Abstract
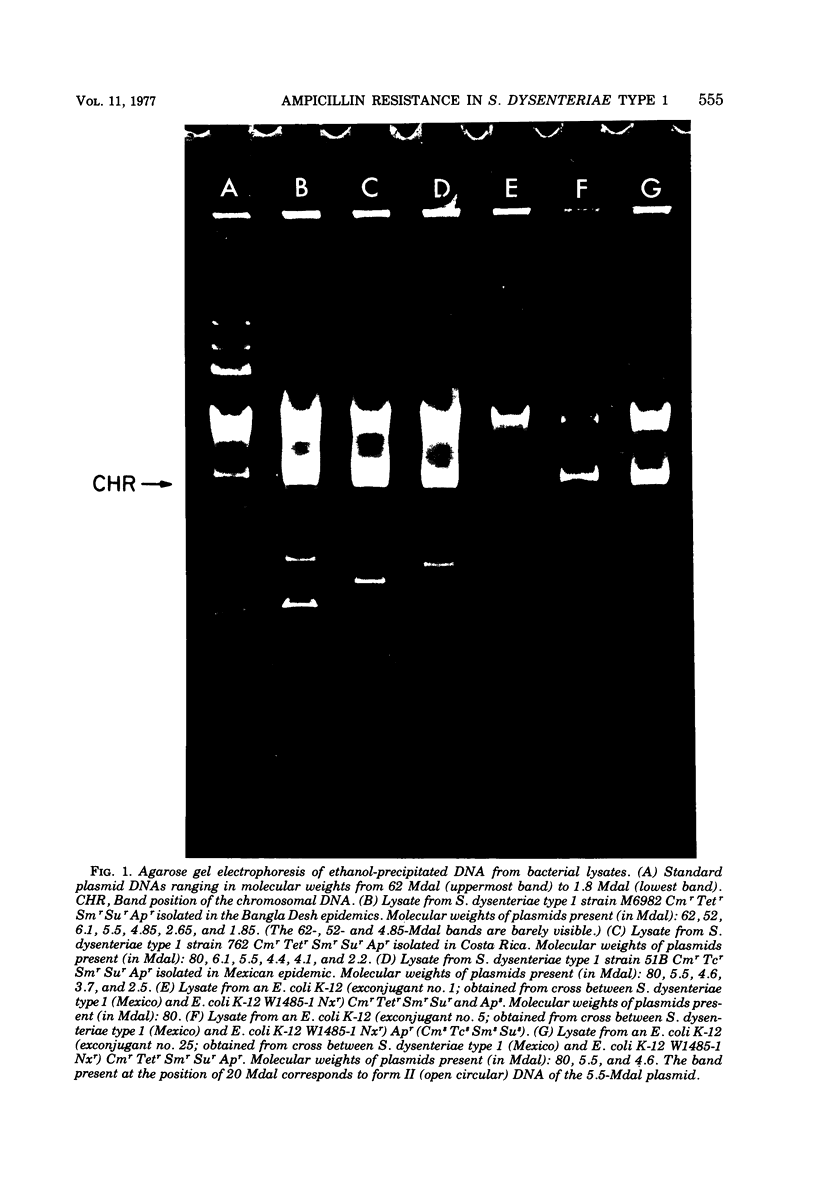
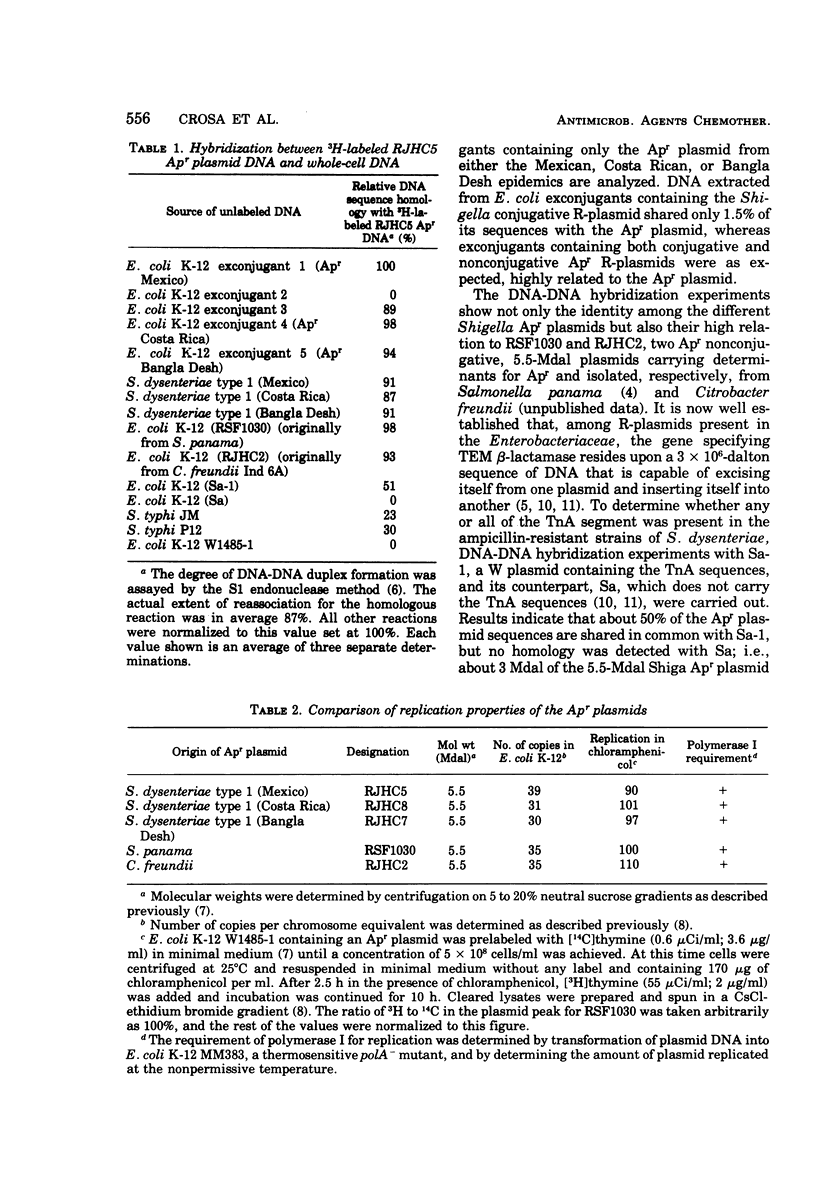
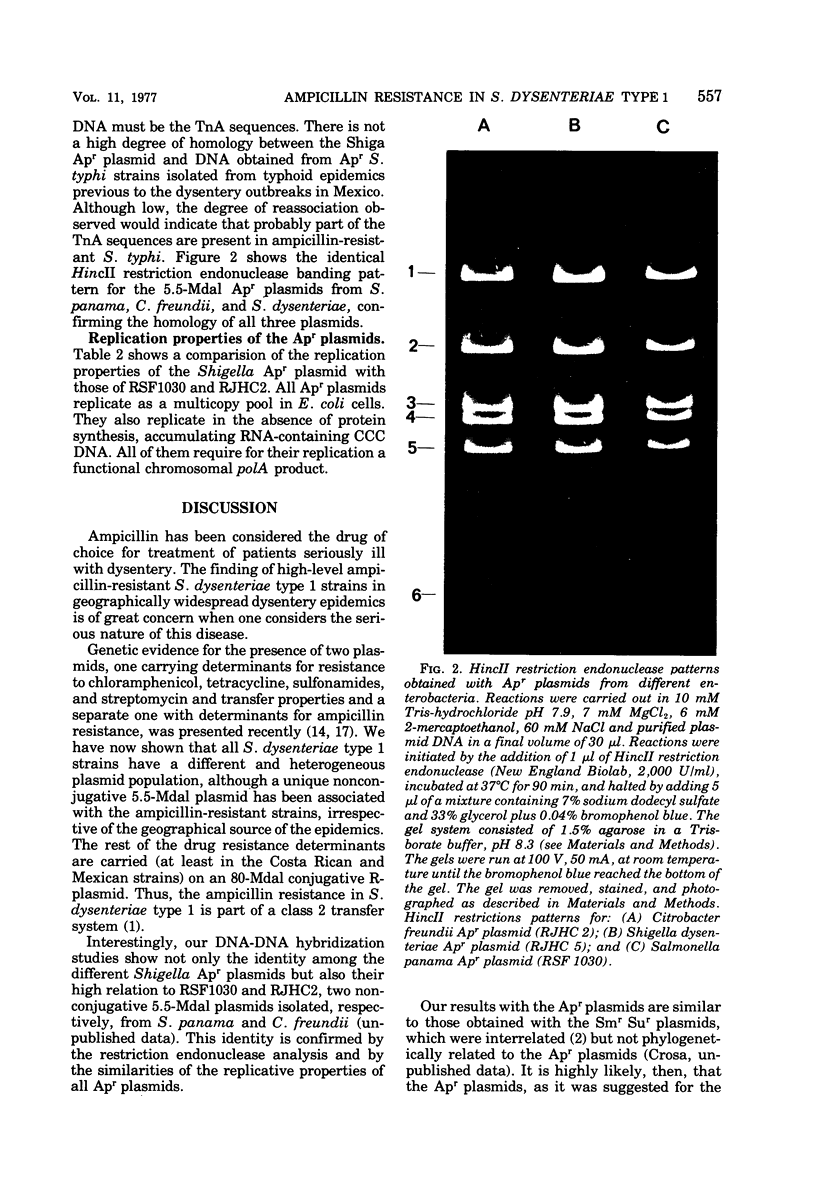
Ampicillin-resistant strains of Shigella dysenteriae type 1 isolated in epidemics in Mexico, Central America, and Bangla Desh were examined for the presence of plasmid deoxyribonucleic acid (DNA) by gel electrophoresis. All strains contained a heterogeneous population of plasmids. Transfer experiments to Escherichia coli K-12 indicated that the ampicillin resistance determinant (Apr) was located on a 5.5-megadalton (Mdal) plasmid identical in all Shiga strains examined, as judged by DNA hybridization and by its molecular properties. This 5.5-Mdal plasmid contained the ampicillin transposon (TnA) sequences. There was not a high degree of homology between the Shiga Apr plasmid DNA and DNA obtained from AprSalmonella typhi strains isolated from typhoid epidemics in Mexico, previous to the dysentery outbreaks. Although low, the degree of reassociation observed indicated that probably part of the TnA sequence was present in S. typhi DNA. The DNA hybridization experiments showed, in addition, that there was a high degree of homology among Apr plasmids isolated from different enterobacteria, and this identity was confirmed by restriction endonuclease activity. These results together with their similarities in molecular and replicative properties indicate that the Apr plasmids, as was suggested for the Smr Sur plasmids, possibly evolved once and then epidemiologically spread in the Enterobacteriaceae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bouanchaud D. H., Chabbert Y. A. Stable coexistence of three resistance factors (fi-) in Salmonella panama and Escherichia coli K12. J Gen Microbiol. 1969 Sep;58(1):107–113. doi: 10.1099/00221287-58-1-107. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Kopecko D. J. Structural evolution of bacterial plasmids: role of translocating genetic elements and DNA sequence insertions. Fed Proc. 1976 Jul;35(9):2031–2036. [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Nature of R-factor replication in the presence of chloramphenicol. Proc Natl Acad Sci U S A. 1975 Feb;72(2):654–658. doi: 10.1073/pnas.72.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata L. J., Gangarosa E. J., Cáceres A., Perera D. R., Mejicanos M. L. Epidemic Shiga bacillus dysentery in Central America. I. Etiologic investigations in Guatemala, 1969. J Infect Dis. 1970 Sep;122(3):170–180. doi: 10.1093/infdis/122.3.170. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olarte J., Filloy L., Galindo E. Resistance of Shigella dysenteriae type 1 to ampicillin and other antimicrobial agents: strains isolated during a dysentery outbreak in a hospital in Mexico City. J Infect Dis. 1976 May;133(5):572–575. doi: 10.1093/infdis/133.5.572. [DOI] [PubMed] [Google Scholar]
- Olarte J., Galindo E. Salmonella typhi resistant to chloramphenicol, ampicillin, and other antimicrobial agents: strains isolated during an extensive typhoid fever epidemic in Mexico. Antimicrob Agents Chemother. 1973 Dec;4(6):597–601. doi: 10.1128/aac.4.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman M. M., Huo I., Dey C. R., Kibriya A. K., Curlin G. Letter: Ampicillin-resistant Shiga bacillus in Bangladesh. Lancet. 1974 Mar 9;1(7854):406–407. doi: 10.1016/s0140-6736(74)93171-7. [DOI] [PubMed] [Google Scholar]