Abstract
Objectives
The aim of the present study was to evaluate DNA damage (micronucleus) and cellular death (pyknosis, karyolysis and karyorrhexis) in exfoliated buccal mucosa cells from adults following cone beam CT exposure.
Methods
A total of 19 healthy adults (10 men and 9 women) submitted to cone beam CT were included.
Results
No significant statistically differences (P > 0.05) in micronucleus frequency were seen before and after cone beam CT exposure. In contrast, the tomography was able to increase other nuclear alterations closely related to cytotoxicity such as karyorrhexis, pyknosis and karyolysis (P < 0.05).
Conclusion
In summary, these data indicate that cone beam CT may not be a factor that induces chromosomal damage, but it is able to promote cytotoxicity.
Keywords: buccal mucosa cells, cone beam computed tomography, micronucleus test
Introduction
In recent years, cone beam CT (CBCT) has been used as a promising new radiographic method in the fields of dentistry for implant treatment, oral surgery, endodontic treatment, orthodontics and temporomandibular joint imaging.1–5 The great advantage of this technology is that it offers three-dimensional (3D) imaging of dental structures and provides clear images of highly contrasted structures, such as bone.6 Compared with conventional CT, CBCT technology in clinical practice has important advantages, such as minimization of the radiation dose, image accuracy, rapid scan time, fewer image artefacts, chair-side image display and real-time analysis.7
Biomonitoring studies have been used in health sciences for many years to assist in diagnosing and staging disease as well as to evaluate the risk assessment. They give information concerning environmental exposure and susceptibility. These studies are divided into three groups: first to define the exposure to mutagenic and/or carcinogenic agents, second to show biological effects on the target tissue and third to give information about the individual susceptibility.8 To date, a variety of assays have been proposed in biomonitoring studies, including those that assess metaphase chromosomal aberrations, sister chromatid exchanges, DNA damage and host cell reactivation. However, these methods are typically laborious and time-consuming or require highly trained technicians to accurately read and interpret slides. For these reasons the application of the micronucleus test to uncultured exfoliated cells has been greeted enthusiastically.9 Micronuclei arise from acentric fragments or whole chromosomes which are not included in the main nuclei of the daughter cells. The formation of micronuclei can be induced by substances that cause chromosome breakage (clastogens) as well as by agents that affect the spindle apparatus (aneugens).10 Recently, we have applied this methodology with success in individuals (adults and children) exposed to dental X-rays11,12 or who have malignant tumours and are undergoing radio- or chemotherapy.13,14 In the present study, we investigated the frequencies of micronucleated cells in oral mucosa from individuals exposed to CBCT. To monitor cytotoxic effects, pyknosis, karyolysis and karyorrhexis were also evaluated in this setting. Certainly, such data will contribute to a better understanding of the effects this new radiographic method upon the cellular system.
Materials and methods
Participants
The participants of this study consisted of 19 healthy adults (10 men and 9 women) with a mean age of 26.8 ± 5 years. All patients submitted to CBCT were outpatients attending the Department of Dental Clinics, University of Sacred Heart, USC, SP, Brazil. Individual characteristics of the participants were collected and included gender, age, habits and exposure to genotoxic agents. CBCT had been requested by a dentist and was performed with an i-CAT CBCT scanner (Image Sciences International, Hatfield, PA). This scanner is used on the dentomaxillofacial region, and has a minute voxel size of 0.2 mm3. The mandible was imaged at a tube voltage of 80 kV, a tube current of 4 mA and exposure time of 40 s. After scanning, contiguous sectional images in three directions, i.e. parallel section (parallel to the dental arch), cross-section (perpendicular to the dental arch) and horizontal section images, were reconstructed from the projection data with a slice width of 1 mm.
Micronucleus test in oral mucosa cells
Exfoliated oral mucosa cells were collected immediately before CBCT exposure and after 10 days. After rinsing the mouth with tap water, cells were obtained by scraping the right/left cheek mucosa with a moist wooden spatula. Cells were transferred to a tube containing saline solution (NaCl at 0.9% concentration), centrifuged (800 rpm) for 5 min, fixed in 3:1 methanol/acetic acid, and dropped on to pre-cleaned slides. Later, the air-dried slides were stained using the Feulgen/fast green method and examined under a light microscope at ×400 magnification to determine the frequency of micronucleated cells, as described elsewhere.15 2000 cells were scored from each patient for each sampling time (before and after CBCT exposure).
Data analysis
Micronuclei were scored according to the criteria described by Sarto et al16 as a parameter for DNA damaging (mutagenicity). For cytotoxicity, the following nuclear alterations were considered: pyknosis, karyolysis and karyorrhexis.17 Results were expressed as a percentage (%). Such analysis has been established in a previous study conducted by our research group.18
Statistical methods
The Wilcoxon test for dependent samples was used to compare the frequencies of micronuclei and other cytotoxic alterations among the samples before and after CBCT exposure. For this purpose, SigmaStat software, version 1.0 (Jadel Scientific, Chicago, IL), was used. The level of statistical significance was set at 5%.
Results
Table 1 shows the frequencies of micronucleated cells in individuals undergoing CBCT. Before CBCT exposure, the mean frequency of micronucleated cells was 0.04%. No statistically significant differences (P > 0.05) were noticed after CBCT exposure. In contrast, an increase in other nuclear alterations was observed after CBCT, as depicted by the frequency of karyorrhexis, pyknosis and karyolysis. These data are summarized in Table 1. Figure 1 shows a micronucleated cell and Figure 2 displays abnormalities closely related to cytotoxicity, i.e. karyorrhexis, pyknosis and karyolysis.
Table 1. Frequency of micronucleated cells (MNCs) and other nuclear alterations (karyorrhexis, pyknosis and karyolysis) in individuals undergoing cone beam CT (CBCT).
| Groups | MNC (%) |
Other nuclear alterations* (%) |
||
| No. of individuals | Mean±SD | No. of individuals | Mean±SD | |
| Individuals prior to CBCT exposure | 19 | 0.04 ± 0.05 | 19 | 7.45 ± 461 |
| Individuals after CBCT exposure | 19 | 0.05 ± 0.06 | 19 | 17.84 ± 5.47† |
*Karyorrhexis, pyknosis and karyolysis
†P < 0.05 compared with individuals prior to CBCT exposure
Figure 1.
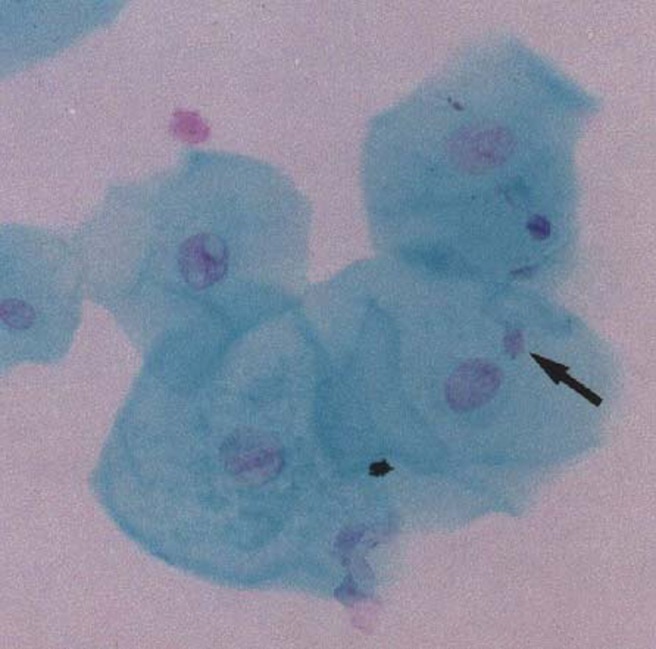
Micronucleated cell (arrow) (×100 magnification, Feulgen/fast green stain)
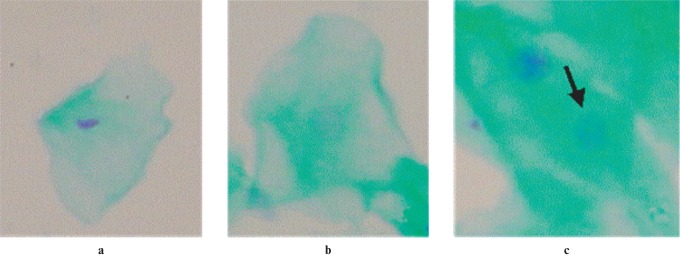
Figure 2.
Cytotoxicity parameters evaluated in this study: (a) pyknosis, (b) karyolysis and (c) karyorrhexis (×100 magnification, Feulgen/fast green stain)
To avoid putative confounding factors, all individual participants in this study were non-smokers. In addition, exposure to known genotoxins was not identified in any of the study participants. A total of seven individuals included in this trial used oral antiseptic solutions (chlorhexidine, Listerine®) regularly. Daily alcohol consumption was not considered in this study because of recall bias.
Discussion
The aim of this study was to employ the micronucleus test to assess chromosome damage and cellular death in adults exposed to CBCT. To the best of our knowledge, the approach has not been addressed in the literature so far.
Damage that leads to the formation of micronuclei takes place in the basal layer of the epithelial tissue, where cells undergo mitosis. Rapid turnover of epithelial tissues brings the cells to the surface, where they exfoliate. As a result, the maximal rate of micronuclei formation in exfoliated cells is seen between 1 and 3 weeks after exposure to the genotoxic agent.19,20 For this reason, a period of 10 days after CBCT exposure was adopted in this study.
Genomic damage is probably the most important fundamental cause of developmental and degenerative diseases. It has been well established that genomic damage is produced by environmental exposure to genotoxins, medical procedures (e.g. radiation and chemicals), micronutrient deficiency (e.g. folate), lifestyle factors (e.g. alcohol, smoking, drugs and stress) and genetic factors such as inherited defects in DNA metabolism and/or repair.21,22 Micronucleated cell indexes may reflect genomic instability, although the mechanisms are not exactly known.23 Overall, the detection of an elevated frequency of micronuclei in a given population indicates increased risk of cancer.24 However, cell types that repair DNA damage efficiently are likely to show lower levels of residual damage than cells less proficient in DNA repair.25 Buccal cells have been shown to have limited DNA repair capacity relative to peripheral blood lymphocytes, and therefore may more accurately reflect genomic instability events in epithelial tissues.26 Our results demonstrated that the micronucleus frequencies were not significantly different before and after CBCT exposure in this trial. By comparison, previous studies conducted by our group have revealed an absence of the clastogenic effect after exposure to panoramic dental radiology.18 Such findings are fully in line with those of other authors.27,28 Biomonitoring studies of populations exposed to some radiographic methods are quite difficult and rather specific because each population is exposed to different doses of radiation. This could explain why some studies find an increase in genetic damage in populations exposed to radiographic procedures. On the basis of our results, we postulated the lack of the clastogenic and/or aneugenic effects related to the CBCT exposure on buccal mucosa cells of healthy individuals. Since the number in the study group is relatively small, further studies are needed to confirm the issue.
To monitor cytotoxic effects, the frequencies of karyorrhexis, karyolysis and pyknosis were evaluated in this experimental design. Despite the lack of cytogenetic damage, our results demonstrated that CBCT was able to induce cellular death as depicted by statistically significant differences (P < 0.05) between values before and after CBCT exposure. It is important to emphasise that this is a new finding in radiation biology and, therefore, the mechanism is as yet unknown. Independent of its mode of action, such results support the notion that CBCT is a cytotoxicant agent. Cytotoxicity interferes with micronucleus induction as some micronucleated cells are inevitably lost after cytotoxic insult, confirming, therefore, the lack of mutagenic effect induced by CBCT. Nevertheless, it has been postulated that repeated exposure to cytotoxicants can result in chronic cell injury, compensatory cell proliferation, hyperplasia and ultimately tumour development.29 In fact, a correlation between cell proliferation and induction of cancer is assumed.30 Proliferation probably increases the risk of mutations within target cells, and may also be important in selective clonal expansion of (exogenously or endogenously) initiated cells from pre-neoplastic foci and eventually tumours.31 Further research will be required to fully elucidate this relationship.
In human cytogenetic studies, it is important to consider some confounding factors. Viruses, alterations in the immune system, failures in the DNA repair system and interindividual variations have already been associated with increased frequencies of chromosome aberrations.32 Furthermore, an age-related increase in micronuclei has been postulated.32 All participants in our study were a similar age, ranging from 18 to 30 years. Moreover, tobacco smoke is usually considered to be a relevant confounding factor.33 Thus, all adults recruited to participate of this study were non-smokers.
In conclusion, the results of the present study suggest that CBCT can induce cytotoxic effects in oral mucosa cells. As cellular death is considered to be a prime mechanism in non-genotoxic mechanisms of carcinogenesis, CBCT should be used only when necessary.
Acknowledgments
This study was supported by grants from FAPESP (Fundação de Amparo a Pesquisa do Estado de Sao Paulo, numbers: 07/00345-7 and 07/01228-4). The authors are thankful to Mr Marcelo Sousa Silva for his technical assistance. AJO is recipient of CNPq student's fellowship (PIBIC). DAR is a recipient of the CNPq fellowship.
References
- 1.Lascala CA, Panelly J, Marques MM. Analysis of the accuracy of linear measurements obtained by cone beam computed tomography (CBCT-NewTom). Dentomaxillofac Radiol 2004;33:291–294 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler CM, Woettche R, Brief J, Hassfeld S. Clinical indications for digital volume tomography in oral and maxillofacial surgery. Dentomaxillofac Radiol 2002;31:126–130 [DOI] [PubMed] [Google Scholar]
- 3.Danforth RA. Cone beam volume tomography: a new digital imaging option for dentistry. J Calif Dent Assoc 2003;31:814–815 [PubMed] [Google Scholar]
- 4.Sukovic P. Cone beam computed tomography in craniofacial imaging. Orthod Craniofac Res 2003;6:31–36 [DOI] [PubMed] [Google Scholar]
- 5.Dudic A, Giannopoulou C, Leuzinger M, Kiliaridis S. Detection of apical root resorption after orthodontic treatment by using panoramic radiography and cone-beam computed tomography of super-high resolution. Am J Orthod Dentofacial Orthop 2009;135:434–437 [DOI] [PubMed] [Google Scholar]
- 6.Marmulla R, Wortche R, Muhling J, Hassfeld S. Geometric accuracy of the NewTom 9000 cone beam CT. Dentomaxillofac Radiol 2005;34:28–31 [DOI] [PubMed] [Google Scholar]
- 7.Scarfe WC, Farman AG, Sukovic P. Clinical applications of conebeam computed tomography in dental practice. J Can Dent Assoc 2006;72:75–80 [PubMed] [Google Scholar]
- 8.Induslki JA, Lutz W. Molecular epidemiology: cancer risk assessment using biomarkers for detecting early health effects in individuals exposed to occupational and environmental carcinogens. Rev Environ Health 1997;12:179–190 [DOI] [PubMed] [Google Scholar]
- 9.Stich HF, Parida BB, Brunnemann KD. Localized formation of micronuclei in the oral mucosa and tobacco-specific nitrosamines in the saliva of “reverse” smokers, Khaini-tobacco chewers and gudakhu users. Int J Cancer 1992;21:172–176 [DOI] [PubMed] [Google Scholar]
- 10.Belien JA, Copper MP, Braakhuis BJ, Snow GB, Baak JP. Standardization of counting micronuclei: definition of a protocol to measure genotoxic damage in human exfoliated cells. Carcinogenesis 1995;16:2395–2400 [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro DA, Angelieri F. Cytogenetic biomonitoring of oral mucosa cells from adults exposed to dental X-rays. Radiat Med 2008;26:325–330 [DOI] [PubMed] [Google Scholar]
- 12.Angelieri F, de Oliveira GR, Sannomiya EK, Ribeiro DA. DNA damage and cellular death in oral mucosa cells of children who have undergone panoramic dental radiography. Pediatr Radiol 2007;37:561–565 [DOI] [PubMed] [Google Scholar]
- 13.Minicucci EM, Kowalski LP, Maia MA, Pereira A, Ribeiro LR, de Camargo JL, et al. Cytogenetic damage in circulating lymphocytes and buccal mucosa cells of head-and-neck cancer patients undergoing radiotherapy. J Radiat Res (Tokyo) 2005;46:135–142 [DOI] [PubMed] [Google Scholar]
- 14.Minicucci EM, Ribeiro DA, de Camargo B, Costa MC, Ribeiro LR, Favero Salvadori DM. DNA damage in lymphocytes and buccal mucosa cells of children with malignant tumours undergoing chemotherapy. Clin Exp Med 2008;8:79–85 [DOI] [PubMed] [Google Scholar]
- 15.Sarto FR, Tomanin L, Giacomelli G, Iannini G, Cupiraggi AR. The micronucleus assay in human exfoliated cells of the nose and mouth: application to occupational exposures to chronic acid and ethylene oxide. Mutat Res 1990;244:345–351 [DOI] [PubMed] [Google Scholar]
- 16.Sarto F, Finotto S, Giacomelli L, Mazzotti D, Tomanin R, Levis AG. The micronucleus assay in exfoliated cells of the human buccal. Mutagenesis 1987;2:11–17 [DOI] [PubMed] [Google Scholar]
- 17.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 1992;271:69–77 [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro DA, de Oliveira G, de Castro G, Angelieri F. Cytogenetic biomonitoring in patients exposed to dental X-rays: comparison between adults and children. Dentomaxillofac Radiol 2008;37:404–407 [DOI] [PubMed] [Google Scholar]
- 19.Fenech M, Holland N, Chang WP. The human micronucleus project - an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res 1999;428:271–283 [DOI] [PubMed] [Google Scholar]
- 20.Majer BJ, Laky B, Knasmuller S. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res 2001;489:147–172 [DOI] [PubMed] [Google Scholar]
- 21.Bukvic N, Bavaro P, Elia G, Cassano F, Fanelli M, Guanti G. Sister chromatid exchange (SCE) and micronucleus (MN) frequencies in lymphocytes of gasoline station attendants. Mutat Res 1998;415:25–33 [DOI] [PubMed] [Google Scholar]
- 22.Pastor S, Gutierrez S, Creus A, Cebulska-Wasilewska A, Marcos R. Micronuclei in peripheral blood lymphocytes and buccal epithelial cells of Polish farmers exposed to pesticides. Mutat Res 2001;495:147–156 [DOI] [PubMed] [Google Scholar]
- 23.Neri M, Fucic A, Knudsen LE, Lando C, Merlo F, Bonassi S. Micronuclei frequency in children exposed to environmental mutagens: a review. Mutat Res 2003;544:243–254 [DOI] [PubMed] [Google Scholar]
- 24.Fenech M. Biomarkers of genetic damage for cancer epidemiology. Toxicology 2002;181–182:411–416 [DOI] [PubMed] [Google Scholar]
- 25.Visvardis EE, Tassiou AM, Piperakis SM. Study of DNA damage induction and repair capacity of fresh and cryopreserved lymphocytes exposed to H2O2 and gamma-irradiation with the alkaline comet assay. Mutat Res 1997;383:71–80 [DOI] [PubMed] [Google Scholar]
- 26.Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res 2008;659:93–108 [DOI] [PubMed] [Google Scholar]
- 27.Cerqueira EM, Gomes-Filho IS, Trindade S. Genetic damage in exfoliated cells from oral mucosa of individuals exposed to X-rays during panoramic dental radiographies. Mutat Res 2004;562:111–117 [DOI] [PubMed] [Google Scholar]
- 28.Popova L, Kishkilova D, Hadjidekova VB, Hristova RP, Atanasova P, Hadjidekova VV, et al. Micronucleus test in buccal epithelium cells from patients subjected to panoramic radiography. Dentomaxillofac Radiol 2007;36:168–171 [DOI] [PubMed] [Google Scholar]
- 29.Mally A, Jagetia JK. Non-genotoxic carcinogens: early effects on gap junctions, cell proliferation and apoptosis in the rat. Toxicology 2002;180:233–248 [DOI] [PubMed] [Google Scholar]
- 30.Jagetia GC, Jayakrishnan A, Fernandes D, Vidyasagar MS. Evaluation of micronuclei frequency in the cultured peripheral blood lymphocytes of cancer patients before and after radiation treatment. Mutat Res 2001;491:9–16 [DOI] [PubMed] [Google Scholar]
- 31.Swenberg JA. Cell proliferation and chemical carcinogenesis: conferences summary and future directions. Environ Health Perspect 1993;101:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.othinfo>Xu GL, Bestor HH, Bourc'his D, Hsich CL, Tommerup N, Bugge M, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene.</othinfo>Nature 1999;402:187–191 [DOI] [PubMed] [Google Scholar]
- 33.Bloching M, Hofmann A, Lautenschlager C, Berghaus A, Grummt T. Exfoliative cytology of normal buccal mucosa to predict the relative risk of cancer in the upper aerodigestive tract using the MN-assay. Oral Oncol 2000;36:550–555 [DOI] [PubMed] [Google Scholar]