Abstract
Objectives
The objective of this study was to determine the ability of two flat panel cone beam CT (CBCT) devices to identify demineralized bone and bone transplants in vivo and in vitro.
Methods
Datasets from patients with autologous bone grafts (n = 9, KaVo 3DeXam (KaVo, Biberach, Germany); n = 38, Accuitomo 40 (Morita, Osaka, Japan)) were retrospectively evaluated. Demineralized and non-demineralized porcine cancellous bone blocks were examined with the two CBCT devices. A SawBone® skull (Pacific Research Laboratories, Vashon, WA) was used as a positioning tool for the bone blocks. Descriptive evaluation and image quality assessment were conducted on the KaVo 3DeXam data (voxel size 0.3 mm) using the OsiriX viewer as well as on the Morita Accuitomo data (voxel size 0.25 mm) using proprietary viewer software.
Results
Both in vivo and in vitro, the descriptive analysis of the images of the two devices showed well-visualized bone transplants with clearly defined cancellous bones and well-defined single bone trabeculae in all cross-sections. In vitro, demineralized samples showed lower radiographic opacity but no significant loss of quality compared with fresh bone (P = 0.070). Single cancellous bone trabeculae were significantly better visualized with the Morita 3D Accuitomo device than with the KaVo 3DeXam device (P = 0.038).
Conclusion
Both the KaVo 3DeXam and Morita 3D Accuitomo devices produce good-quality images of cancellous bones in in vivo remodelling as well as after in vitro demineralization.
Keywords: cone beam computed tomography, bone augmentation, implantology
Introduction
Cone beam CT (CBCT) is an imaging technology based on the axial cross-sectional reconstruction of radiographic images of cylindrical or spherical volumes.1 CBCT is primarily used as an alternative to multidetector CT (MDCT) to generate planar cross-sections and three-dimensional reconstructions of bone situations with a lower radiation dose. A further advantage of the CBCT device is its general availability in dental practices.2–5 Multidimensional imaging technologies are crucial for meeting the requirements of modern dental implantology and oral surgery.2
CBCT has been assumed to be inappropriate for the imaging of partly demineralized bone, including bone transplants in the remodelling phase after augmentation.3
However, CBCT devices have improved significantly in recent years. Several manufacturers have developed more advanced image intensifiers, and flat panel technology has become a further detector type in sectional radiography.4–7 Current flat panel volume CT devices in dentistry include, for instance, the Accuitomo (Morita, Osaka, Japan), NewTom VG (Quantitative Radiology, Verona, Italy), ProMax 3D (Planmeca, Helsinki, Finland), Picasso (E-Woo, Giheung-gu, South Korea), KaVo 3DeXam (former i-CAT) (KaVo, Biberach, Germany), and several Orange Dental devices including Picasso Master (Orange Dental, Bora-dong, South Korea), Scanora 3D (Soredex, Subang Jaya, Malaysia) and Iluma (Kodak Dental Systems, Rochester, NY).
This study further evaluates cancellous bone imaging during the remodelling phase by testing two CBCT devices, i.e. the Morita 3D Accuitomo 40 and the KaVo 3DeXam, in an in vitro model of demineralized bone. Data from patients with autologous bone grafts were evaluated retrospectively.8 Data from patients were used to provide imaging results of cancellous bone with both devices. The in vitro model presented here is one method to standardize the imaging of demineralized bone.
Materials and methods
Study design
The first part of the study retrospectively evaluated patient data for both CBCT devices. The second part evaluated freshly frozen and demineralized porcine spine bone specimens (n = 20 per group) for both CBCT devices; a SawBone® skull (Pacific Research Laboratories, Vashon, WA) was used as a positioning tool for the bone specimens.
Patient data
We retrospectively evaluated CBCT data of patients from the University of Mainz who had autologous bone grafts but no additional alloplastic ceramic material (n = 9 for the KaVo 3DeXam and n = 38 for the Morita Accuitomo). The KaVo 3DeXam group consisted of 6 women aged 49–77 years and 3 men aged 48–73 years; the Morita Accuitomo group consisted of 26 women aged 44–79 years and 12 men aged 43–75 years. Patient data were used in compliance with current ethics guidelines and laws.
SawBone® based skull model
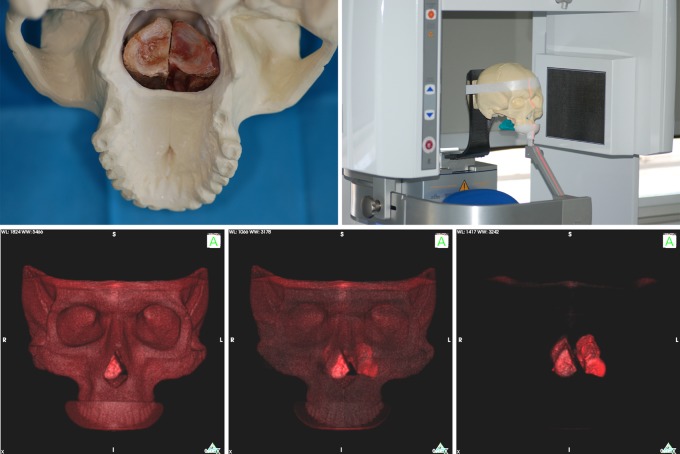
A SawBone® skull was modified by cutting off the dorsal wall of the maxilla to make room for half a porcine spine vertebra. We did not choose a real bone skull to avoid imaging interference with the samples. Other commercially available models, such as the full skull RANDO® phantom, were not appropriate for the purpose of this study since no samples can be placed in this model. The SawBone® skull allowed bone grafts to be placed at the correct positions equivalent to those in patients (Figure 1).
Figure 1.
Experimental set-up for the in vitro experiments. Top left: position of both samples in the maxillary sinus of the SawBone®. Top right: position of the SawBone® equivalent to the position in a patient (here the KaVo 3DeXam). Lower left to right: OsiriX 3D imaging of the experimental set-up with the SawBone® fading away to show the samples in the correct position
In vitro testing of porcine bone material
Porcine spines, already cut in half, were obtained from a Munich slaughterhouse. Half pieces of vertebral bone were mechanically separated and freed from connective tissue. 20 pieces were freshly frozen at −30°C; 20 other specimens were demineralized in 10% ethylenediaminetetraacetic acid/phosphate-buffered saline (EDTA-PBS; pH 7.4) for 7 days, and the solution was changed daily as previously described.9 The demineralized porcine bone pieces showed 75% mineralization in densitometry measurements. The pieces were then defatted with acetone. CBCT scanning was carried out with a SawBone® skull as a positioning device (Figure 1). Specimens were packed in a latex cover for hygiene reasons.
CBCT imaging data analysis
The devices used in this study were the 3D Accuitomo 40 and the KaVo 3DeXam. Data from both study groups were evaluated in the same manner. KaVo 3DeXam data (voxel size 0.3 mm; 120 kV and approximately 5 mAs) were descriptively evaluated by means of multiplanar reconstruction images of each dimension, which were produced with the OsiriX software using a bone window setting (OsiriX version 2.7.5 (OsiriX Foundation, Geneva, Switzerland); window level: 300.00; window width: 1500.00; resulting in a window between −450 and +1050 Hounsfield units).10 Data from the Morita Accuitomo (voxel size 0.25 mm; approximately 85 kV and 3.5 mAs) were analysed with proprietary viewer software. All image-processing steps were of clinical standard and did not result in contrast or pixel loss.
Image quality assessment
Two equal sets of images of patient data and of both in vitro experiment groups were evaluated by four independent observers (dentists certified by the German Board for CBCT imaging). Images such as those demonstrated in Figures 2–5, including two orthogonal displays, were presented to the examiners. Demineralized samples from each device were compared with fresh ones with the following standardized rating:
Figure 2.
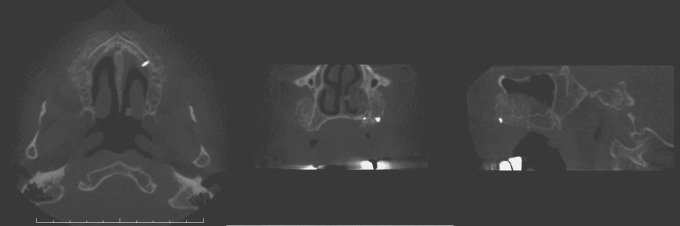
Example from the KaVo 3DeXam device the three radiological imaging cross-sections show the bone transplant in the area of the left alveolar rim in a patient (100 days after transplantation from the iliac crest)
Figure 5.
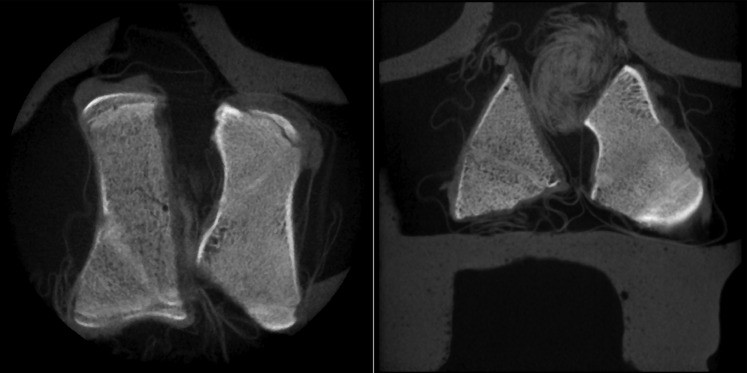
The in vitro test with the 3D Accuitomo device shows better contrast and sharper details at the bone trabeculae. Left, axial; right, coronal
0 = cancellous bone not circumscribed
1 = cancellous bone circumscribed
Clinical images of the Morita were compared with the KaVo 3DeXam with the following rating:
0 = augmentation circumscribed
1 = augmentation imaging completely satisfying
Results were statistically analysed with the unpaired t-test for patient data analysis and one-way ANOVA for the in vitro experiment. Statistical significance of differences between results was assumed if P < = 0.05.
Results
First part of the study: evaluation of patient data
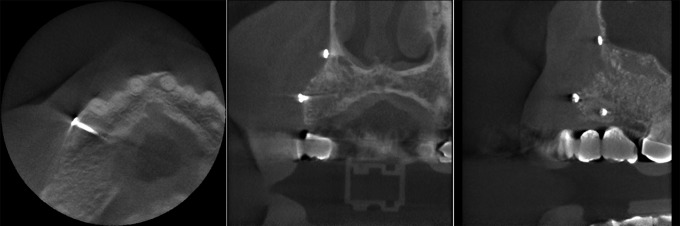
The descriptive analysis of the images showed well-visualized bone transplants in all cross-sections. The cancellous bone was clearly circumscribed and separated from the surrounding connective tissue. The images of the single bone trabeculae were of good quality. The images produced with the KaVo 3DeXam device showed softer borders and margins, whereas the Morita Accuitomo device showed better contrast and brightness (Figures 2 and 3). All images were sufficient for clinical use and implant planning.
Figure 3.
Example from the 3D Accuitomo device: the three radiological imaging cross-sections show the bone transplant in the frontolateral right alveolar rim in a patient (130 days after transplantation from the iliac crest)
Second part of the study: in vitro testing of porcine bone material in CBCT devices
EDTA treatment reduced mineralization by 25% in cancellous bone samples. These samples were compared with the non-demineralized freshly frozen bone samples, as described above (Figures 4 and 5). Images of each CBCT device showed good visualization in both sample groups. Demineralized samples showed lower radiographic opacity but still enabled visibility of large bone areas. Cancellous bone trabeculae were more easily seen with the Morita 3D Accuitomo device than with the KaVo 3DeXam device. However, contrast and brightness adjustments in the viewer program OsiriX allowed a similar overall definability of the entire bone block.
Figure 4.
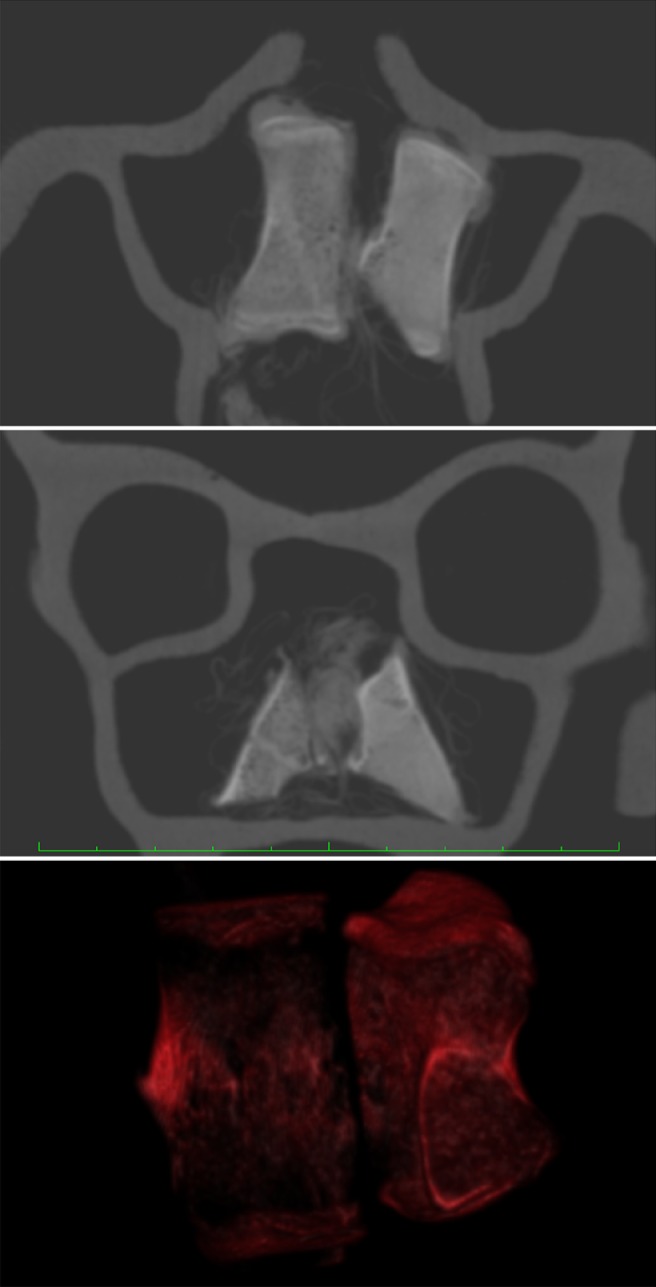
The in vitro test with the KaVo 3D shows both the freshly frozen and the partly demineralized bone sample in the OsiriX program. Top, axial view; middle, coronal view; bottom, 3D reconstruction
Image quality assessment
The analysis of patient data shows a significant difference between the Morita Accuitomo and the KaVo 3DeXam devices in the unpaired t-test (P = 0.038) (Figure 6). Data comparison of the in vitro image assessment by one-way ANOVA shows no difference between the data (P = 0.070).
Figure 6.
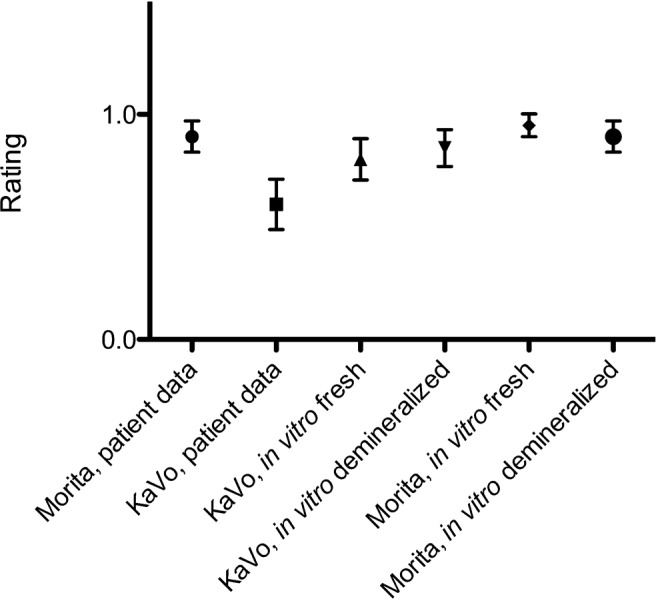
Image quality assessments. Patient data: 0, cancellous bone not circumscribable; 1, cancellous bone circumscribable. In vitro study: 0, augmentation definable from soft tissue; 1, augmentation imaging completely satisfactory
Discussion
Images of dentomaxillofacial structures were evaluated with CBCT.3,7,10–15 Bone transplants are important in dental implantology, but only a few reports on CBCT imaging quality have been published.14–17 A retrospective study examined the imaging quality of autologous bone grafts with the NewTom® 9000 CBCT device, describing a limited definition of cancellous bone in the remodelling phase.14,16 As a sequel to this study, we evaluated the imaging of bone transplants with the Morita 3D Accuitomo 40 and the KaVo 3DeXam CBCT devices in a two-part study. The examination included retrospective patient data and a new in vitro model with porcine spine bone blocks.
We applied a usual evaluation procedure for both CBCT devices. These methods of analysis were combined with a voxel size of 0.3 mm3 for the KaVo 3DeXam and 0.25 mm3 for the Morita Accuitomo to achieve the same standard of quality as in our clinical practice. Equivalence of the images with the OsiriX DICOM viewer compared with the primary software of the manufacturer was validated for the KaVo 3DeXam data.
Evaluation of skull bone imaging in CBCT devices has been described previously.18 The descriptive evaluation presented here is a common methodology.19 Some authors based quality assessments on density measurements.3 We did not consider Hounsfield values because of their inconsistency between the different CBCT devices; additionally, voxels are not always isotropic, as has been shown by MDCT.2 We combined the clinical descriptive results with a reproducible in vitro model that can be easily applied in other studies, which provides an interesting experimental tool in addition to those already available.
This retrospective study indicated that the Morita 3D Accuitomo and the KaVo 3DeXam devices provide appropriate imaging techniques for the imaging of cancellous bone grafts in in vivo remodelling. Both devices allowed a clear definition of the graft border towards the soft tissue. The Morita 3D Accuitomo showed sharper contrast and a clear definition of single bone trabeculae. The descriptive results were significant in the related image quality assessment. We assume that the wide range of manual adjustments available with the Accuitomo are the main reason for this advantage, whereas the KaVo settings are widely automated for faster processing. These results correspond to a previous study on the NewTom® 9000 CBCT device.3
The in vitro results show analogous outcomes. However, no significant difference existed between the two devices or between fresh and demineralized samples. The good overall visualization of freshly frozen and demineralized samples indicates the suitability of both devices for the imaging of cancellous bone transplants in remodelling and for the related planning of implant procedures. The difference between the two CBCT devices allows an estimation of quality differences in the detail resolution. These results indicate that the Morita Accuitomo device might be more appropriate for the imaging of particular trabecular bone structures. Thus, a larger number of patients were examined in vivo (n = 38) compared with the number of patients examined with the KaVo 3DeXam (n = 9).
In conclusion, it was shown that both the KaVo 3DeXam and the Morita 3D Accuitomo devices allow good visualization of cancellous bones, in in vivo remodelling and after in vitro demineralization.
References
- 1.Mozzo P, Procacci C, Tacconi A, Martini PT, Andreis IA. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur Radiol 1998;8:1558–1564 [DOI] [PubMed] [Google Scholar]
- 2.Harris D, Buser D, Dula K, Grondahl K, Haris D, Jacobs R, et al. E.A.O. guidelines for the use of diagnostic imaging in implant dentistry. A consensus workshop organized by the European Association for Osseointegration in Trinity College Dublin. Clin Oral Implants Res 2002;13:566–570 [DOI] [PubMed] [Google Scholar]
- 3.Draenert FG, Gebhart F, Neugebauer C, Coppenrath E, Mueller-Lisse U. Imaging of bone transplants in the maxillofacial area by NewTom 9000 cone-beam computed tomography: a quality assessment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:e31–e35 [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Grasruck M, Suess C, Bartling SH, Schmidt B, Stierstorfer K, et al. Ultra-high resolution flat-panel volume CT: fundamental principles, design architecture, and system characterization. Eur Radiol 2006;16:1191–1205 [DOI] [PubMed] [Google Scholar]
- 5.Kalender WA, Kyriakou Y. Flat-detector computed tomography (FD-CT). Eur Radiol 2007;17:2767–2779 [DOI] [PubMed] [Google Scholar]
- 6.Mahnken AH, Grasruck M, Schmidt B, Gunther RW, Wildberger JE. Flat panel computed tomography for non-invasive flow measurement: initial results in in-vitro studies. Eur Radiol 2008;18:747–752 [DOI] [PubMed] [Google Scholar]
- 7.Heiland M, Pohlenz P, Blessmann M, Habermann CR, Oesterhelweg L, Begemann PC, et al. Cervical soft tissue imaging using a mobile CBCT scanner with a flat panel detector in comparison with corresponding CT and MRI data sets. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:814–820 [DOI] [PubMed] [Google Scholar]
- 8.Draenert GF, Gebhart F, Al Nawas B, Wagner W. Demineralized bone and bone transplants in vivo/vitro in CBCTs (#1980). IADR/AADR/CADR 87th General Session and Exhibition (1–4 April 2009). Miami, USA; 2009. [Google Scholar]
- 9.Belanger LF, Copp DH, Morton MA. Demineralization with EDTA by constant replacement. Anat Rec 1965;153:41–47 [DOI] [PubMed] [Google Scholar]
- 10.Jalbert F, Paoli JR. Osirix: free and open-source software for medical imagery. Rev Stomatol Chir Maxillofac 2008;109:53–55 [DOI] [PubMed] [Google Scholar]
- 11.Prokop M. New challenges in MDCT. Eur Radiol 2005;15:E35–E45 [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Endo M, Obata T, Tsunoo T, Susumu K, Tanada S. Properties of the prototype 256-row (cone beam) CT scanner. Eur Radiol 2006;16:2100–2108 [DOI] [PubMed] [Google Scholar]
- 13.Kyriakou Y, Kalender WA. X-ray scatter data for flat-panel detector CT. Phys Med 2007;23:3–15 [DOI] [PubMed] [Google Scholar]
- 14.Loubele M, Guerrero ME, Jacobs R, Suetens P, van Steenberghe D. A comparison of jaw dimensional and quality assessments of bone characteristics with cone-beam CT, spiral tomography, and multi-slice spiral CT. Int J Oral Maxillofac Implants 2007;22:446–454 [PubMed] [Google Scholar]
- 15.Loubele M, Van Assche N, Carpentier K, Maes F, Jacobs R, van Steenberghe D, et al. Comparative localized linear accuracy of small-field cone-beam CT and multislice CT for alveolar bone measurements. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:512–518 [DOI] [PubMed] [Google Scholar]
- 16.Schulze D, Heiland M, Blake F, Rother U, Schmelzle R. Evaluation of quality of reformatted images from two cone-beam computed tomographic systems. J Craniomaxillofac Surg 2005;33:19–23 [DOI] [PubMed] [Google Scholar]
- 17.Loubele M, Maes F, Schutyser F, Marchal G, Jacobs R, Suetens P. Assessment of bone segmentation quality of cone-beam CT versus multislice spiral CT: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:225–234 [DOI] [PubMed] [Google Scholar]
- 18.Shapurian T, Damoulis PD, Reiser GM, Griffin TJ, Rand WM. Quantitative evaluation of bone density using the Hounsfield index. Int J Oral Maxillofac Implants 2006;21:290–297 [PubMed] [Google Scholar]
- 19.Stoppie N, Pattijn V, Van Cleynenbreugel T, Wevers M, Vander Sloten J, Ignace N. Structural and radiological parameters for the characterization of jawbone. Clin Oral Implants Res 2006;17:124–133 [DOI] [PubMed] [Google Scholar]