Abstract
Objectives
This study was undertaken to investigate the relationship between radiographic appearance and epithelial cell proliferations in keratocystic odontogenic tumours (KCOTs).
Methods
A retrospective radiographic analysis was performed on 284 cases of KCOT to gain insight into the radiographic characteristics. Expression of Ki-67 in 30 of the 284 cases was detected by the labelled streptavidin–biotin (LSAB) method and evaluated by an image analysis system.
Results
The radiographic presentation of KCOT was divided into four types: unilocular, multilocular, multiple and naevoid basal cell carcinoma syndrome (NBCCS). The expression of Ki-67 in NBCCS was significantly different from the solitary and multiple KCOTs (P = 0.018, 0.002). In multilocular KCOTs it was also significantly different from the unilocular and syndrome-associated lesions (P = 0.000). In contrast, no significant differences were observed between the solitary and multiple lesions (P = 0.220).
Conclusions
A high correlation exists in KCOT between its biological behaviour and imaging features. The solitary KCOT seems less biologically aggressive and it should be classified as a cyst rather than a tumour. This means that more than half of KCOTs manifest themselves as ordinary cysts.
Keywords: keratocystic odontogenic tumour, imaging feature, Ki-67 antigen, immunohistochemistry
Introduction
First described by Philipsen in 1956,1 the odontogenic keratocyst (OKC) constitutes approximately 11% of all cysts of the jaws.2 It is an aggressive cystic lesion with a predilection for recurrence, and the recurrence rate is higher than that of other odontogenic cysts.3 In 1967, Toller suggested that the OKC should best be regarded as a benign cystic neoplasm, rather than simply as an odontogenic cyst.4 In the years since, numerous reports influenced the World Health Organization (WHO) to reclassify this lesion in 2005 as a keratocystic odontogenic tumour (KCOT), and define it as a benign uni- or multicystic intraosseous tumour of odontogenic origin.5 WHO recommends the term KCOT as it better reflects the neoplastic nature of the lesion;5 however, this reclassification has not yet been universally accepted.
In clinical studies, imaging can help narrow the differential diagnosis, thereby helping to guide patient treatment. However, the radiographic appearances of KCOTs are various, and it is difficult to distinguish KCOTs from other cysts or neoplastic lesions on the basis of their radiographic features alone. The potentially aggressive, infiltrative behaviour of KCOT is universally acknowledged, and the WHO's reclassification emphasizes this nature. Of the immunohistochemical cell cycle markers, the most widely used has been the monoclonal antibody Ki-67, which labels all active parts of the cell cycle, rises during the second half of the S phase, reaches a peak in the G2 and M phases, and rapidly degrades after mitosis.6 It can be expressed actively in proliferating cells, in particular neoplasms. A number of papers have been published during the past decade on the expression of Ki-67 in OKCs. Nevertheless, it remains unclear whether the proliferation of KCOT has a basic relationship with its radiographic appearances.
In this study, an attempt was made to evaluate the relationship between radiographic patterns of KCOT and immunocytochemical expression of Ki-67 in its epithelial cells. The aim was to find the epithelial cell proliferation in different radiographic types, and verify whether WHO's reclassification of KCOT from cyst to tumour is appropriate.
Materials and methods
Patients and data collection
Radiographs of 284 patients (181 males and 103 females, a 1.76:1 ratio) were included in this study. The patients were diagnosed histopathologically as having a KCOT (orthokeratinized KCOTs were excluded) in West China Hospital of Stomatology, Sichuan University, during the period 1987–2007. The mean patient age was 32 years with a range of 9–87 years. Additionally, 30 cases of the total 284 patients (18 males and 12 females, a 1.5:1 ratio) obtained from excisional biopsy were selected from the files of the histopathology laboratory, West China College of Stomatology, Sichuan University. The mean age of these patients was 34.5 years (range 9–66 years).
Imaging
A retrospective radiographic analysis was performed on 284 cases to gain insight into the radiographic characteristics. The radiographs consisted of panoramic radiographs, lateral and posteroanterior skull conventional radiographs and posteroanterior chest radiographs.
Immunohistochemical evaluation
Expressions of Ki-67 were assessed on 30 out of the 284 cases by immunohistochemistry performed with labelled streptavidin–biotin (LSAB) method. Mouse monoclonal antibodies (MoAb) to Ki-67 (clone MO722) and reagent kit were obtained from Dako (Carpinteria, CA). Formalin-fixed and paraffin-embedded specimens were cut at a thickness of 4 μm and sections were deparaffined for antigen retrieval. The procedure for the LSAB method was carried out with the primary antibody for Ki-67 MoAb. Counterstaining was performed by Mayer's hematoxylin. The slides of each case were observed under a light microscope, five randomly selected equidistant fields were assessed at 20×10 magnification. All slides were independently scored by one observer. The images were captured by the Olympus DP70 camera using the manufacturer's DP Controller 3.1.1.267 software (Olympus, Center Valley, PA). Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD) was used for image analysis. In this study, the immunohistochemical expression of Ki-67 was evaluated comprehensively using the following scores: area, density mean and integrated optical density (IOD).
Statistical analysis
All calculations were performed with SPSS 11.0 for Windows (Northwestern University Information Technology, Evanston, IL). ANOVA (by means of the least significant difference test) was used to compare the scores among the cohorts. Differences were considered significant at the level of P < 0.05. Among the three scores (area, density mean and IOD) at least two were statistically different and could be considered significant. All values are shown as mean±SD.
Results
The radiographic presentation of KCOT could be broadly divided into four types:
a) Unilocular KCOT: the radiograph shows a well-circumscribed radiolucent cystic lesion surrounded by a thin smooth or scalloped radiopaque border that is often associated with impacted teeth (Figure 1).
Figure 1.
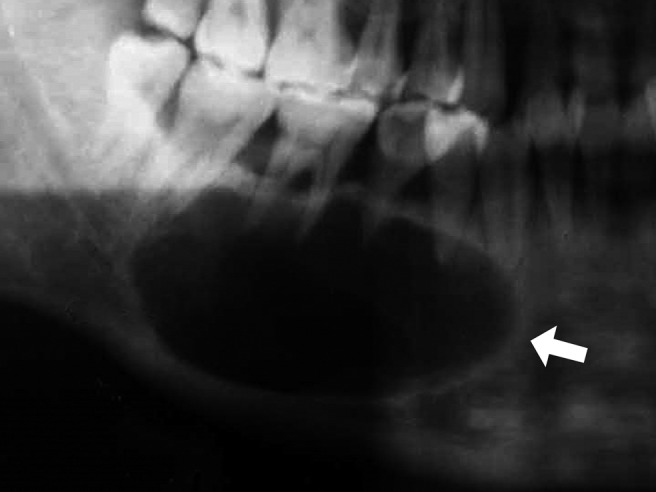
Panoramic radiograph shows an ellipsoid, well-demarcated, corticated, radiolucent lesion (arrow)
b) Multilocular KCOT: the radiograph shows at least two round or ovoid radiolucent cystic lesions, which are uniform or different in size, and overlap each other. Their interiors are partially divided by two kinds of septa: the higher-density, clear and sharp ones are bony septa; and the lower-density and discontinuous ones are fibrous septa (Figure 2).
Figure 2.
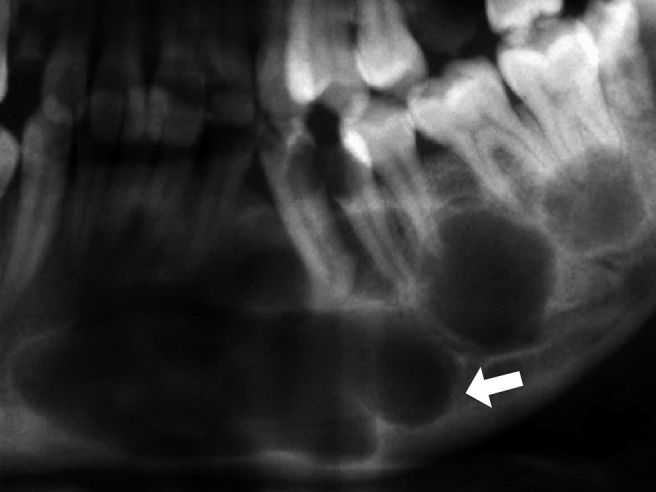
Panoramic radiograph demonstrating several round radiolucent cystic lesions within the mandible, which overlap each other, with the bony septa visible (arrow)
c) Multiple KCOT (excluding naevoid basal cell carcinoma syndrome (NBCCS)): several separate cystic images are distributed in the jaws and it is not associated with a variety of developmental anomalies and neoplasias (Figure 3).
Figure 3.
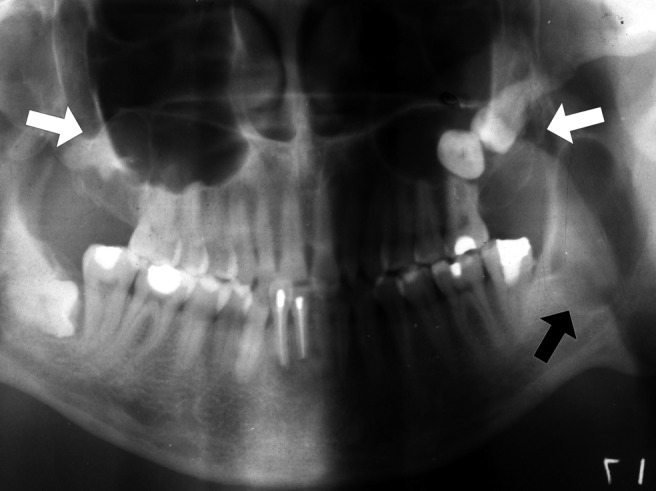
Panoramic radiograph showing radiolucent well-defined multilocular lesions in both jaws, associated with two unerupted teeth within the maxilla. An ellipsoid lesion with no internal structure is detected in the left ascending ramus (arrows)
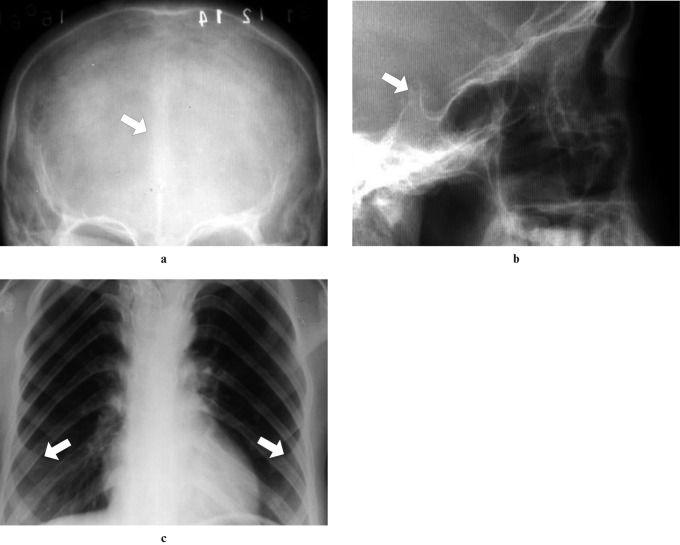
d) NBCCS or Gorlin–Goltz syndrome is characterized by five main components: multiple naevoid basal cell carcinomas, jaw cysts (also known as multiple KCOTs), congenital skeletal anomalies, ectopic calcifications and palmar and plantar pits7 (Figure 4).
Figure 4.
(a) Frontal radiograph showing calcification of the falx cerebri, (b) lateral conventional radiograph demonstrating a calcified sella turcica and (c) posteroanterior chest radiograph view also showing bifid ribs (fifth costal arch bifid) on both sides (arrows)
Regarding the types of KCOT, the unilocular ranked first (64.79%), followed by the multilocular (21.90%); multiple and NBCCS were relatively rare (9.79% and 3.52%, respectively), as shown in Table 1.
Table 1. Radiographic appearance of 284 patients with keratocystic odontogenic tumours (KCOTs).
| Radiographic appearance | Number of cases | Incidence (%) |
| Unilocular | 184 | 64.79 |
| Multilocular | 62 | 21.90 |
| Multiple (excluding NBCCS) | 28 | 9.79 |
| NBCCS | 10 | 3.52 |
Expression of Ki-67 in epithelial cells
The brown Ki-67-positive cells were unevenly distributed within the epithelium; some regions were clustered (Figure 5) and some were scattered (Figure 6). They were localized in basal and suprabasal layers, and nuclei were granular and heterogeneous (Figures 7–10).
Figure 5.

Clustered region of Ki-67 expression in epithelial cells
Figure 6.
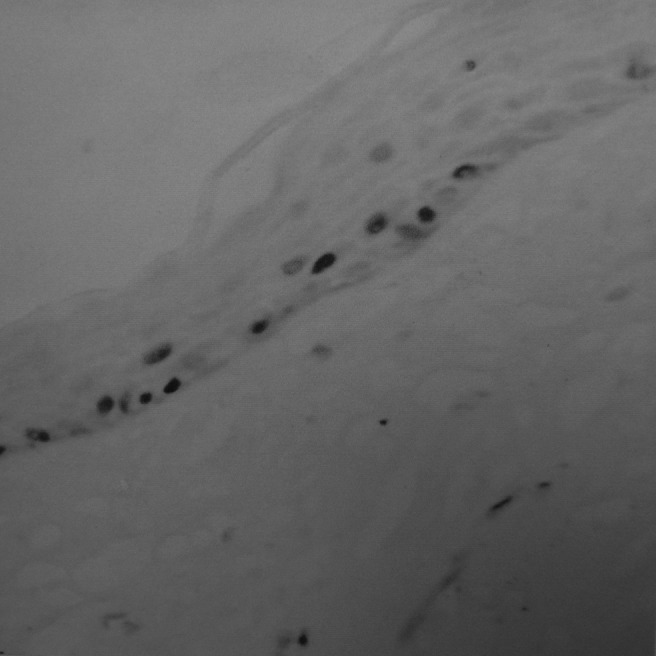
Scattered region of Ki-67 expression of epithelial cells
Figure 7.
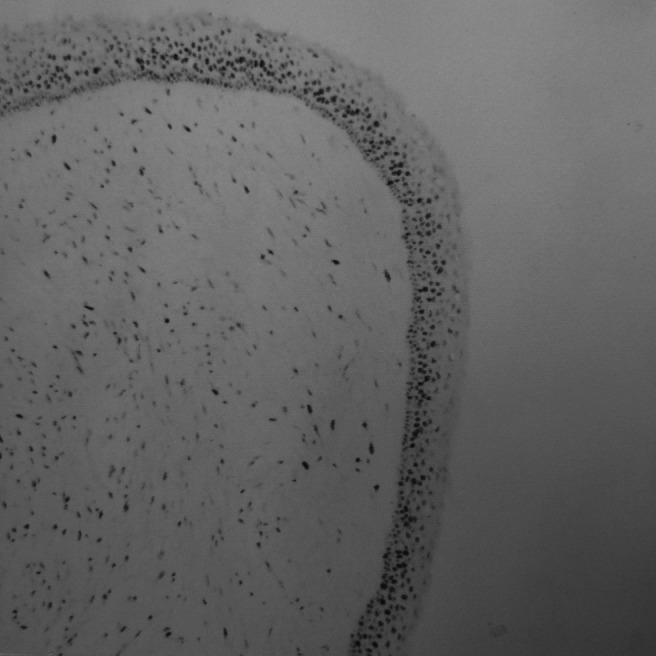
Expression of Ki-67 in unilocular keratocystic odontogenic tumours
Figure 10.
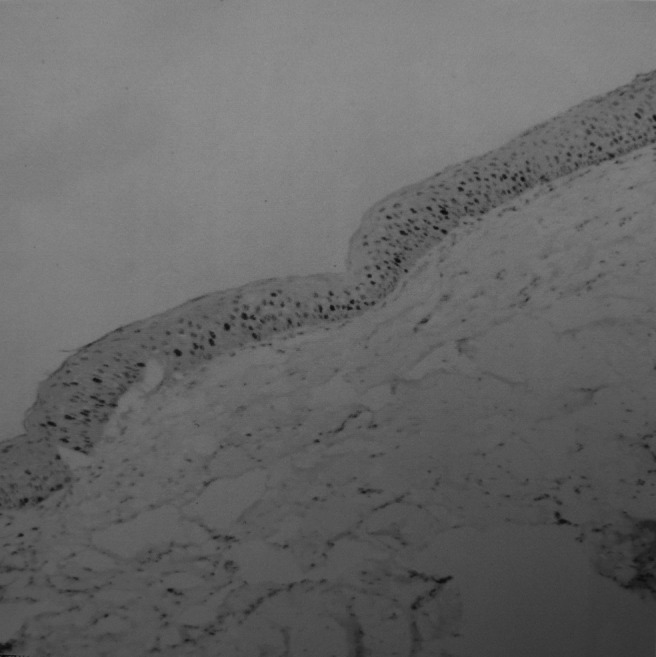
Expression of Ki-67 in naevoid basal cell carcinoma syndrome
Figure 8.
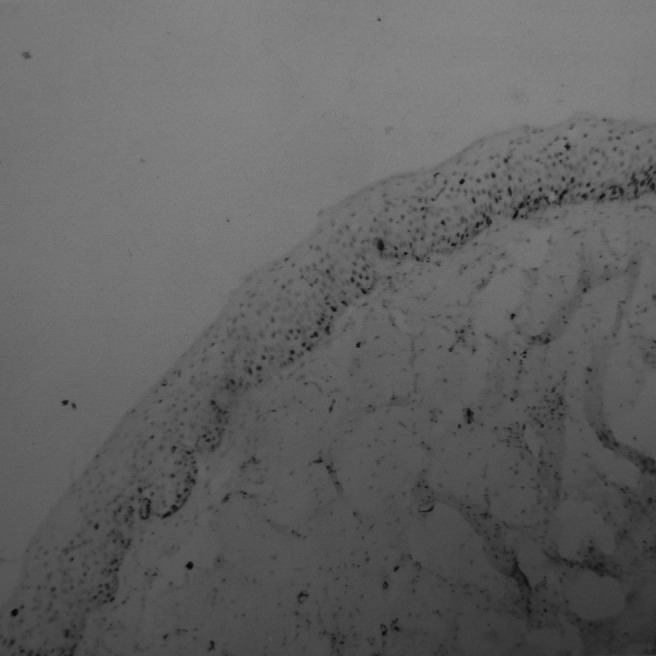
Expression of Ki-67 in multilocular keratocystic odontogenic tumours
Figure 9.
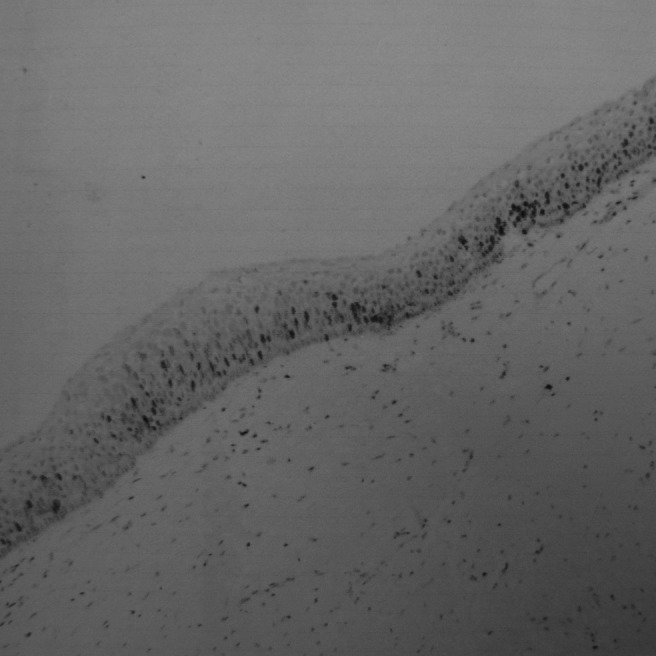
Expression of Ki-67 in multiple keratocystic odontogenic tumours
Relationship between expression of Ki-67 and radiographic features
The 30 cases for Ki-67 analysis were chosen at random from the total of 284 cases. They included nine unilocular cases, seven multilocular cases, seven multiple cases (excluding NBCCS) and seven NBCCS cases. The results of immunohistochemical evaluations are shown in Tables 2 and 3. When comparing the four types of KCOTs, the expression of Ki-67 in the multilocular group was significantly different from that in the unilocular and NBCCS groups (P = 0.000, Table 2). Moreover, there was a significant difference between the multiple and syndrome-associated groups (P = 0.002, Table 2). When comparing the solitary and NBCCS groups, a significant difference between them was found (P = 0.018, Table 3). In contrast, no significant differences were observed between the solitary and multiple KCOT groups (P = 0.220; Table 3).
Table 2. Comparison of unilocular, multilocular, multiple keratocystic odontogenic tumours (KCOTs) and naevoid basal cell carcinoma syndrome (NBCCS).
| Radiographic appearance | Area | Density mean | IOD |
| Unilocular | 75.3533 ± 18.6387 | 0.4375 ± 0.0450 | 45.4875 ± 12.5480 |
| Multilocular | 83.0582 ± 19.2774 | 0.3145 ± 0.1227 | 28.8200 ± 15.6948 |
| Multiple (excluding NBCCS) | 90.0200 ± 14.1874 | 0.3600 ± 0.0354 | 33.2620 ± 5.2980 |
| NBCCS | 90.5200 ± 10.0778 | 0.5050 ± 0.0663 | 54.6650 ± 12.1198 |
The expression of Ki-67 in the multilocular group was significantly different from that in the unilocular and NBCCS groups (P = 0.000); moreover, there was a significant difference between the multiple and syndrome-associated groups (P = 0.002). IOD, integrated optical density
Table 3. Comparison of solitary, multiple keratocystic odontogenic tumours (KCOTs) and naevoid basal cell carcinoma syndrome (NBCCS).
| Radiographic appearance | Area | Density mean | IOD |
| Solitary | 79.0383 ± 18.9236 | 0.3787 ± 0.1086 | 37.5161 ± 16.2223 |
| Multiple (excluding NBCCS) | 90.0200 ± 14.1874 | 0.3600 ± 0.0354 | 33.2620 ± 5.2980 |
| NBCCS | 90.5200 ± 10.0778 | 0.5050 ± 0.0663 | 54.6650 ± 12.1198 |
There was a significant difference in the expression of Ki-67 between the solitary KCOT and NBCCS groups (P = 0.018); however, no significant differences were observed between the solitary and multiple KCOT groups (P = 0.220). IOD, integrated optical density
Discussion
KCOT is officially known as a true benign tumour, which its aggressive nature. Numerous studies have been performed to analyse the expression in actively proliferating cells; however, few have involved the relation to its imaging features, and it is, therefore, not clear whether the proliferation of KCOT has a relationship with its radiographic appearances.
The typical radiographic features of KCOT are unilocular, multilocular or multiple well-circumscribed radiolucent lesions surrounded by a thin radiopaque border with a smooth or loculated periphery.8 Frequently, the lumen, densely filled with keratin, will cause the image to show a hazy appearance. An important characteristic of KCOT is its trend to grow along the major axis of the jaws. Occasionally KCOTs may erode through the buccal plate or mandibular lingual cortex; on radiographs a radiolucent shadow is frequently seen in the mandible.9 KCOTs can displace and resorb teeth, and the inferior alveolar nerve canal may be displaced. In the maxilla they can invaginate and occupy the entire maxillary antrum. Multiple KCOTs in a young patient should highlight the possibility of NBCCS. NBCCS is a rare autosomal dominant disorder with a prevalence of 1 per 57 000 and a 3:1 male to female gender predilection.10 This syndrome is characterized by up to 100 less common features.11 Patients may have various developmental anomalies and an increased risk of other types of neoplasias.12 KCOT is the second most common feature associated with NBCCS.13 Some authors suggest that as many as half of KCOTs are related to NBCCS.14
A number of papers have been published during the past decade on the expression of Ki-67 in odontogenic cysts, and more specifically in OKCs. This marker is expressed in actively proliferating cells, in particular neoplasms. Given the evidence that the OKC is an aggressive lesion, and the suggestion that it might be regarded as a neoplasm, a spate of studies applied these methodologies to this cyst. The number of Ki-67-positive cells is higher in KCOTs than in dentigerous and radicular cysts.6 This reflects a higher mitotic count and turnover rate in KCOT epithelium.15 Moreover, the uneven distribution of Ki-67-positive cells within the epithelium reflects the fact that the growth pattern of KCOT epithelium is characterized by heterogeneity, and explains the infiltrative growth in KCOTs in contrast to the expansive growth in other cysts. In KCOTs, the positivity is expressed mostly in the suprabasal layer of epithelium, but in other cysts it is mainly in the basal layer.16 The characteristic distribution also suggests that the course of epithelium differentiation and maturation in KCOTs is different from that of other cysts. Thus, the distinctive epithelial cell proliferation in KCOT may determine its particularity of growth and behaviour.
According to the immunohistochemical analysis of 30 cases, the expression of Ki-67 in NBCCS was found to be significantly different from that in solitary and multiple KCOTs (P = 0.018 and 0.002, respectively) and in multilocular KCOTs it was also significantly different from unilocular and syndrome-associated lesions (P = 0.000). However, no significant difference was observed between solitary and multiple lesions (P = 0.220). With KCOTs, different radiographic appearances are associated with different epithelial cell proliferations. By contrast, the expression of Ki-67 in both unilocular and multilocular lesions was lower, the lower proliferation and recurrence making them look more like cysts than tumours. The multiple lesions, which were not associated with developmental anomalies and neoplasias, were not a type of more aggressive KCOTs, but rather they were quite distinct from syndrome-associated lesions. The expression in syndrome-associated lesions was significantly higher than in the solitary lesions. The higher expression of cell proliferation markers in syndromic patients reflecting the abnormal genetics of these lesions, especially in terms of a greater tendency to recurrence.
In conclusion, the above findings suggest that the proliferation of KCOTs is related to its imaging features. Although WHO's reclassification of KCOT from cyst to tumour can motivate clinicians to manage the disease in a correspondingly aggressive manner and decrease unnecessary recurrences, this study has found that the solitary KCOT seems to be less biologically aggressive and should be classified as a cyst rather than a tumour. This means that more than half of KCOTs manifest as ordinary cysts. However, it has not been possible to identify the relationship between imaging features and molecular biology in KCOTs. Thus, further studies are required to clarify this issue.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 10675087 and 10574095) and State Key Laboratory of Oral Diseases, Sichuan University, Chengdu, Sichuan, China.
References
- 1.Philipsen HP. Om keratocyster (kolesteatomer) i kaeberne. Tandlaegebladet 1956;60:963–980 [Google Scholar]
- 2.Maurette PE, Jorge J, de Moraes M. Conservative treatment protocol of odontogenic keratocyst: a preliminary study. J Oral Maxillofac Surg 2006;64:379–383 [DOI] [PubMed] [Google Scholar]
- 3.Ahlfors E, Larsson A, Sjögren S. The odontogenic keratocyst: a benign cystic tumor? J Oral Maxillofac Surg 1984;42:10–19 [DOI] [PubMed] [Google Scholar]
- 4.Toller P. Origin and growth of cysts of the jaws. Ann R Coll Surg Engl 1967;40:306–336 [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. Lyon: IARC Verlag, 2005, pp 306–307. [Google Scholar]
- 6.Li TJ, Browne RM, Matthews JB. Epithelial cell proliferation in odontogenic keratocysts: a comparative immunocytochemical study of Ki-67 in simple, recurrent and basal cell naevus syndrome (BCNS)-associated lesions. J Oral Pathol Med 1995;24:221–226 [DOI] [PubMed] [Google Scholar]
- 7.Gorlin RJ. Nevoid basal cell carcinoma syndrome. Dermatol Clin 1995;13:113–125 [PubMed] [Google Scholar]
- 8.Blanchard SB. Odontogenic keratocysts: review of the literature and report of a case. J Periodontol 1997;68:306–311 [DOI] [PubMed] [Google Scholar]
- 9.Stoelinga PJ. Long-term follow-up on keratocysts treated according to a defined protocol. Int J Oral Maxillofac Surg 2001;30:14–25 [DOI] [PubMed] [Google Scholar]
- 10.Lovin JD, Talarico CL, Wegert SL, Gaynor LF, Sutley SS. Gorlin's syndrome with associated odontogenic cysts. Pediatr Radiol 1991;21:584–587 [DOI] [PubMed] [Google Scholar]
- 11.Amlashi SF, Riffaud L, Brassier G, Morandi X. Nevoid basal cell carcinoma syndrome: relation with desmoplastic medulloblastoma in infancy: a population-based study and review of the literature. Cancer 2003;98:618–624 [DOI] [PubMed] [Google Scholar]
- 12.Kimonis VE, Goldstein AM, Pastakia B, Yang ML, Kase R, DiGiovanna JJ. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet 1997;69:299–308 [PubMed] [Google Scholar]
- 13.Melo ES, Kawamura JY, Alves CA, Nunes FD, Jorge WA, Cavalcanti MG. Imaging modality correlations of an odontogenic keratocyst in the nevoid basal cell carcinoma syndrome: a family case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:232–236 [DOI] [PubMed] [Google Scholar]
- 14.Oda D, Rivera V, Ghanee N, Kenny EA, Dawson KH. Odontogenic keratocyst: the northwestern USA experience. J Contemp Dent Pract 2000;1:60–74 [PubMed] [Google Scholar]
- 15.Bornstein MM, Filippi A, Altermatt HJ, Lambrecht JT, Buser D. The odontogenic keratocyst—odontogenic cyst or benign tumor? (Article in French and German). Schweiz Monatsschr Zahnmed 2005;115:110–128 [PubMed] [Google Scholar]
- 16.Slootweg PJ. p53 protein and Ki-67 reactivity in epithelial odontogenic lesions: an immunohistochemical study. J Oral Pathol Med 1995;24:393–397 [DOI] [PubMed] [Google Scholar]