Abstract
A case of Gorham's disease in the maxilla of a 56-year-old male patient is described. The clinical presentation, radiographic and histopathological features and treatment are presented. A discussion of the current understanding of this rare disease, based on review of the literature, is offered.
Keywords: Gorham's disease; osteolysis; massive; maxilla; radiology, diagnostic X-ray
Case report
History and clinical examination
In 2003, radiographs of a 56-year-old man, complaining of pain and loose teeth in his left maxilla, were submitted for radiological consultation. Based on the ill-defined bone destruction seen radiographically (Figure 1a–c), a provisional diagnosis of a malignant neoplasm was made. A biopsy was taken from the periapical region of the left maxillary first molar. This tooth was vital, but root canal treatment was performed at the time of the periapical biopsy because of the possibility of devitalization of the tooth. No evidence of neoplasia or cellular atypia was seen. A diagnosis of a cholesterol granuloma of the maxillary sinus was made.
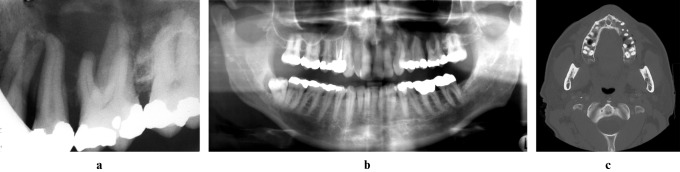
Figure 1.
Digitally enhanced periapical (a), digitally enhanced panoramic (b), and axial bone window CT (c) images from initial presentation (2003) depicting a region of ill-defined bone loss in the left maxillary alveolar process around the premolars and first molar
2 years later (2005) the patient was referred to the radiology service complaining of progressive loosening of the teeth in his left maxilla. He described feeling a non-painful fullness over the left maxillary canine. Upon examination a firm, non-mobile, non-tender swelling in the left nasolabial fold region was palpated. The entire left maxillary posterior segment was mobile and could be displaced as a unit. The teeth in the region were not sensitive to percussion. There was no regional lymphadenopathy. The patient was otherwise in good general health and was a non-smoker.
Radiographic examination
Plain radiographs and CTs revealed generalized ill-defined destruction of the left maxilla (Figure 2a–e). The osseous structures of the alveolar process on the left side were absent from the midline to the tuberosity, and the hard palate was resorbed nearly to the midline. The anterior wall of the maxillary sinus was missing and there was a small (1.7 cm) non-enhancing soft tissue mass in this region, corresponding to the swelling detected clinically. Soft tissue thickening was also noted along the buccal aspect of the roots of the maxillary teeth. The zygomatic process of the maxilla was partially destroyed, and the inferior extent of the posterolateral wall of the sinus was absent (Figure 2d). There was no adjacent soft tissue mass, either within the sinus or in the retroantral fat, to indicate an associated tumour as the cause of the bone destruction. There was no displacement or resorption of the teeth. No lymphadenopathy or masses elsewhere in the neck were identified.
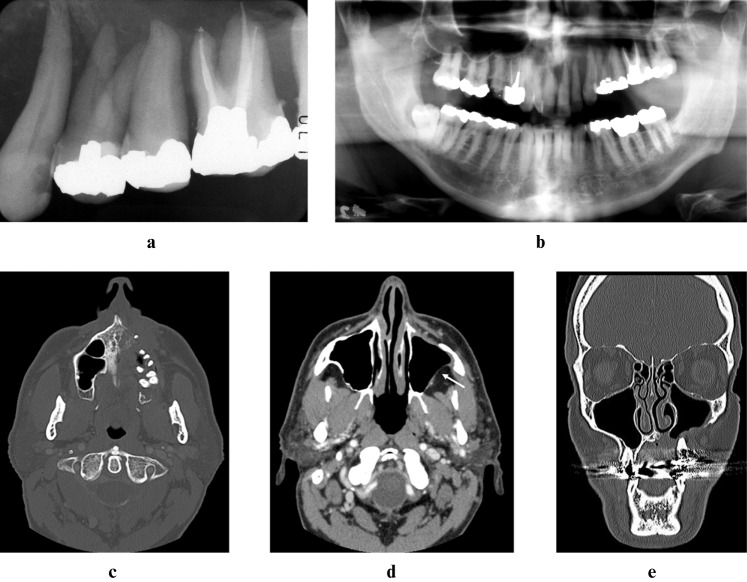
Figure 2.
Digitally enhanced periapical (a), digitally enhanced panoramic (b), axial bone window computed tomography (CT) (c), axial soft tissue-window CT (d) and coronal bone-window CT (e) images at second presentation (2005) demonstrating massive bone destruction of the left maxilla. Despite loss of the cortical boundaries, there is no soft tissue mass present in the maxillary sinus or in the retroantral fat (d, white arrow)
A provisional diagnosis of Gorham's massive osteolysis was made owing to the lack of clinical or radiological findings to suggest a neoplastic, inflammatory, infectious or metabolic condition. An incisional biopsy was made at the leading edge of the bony destruction, apical to the maxillary left lateral incisor and canine, in the region of the soft tissue mass.
Histological examination
The histological sections examined showed fibrous connective tissue with a patchy chronic inflammatory infiltrate consisting of lymphocytes, plasma cells and histiocytes (Figure 3a–c). The fibroblasts were spindled to stellate in appearance, with rare mitoses. Dispersed throughout the tissue were small blood vessels and trabecular bone with osteolysis. Osteoclasts were seen in areas of bone resorption, but they were not prominent. Reactive new bone formation was also noted. No evidence of granulomatous inflammation or neoplasia was detected. Fungal stains were negative.
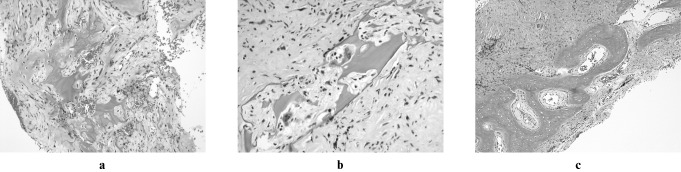
Figure 3.
Histological sections, haematoxylin and eosin. (a) Fibrous connective tissue with trabecular bone and small blood vessels (low power); (b) trabecular bone with osteolysis (high power); (c) reactive new bone (medium power)
Treatment and follow-up
The patient was referred to a tertiary oncology hospital for radiation therapy. He was planned to receive 45 Gy in 25 fractions but received 43.21 Gy in 24 fractions. Follow-up CT studies were obtained at 1, 10, 18 and 21 months after completion of treatment. There was neither progression nor resolution of the disease for 1.5 years. At 21 months further bone destruction was detected across the midline of the palate (Figure 4a–c). There remained no involvement of the mandible or skull base. The patient then completed 1 year of bisphosphonate therapy (pamidronate 90 mg intravenously every 3 months). A recent follow-up CT scan demonstrated progressive destruction of the maxilla as well as involvement of the floor of the orbit, suggesting that the therapy was not successful.
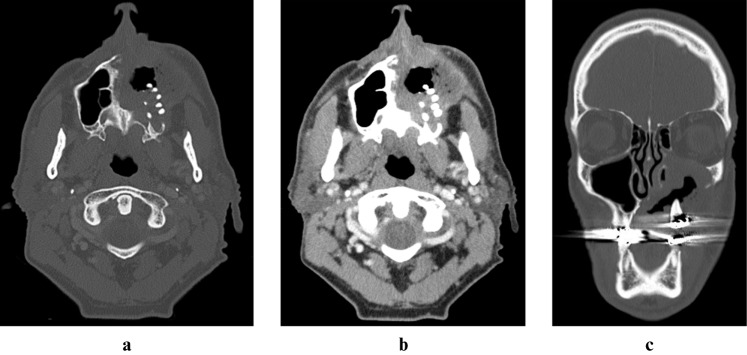
Figure 4.
Axial bone window (a), axial soft tissue window (b) and coronal bone window (c) CT images from 21 months after radiation therapy (2008) demonstrate progressive bone destruction of the maxilla and soft tissue thickening
A resin-bonded splint was used to stabilize the affected dentition; however, the patient required extraction of multiple teeth in the left maxilla because of loss of bone support. An oral antral fistula developed, subsequent to the extractions and because of the absence of the underlying bone. The patient's current concerns are severe pain in the left maxilla and an enlarging oral antral communication, which he manages with irrigation.
Discussion
Gorham's disease, also termed massive osteolysis and vanishing bone disease, is a very rare and destructive condition, resulting in the spontaneous and progressive regional resorption of osseous structures with replacement by fibrous tissue. This entity is named after the author who described two cases of patients with massive osteolysis involving the clavicle and scapula in 1954.1 Since then fewer than 150 cases have been reported in the literature2 with approximately 40 cases involving the maxillofacial skeleton.3
Children and young adults are most commonly affected, although patients ranging in age from 18 months to 72 years have been reported.4, 5 There is a slight male predilection.6 The most common sites of involvement are the shoulder and pelvis,7 although any bone may be affected. All previously reported cases involving the maxillofacial region have affected the mandible alone or with the maxilla, but never the maxilla alone,6, 8, 9 as in the case presented here. The clinical presentation is variable, and, in the jaws, pain, tooth mobility, pathological fracture, facial deformity and malocclusion are early signs.3, 6 Laboratory findings are unremarkable. Although the disease is progressive and can be severely disfiguring, it is rarely fatal. It may progress unremittingly over years but can also spontaneously arrest. The most common complications leading to death are chylothorax, when the ribs or thoracic vertebrae are involved, and transection of the spinal cord because of spine destruction.10
The cause of Gorham's disease is unknown. In 1955, Gorham and Stout11 characterized the histopathological findings in this condition and attributed the bone destruction to local vascular angiomatosis. They postulated that the proliferation of vessels creates a hyperaemia that could disturb the osteoblast to osteoclast balance and result in increased bone resorption. More recently, Hirayama et al12 reported that the number of circulating osteoclast precursors was no higher in a patient with massive osteolysis than in age- and sex-matched control subjects. Rather, the osteoclast precursors of the patient with osteolysis were found to be more sensitive to stimulating and humoral factors, such as receptor activator of nuclear factor kappa B ligand (RANKL) and interleukin-6, and produce greater lacunar resorption than the control cells. Devlin et al13 suggested that enhanced osteoclast activity, related to elevated serum interleukin-6, contributes to the bone resorption of Gorham's disease. Trauma has also been implicated as an aetiological factor2, 4, 13 although it is not a consistent historical finding6, 14, 15 and may be incidental.1
Radiological findings are dramatic. A large localized region of bone destruction with possible involvement of contiguous bones, even with extension across joint spaces, may be seen. Osseous structures are replaced with fibrous tissue. Associated soft tissue masses are rarely seen. MRI findings of Gorham's disease are non-specific and variable16 and advanced imaging studies may fail to reveal the presumed vascular nature of this condition.17 Given the rarity of this disease, other more common conditions must be considered in a differential diagnosis. Malignant disease must be ruled out histologically, although lack of a soft tissue mass points away from the presence of a neoplasm. Sclerosis and periosteal bone formation, seen radiographically in osteomyelitis, are not features of Gorham's disease. There are no serum abnormalities or generalized osseous changes as with metabolic conditions. Additionally, other rare conditions, such as granulomatous or osteolytic diseases, may present with concurrent effects on other organ systems or only in specific anatomical sites, which helps differentiate them from Gorham's disease.
Histological examination of tissue from an involved area reveals a non-specific vascular proliferation with fibrous connective tissue, sometimes containing lymphocytes and plasma cells. The vascular proliferation consists of thin-walled, often dilated, vascular or lymphatic channels of varying sizes. Angiomatosis, however, is not always a histopathological feature and has been questioned in the literature as an intrinsic characteristic.14 Some authors have reported cases of widespread, multicentric organ, osseous and/or skin involvement related to large lymphangiomatous malformations,18–20 whereas other cases, such as the one presented here, are more localized and lack significant vascular proliferation or soft tissue involvement. Vigorita et al18 proposed that the multicentric-type disease may develop secondary to a dysplastic lymphatic system and suggested that this should be considered a separate subtype of Gorham's disease. The presence of osteoclasts adjacent to bone fragments may or may not be a prominent feature.17 This may be related to the site of histological evaluation.13 New bone formation has been variably reported and may represent a periosteal reaction to the altered forces on the affected bone.14 Cellular atypia is absent. Definitive diagnosis of Gorham's disease is a diagnosis of exclusion.3
Treatment of Gorham's disease has met with variable success. Surgical resection of the affected area with osseous graft placement has often failed because of resorption of the graft. Radiation therapy has been advocated as a treatment of choice; however, in young patients the increased risk of developing a secondary malignancy must be considered. Other suggested treatments include bisphosphonates, calcitonin, vitamin D and alpha-2b-interferon therapy. The goal of treatment is to arrest progression of the osteolysis, as remineralization or reformation of affected bones is not seen. Currently, there is no universally accepted treatment for patients with Gorham's disease.
In conclusion, this report documents a case of Gorham's disease arising in the maxilla of a 56-year-old man. This appears to be the first published case of involvement of the maxilla without concurrent mandibular disease. Essentially, the imaging characteristics include bone resorption without an associated soft tissue mass or other evidence of a neoplasm or any sign of inflammatory disease. The aetiology and details of the disease mechanism remain controversial. Gorham's disease should be considered as a possible diagnosis when significant bone destruction occurs without an apparent cause.
References
- 1.Gorham LW, Wright AW, Shultz HH, Maxon FC., Jr Disappearing bones: a rare form of massive osteolysis; report of two cases, one with autopsy findings. Am J Med 1954;17:674–682 [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg 2003;129:1340–1343 [DOI] [PubMed] [Google Scholar]
- 3.Mignogna MD, Fedele S, Lo Russo L, Lanza A, Marenzi G, Sammartino G. Gorham's disease of the mandible mimicking periodontal disease on radiograph. J Clin Periodontol 2005;32:1022–1026 [DOI] [PubMed] [Google Scholar]
- 4.Motamedi MH, Homauni SM, Behnia H. Massive osteolysis of the mandible: a case report. J Oral Maxillofac Surg 2003;61:957–963 [DOI] [PubMed] [Google Scholar]
- 5.Fisher KL, Pogrel MA. Gorham's syndrome (massive osteolysis): a case report. J Oral Maxillofac Surg 1990;48:1222–1225 [DOI] [PubMed] [Google Scholar]
- 6.Anavi Y, Sabes WR, Mintz S. Gorham's disease affecting the maxillofacial skeleton. Head Neck 1989;11:550–557 [DOI] [PubMed] [Google Scholar]
- 7.Patel DV. Gorham's disease or massive osteolysis. Clin Med Res 2005;3:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benhalima H, Lazrak A, Boulaich M, Mezahi M, Ourlai A, Kzadri M, et al. Massive osteolysis of the maxillo-facial bones: case report and review of the literature. Odontostomatol Trop 2001;24:35–40 [PubMed] [Google Scholar]
- 9.Tsang WM, Tong AC, Chow LT, Ng IO. Massive osteolysis (Gorham disease) of the maxillofacial skeleton: report of 2 cases. J Oral Maxillofac Surg 2004;62:225–230 [DOI] [PubMed] [Google Scholar]
- 10.Holroyd I, Dillon M, Roberts GJ. Gorham's disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:125–129 [DOI] [PubMed] [Google Scholar]
- 11.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am 1955;37:985–1004 [PubMed] [Google Scholar]
- 12.Hirayama T, Sabokbar A, Itonaga I, Watt-Smith S, Athanasou NA. Cellular and humoral mechanisms of osteoclast formation and bone resorption in Gorham-Stout disease. J Pathol 2001;195:624–630 [DOI] [PubMed] [Google Scholar]
- 13.Devlin RD, Bone HG, 3rd , Roodman GD. Interleukin-6: a potential mediator of the massive osteolysis in patients with Gorham-Stout disease. J Clin Endocrinol Metab 1996;81:1893–1897 [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K, Ito T, Tsuchiya T, Takahashi H. Is angiomatosis an intrinsic pathohistological feature of massive osteolysis? Report of an autopsy case and a review of the literature. Virchows Arch 2003;442:400–406 [DOI] [PubMed] [Google Scholar]
- 15.Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1983;55:331–343 [DOI] [PubMed] [Google Scholar]
- 16.Yoo SY, Hong SH, Chung HW, Choi JA, Kim CJ, Kang HS. MRI of Gorham's disease: findings in two cases. Skeletal Radiol 2002;31:301–306 [DOI] [PubMed] [Google Scholar]
- 17.Lo CP, Chen CY, Chin SC, Juan CJ, Hsueh CJ, Chen A. Disappearing calvarium in Gorham disease: MR imaging characteristics with pathologic correlation. AJNR Am J Neuroradiol 2004;25:415–418 [PMC free article] [PubMed] [Google Scholar]
- 18.Vigorita VJ, Magitsky S, Bryk E. Gorham's disease: an autopsy report. Clin Orthop Relat Res 2006;451:267–273 [DOI] [PubMed] [Google Scholar]
- 19.Bruch-Gerharz D, Gerharz CD, Stege H, Krutmann J, Pohl M, Koester R, et al. Cutaneous lymphatic malformations in disappearing bone (Gorham-Stout) disease: a novel clue to the pathogenesis of a rare syndrome. J Am Acad Dermatol 2007;56:S21–25 [DOI] [PubMed] [Google Scholar]
- 20.Aviv RI, McHugh K, Hunt J. Angiomatosis of bone and soft tissue: a spectrum of disease from diffuse lymphangiomatosis to vanishing bone disease in young patients. Clin Radiol 2001;56:184–190 [DOI] [PubMed] [Google Scholar]