggregations from lymphoepithelial cysts;
and serial ultrasound scans to monitor changes in ultrasound pattern in the parotids, particularly in relation to drug treatment.
In conclusion diagnostic ultrasound is the most appropriate imaging modality to investigate the parotid glands in HIV-positive patients. Even patients with no visible parotid enlargement are likely to have abnormalities that can be detected sonographically. There is a wide spectrum of ultrasound patterns, which can be categorized into four main groups (ten subgroups). Lymphocytic aggregations have some sonographic features that differentiate them from lymphoepithelial cysts. Our study of 200 patients, probably the largest such study in the English language literature, has found a high prevalence of lymphoepithelial cysts and lymphocytic aggregations in patients with and without parotid enlargement, as well as a high prevalence of FI in patients on highly active antiretroviral therapy.
Acknowledgments
The authors wish to acknowledge Mr William Nganwa, Consultant Oral and Maxillofacial Surgeon, Mulago Hospital, Dr Andrew Kambugu, Head, Clinical Services, Infectious Disease Institute, Mulago, Mr Dunstan Bagenda, statistician, MUJU, Mulago, and Ms Agnes Kiraga, statistician, Infectious Disease Institute, Mulago Hospital. This paper was the recipient of first prize in the Research Award competition at the 17th International Congress of Dentomaxillofacial Radiology.
References
- 1.Tindyebwa D, Kayita J, Musoke P, Eley B, Nduati R, Coovadia H, et al(eds) Handbook on paediatric AIDS in Africa. The African Network for the Care of Children affected with AIDS (ANECCA), 2006. Available from: http://www.fhi.org/en/HIVAIDS/pub/guide/mans1.htm/ [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Estimated number of people living with HIV by country, 1990–2007. Available from: http://www.unaids.org/en/KnowledgeCentre/HIVData/Epidemiology/latestEpiData.asp/ [Google Scholar]
- 3.Schiodt M, Greenspan D, Daniels TE, Nelson J, Leggott PJ, Wara DW, et al. Parotid gland enlargement and xerostomia associated with labial sialadenitis in HIV-infected patients. J Autoimmun 1989;2:415–425 [DOI] [PubMed] [Google Scholar]
- 4.Soberman N, Leonidas JC, Berdon WE, Bonagura V, Haller JO, Posner M, et al. Parotid enlargement in children seropositive for human immunodeficiency virus: imaging findings. Am J Roentgenol 1991;157:553–556 [DOI] [PubMed] [Google Scholar]
- 5.Williams MA. Head and neck findings in pediatric acquired immune deficiency syndrome. Laryngoscope 1987;97:713–716 [PubMed] [Google Scholar]
- 6.Vargas PA, Mauad T, Bohm GM, Saldiva PHN, Almeida OP. Parotid gland involvement in advanced AIDS. Oral Diseases 2003;9:55–61 [DOI] [PubMed] [Google Scholar]
- 7.Itescu S, Brancato LJ, Buxbaum J, Gregersen PK, Rizk CC, Croxson TS, et al. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med 1990;112:3–10 [DOI] [PubMed] [Google Scholar]
- 8.Martinoli C, Pretolesi F, Del Bono V, Derchi LE, Mecca D, Chiaramondia M. Benign lymphoepithelial parotid lesions in HIV-positive patients: spectrum of findings at gray-scale and Doppler sonography. AJR Am J Roentgenol 1995;165:975–979 [DOI] [PubMed] [Google Scholar]
- 9.Vona S, Colombo E, Damiani G, Bianco R, Cornalba GP. Salivary gland lesions in HIV positive patients. Eur Radiol 1994;4:434–438 [Google Scholar]
- 10.Mandel L. Ultrasound findings in HIV positive patients with parotid gland swellings. J Oral Maxillofac Surg 2001;59:283–286 [DOI] [PubMed] [Google Scholar]
- 11.Goddart D, Francois A, Ninane J, Vermylen Ch, Cornu G, Clapuyt Ph, et al. Parotid gland abnormality found in children seropositive for the human immunodeficiency virus (HIV). Pediatr Radiol 1990;20:355–357 [DOI] [PubMed] [Google Scholar]
- 12.Gooding GAW, Sooy CD, Hybarger CP. Ultrasonography of cystic parotid lesions in HIV infection. Similarity of sonographic appearance with Sjogren's syndrome. J Ultrasound Med 1992;11:35–39 [DOI] [PubMed] [Google Scholar]
- 13.Dave SP, Pernas FG, Roy S. The benign lymphoepithelial cyst and a classification system for lymphocytic parotid gland enlargement in the pediatric HIV population. Laryngoscope 2007;117:106–113 [DOI] [PubMed] [Google Scholar]
- 14.Shugar JM, Som PM, Jacobson AL, Ryan JR, Bernard PJ, Dickman SH. Multicentric parotid cysts and cervical adenopathy in AIDS patients. A newly recognized entity: CT and MR manifestations. Laryngoscope 1998;98:772–775 [DOI] [PubMed] [Google Scholar]
- 15.Owotade FJ, Fatusi OA, Adebiyi KE, Ajike SO, Folayan MO. Clinical experience with parotid gland enlargement in HIV infection: a report of five cases in Nigeria. J Contemp Dent Pract 2005;6:136–145 Available from: http://www.thejcdp.com/issue021/owotade/index_nlm.htm/. [PubMed] [Google Scholar]
- 16.Mandel L, Surattanont F. Regression of HIV parotid swellings after antiviral therapy: case reports with computed tomographic scan evidence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:454–459 [DOI] [PubMed] [Google Scholar]
- 17.Olive A, Salavert A, Manriquez M, Clotet B, Moragas A. Parotid lipomatosis in HIV positive patients: a new clinical disorder associated with protease inhibitors. Ann Rheum Dis 1998;57:749. [DOI] [PMC free article] [PubMed] [Google Scholar]