Abstract
Objectives
The aim of this study was to evaluate the antioxidant substances present in the human diet with an antimutagenic protective capacity against genotoxic damage induced by exposure to X-rays in an attempt to reduce biological damage to as low a level as reasonably possible.
Methods
Ten compounds were assessed using the lymphocyte cytokinesis-block micronucleus (MN) cytome test. The compounds studied were added to human blood at 25 μM 5 min before exposure to irradiation by 2 Gy of X-rays.
Results
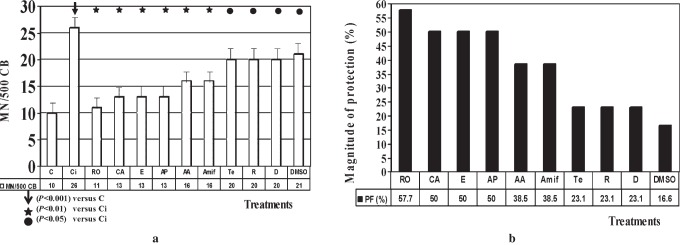
The protective capacity of the antioxidant substances assessed was from highest to lowest according to the frequency of the MN generated by X-ray exposure: rosmarinic acid = carnosic acid = δ-tocopherol = l-acid ascorbic = apigenin = amifostine (P < 0.001) > green tea extract = diosmine = rutin = dimetylsulfoxide (P < 0.05) > irradiated control. The reduction in genotoxic damage with the radiation doses administered reached 58%, which represents a significant reduction in X-ray-induced chromosomal damage (P < 0.001). This degree of protection is greater than that obtained with amifostine, a radioprotective compound used in radiotherapy and which is characterised by its high toxicity.
Conclusion
Several antioxidant substances, common components of the human diet and lacking toxicity, offer protection from the biological harm induced by ionizing radiation. Administering these protective substances to patients before radiological exploration should be considered, even in the case of small radiation doses and regardless of the biological damage expected.
Keywords: radiation effects, antimutagenic agents, antioxidant, micronuclei
Introduction
In developed countries, it is estimated that 440 dental radiology sets exist per 1 000 000 inhabitants, which represents 57% of all X-ray equipments used in medical radiology. The number of dental radiological explorations—310 000 per 1 000 000 inhabitants—represents 25.25% of all radiodiagnostic examinations made in the world's population. The annual mean effective dose is in the order of 1 mSv per year, of which about 90% is as a result of medical or dental radiodiagnosis.1-3 In Spain, professionally exposed workers (PEW) in dental installations account for only about 14% of all PEW in Spain in 2005, although 86.8% of PEW show exposures greater than 1 mSv per year. The number of radiological explorations and the number of PEW have both steadily increased in recent years,3 suggesting that an effort should be made to minimize the radiation doses administered and the subsequent appearance of lesions induced by ionizing radiation.2
With the doses administered in radiodiagnostics, deterministic effects (non-stochastic) should not appear, although at very low doses non-deterministic (stochastic) lesions may appear, including neoplastic diseases and somatic mutations that may contribute to other illnesses and heritable mutations that may increase the risk in future generations.4 To diminish the growing concern of radiation-induced somatic and heritable mutations, the concept of “as low as reasonably achievable” (ALARA) with respect to the administered dose was recommended by national and international agencies for both PEW and patients. The initial concept of radiation protection involved three physical principles: (1) shielding (usually by lead) of unexposed areas, especially radiosensitive organs; (2) increased distance between the radiation source and patients of PEW; and (3) reduction of exposure time.2,4 However, even though each of these factors has been useful, they have serious limitations in clinical practice.2-4
For this reason, new strategies for biological protection are needed to improve the efficacy of current efforts in reducing biological damage. In this study, we assess different antioxidant compounds that show a protective capacity against the chromosome damage induced by ionizing radiation and relate these effects with a new strategy for diminishing biological damage induced by ionizing radiation.
Materials and methods
Chemicals
82% carnosic acid (CA), green tea extract (90% catechins) (TE), apigenin (API) and diosmine (D) were supplied by Nutrafur-Furfural Español S.A. (Murcia, Spain), 95% rosmarinic acid (RO) was obtained from Extrasynthèse (Genay, France) and 99% l-ascorbic acid (AA) and 99% δ-tocopherol (E) were obtained from Sigma Co. (Madrid, Spain). Dimethylsulphoxide (DMSO) and rutin (R) were obtained from Merck (Darmstadt, Germany) and amifostine (AMF) was obtained from Schering-Plough, SA (Ethyol® injectable, Madrid, Spain).
Blood samples and irradiation procedure
Heparinized samples of human peripheral blood were obtained from two healthy, young, non-smoking female donors. The CA, E, R and D were dissolved in 5% aqueous DMSO mg ml–1, and AA, TE, API, AMF and RO were dissolved in water; 20 μl of these solutions were added to 2 ml of human blood to obtain a 25 μM concentration 5 min before irradiation. The DMSO group was included in this study, not only because it was added as a solvent, but also because it is generally considered to be a classical radical scavenger and radioprotective agent according to structural and experimental data.5,6
The blood samples were exposed to X-rays with an Andrex SMART 200E machine (YXLON International, Hamburg, Germany) operating at 120 kV, 4.5 mA, focus-object distance 74.5 cm at room temperature for 19 min 29 s with a dose rate of 103 mGy min–1 at a dose of 2 Gy ± 3%. The radiation doses were monitored by a UNIDOS® universal dosimeter with PTW Farme® ionization chamber TW 30010 (PTW-Freiburg, Freiburg, Germany) in the radiation cabin and the dose of radiation of X-ray was confirmed by means of thermoluminescent dosimeters (TLDs) (GR-200®, Conqueror Electronics Technology Co. Ltd, China). The TLDs were supplied and measured by CIEMAT (Ministry of Industry and Energy, Spain).
Culture technique
The micronucleus (MN) assessment was carried out on the human irradiated lymphocytes after X-irradiation (with the pre-treatment substances added), with the following cytokinesis-blocking (CB) method described by Fenech and Morley7 and adapted by the International Atomic Energy Agency (IAEA).8 Whole blood (1 ml) was cultured at 37°C for 72 h in 9 ml of F-10 medium (Sigma Co.) containing 15% fetal bovine serum (Sigma Co.), 1.6% phytohaemaglutinin (Sigma Co.) and 1% penicillin/streptomycin (Sigma Co.). 44 h after initiation of the culture of the lymphocytes, cytochalasin B (Cyt. B) (Sigma Co.) was added at a concentration of 3 μg ml–1. At 72 h the lymphocytes were treated with hypotonic solution (KCL, 0.075 M) for 3 min and fixed using methanol:acetic acid (3:1). Air-dried preparations were made and slides were stained with May–Grunwald–Giemsa.
Scoring of micronuclei
Triplicate cultures were analysed for each substance. In each, at least 500 CB cells (MN/500 CB) were examined by two specialists using a Zeiss light microscope (Oberkochem, Germany) with 400×magnification to examine the slides and 1000× magnification to confirm the presence or absence of MN in the cells (3000 CB/substance studied) according to the published criteria.7,9
Statistical analysis
The degree of dependence and correlation between variables was assessed using analysis of variance complemented by a contrast of means using P < 0.05. Quantitative means were compared by regression and lineal correlation analysis.
Their results were used to obtain the magnitude of protection:
 |
where Fcontrol = frequency of MN in irradiated blood lymphocytes and Ftreated = frequency of MN in blood lymphocytes treated pre- and post-γ irradiation as described previously.5,10
Results
The basal frequency of the MN/500 CB in this study was 10 ± 2 MN/500 CB for the non-irradiated control blood samples and 28 ± 4 MN/500 CB for the control samples irradiated with 2 Gy (P < 0.001). The addition of the substances assessed produced no significant differences with respect to the non-irradiated controls. Figure 1 shows a photomicrograph of lymphocytes cultured with CB cells stained with May–Grünwald–Giemsa.
Figure 1.
Photomicrograph of lymphocytes cultured with cytokinesis-blocked (CB) cells stained with May–Grünwald–Giemsa (400×)(CB: cytokinesis-blocked cells; CBMN: CB cells with micronuclei; E: sample of haemolysed erythrocytes)
Figure 2a shows the frequency of appearance of micronuclei and the statistical significance obtained with a frequency of MN/500 CB. Figure 2b depicts the magnitude of protection calculated for each of the treatments. In the X-ray treatments, the capacity of protection, from highest to lowest according to the frequency of the MN generated by X-rays exposition, was: RO = CA = E = AA = API = AMF (P < 0.001) > TE = D = R = DMSO (P < 0.05) > irradiated control.
Figure 2.
(a) Frequency of micronuclei in 500 cytokinesis-blocked (CB) cells. (b) Magnitude of protection (%) of different treatments administered before irradiation with X-rays. Control (C); control irradiated (Ci); carnosic acid (CA); green tea extract (TE); apigenin (API); diosmine (D); rosmarinic acid (RO); l-ascorbic acid (AA); δ-tocopherol (E); dimethylsulphoxide (DMSO); rutin (R); amifostine (AMF)
Discussion
Two opposing hypotheses on the potential risk of low doses of radiation in humans are the subject of debate: most radiobiologists believe that diagnostic doses of ionizing radiation should not be considered insignificant for risks of somatic and heritable mutations or neoplastic and non-neoplastic diseases in humans,4 although some radiation scientists suggest that diagnostic doses of radiation do not contribute to health risks in humans.11
Two cytogenetic tests based on the increased frequency of appearance of MN are the most commonly used tests for determining the mutagenic capacity of a chemical or physical genotoxic agent: the in vivo test on mouse bone medulla12 and the in vitro test on human lymphocytes irradiated with the cytogenetic blocking test.7-9 With these two tests, the protective effect of different compounds has been established from the reduction in MN levels after exposure to different genotoxic agents (chemical and physical), among them ionizing radiation, as is the case this study.5,11,12,13-18
With the low doses of ionizing radiation administered in simple dental radiodiagnostic exposures, contradictory effects have been described with the in vitro micronucleus test.19,20 We used the MN test to reveal the absence of any genotoxic lesion induced in patients who have been submitted to diagnostic nuclear medicine explorations,21 but also to describe the genotoxic effect of higher doses than those used in ablative treatments for thyroid cancer or complex radiodiagnostic explorations.22,23 In agreement with the observations of other authors, we described how the sensitivity of the MN test still does not allow us to detect the possible effect of ionizing radiation when using doses as low as those used in simple radiodiagnostic explorations, in spite of the modifications of the test that have been carried out during recent years.9,22
When this threshold of sensitivity is reached in the MN test, ionizing radiation shows a significant genotoxic capacity with the number of MN that show radiation-induced chromosomal damage increasing. We also used the MN test to evaluate the protection capacity of several antioxidant compounds against gamma radiation. It was seen how some pure flavonoids (diosmine and rutin) and polyphenolic extracts show greater protective capacity than traditional radioprotectors, for example sulfhydryl compounds and even vitamin C, against X-rays in vivo5,6,24 and γ-radiation in vitro.25 In the present, we use different substances in vitro with X-rays and obtain similar results of genoprotective effects.
We have previously described how this antimutagenic effect is proportional to the antioxidant capacity,26 although dependent on the bio-availability in the medium tested. Accordingly, we observed that the flavan-3-ols show the greatest protective capacity of all the polyphenols,5 while other flavonoids with a greater antineoplastic and antiproliferative capacity show a lower antimutagenic capacity.27-29 Continuing the search for compounds with a greater antioxidant capacity, we have described other substances with a different chemical structure (RO) that show greater genoprotective capacity.18 This capacity also depends on the degree of polymerization and solubility of the substances tested, since both modify their bio-availability.17,30,31
The different protection mechanisms and their relation with the chemical structure and the moment of exposure to gamma radiation have been described previously,31 and we consider that the same applies in the case of exposure to X-rays. In radiodiagnosis and nuclear medicine the moment of exposure can be chosen, which means that it is possible to select non-toxic, water-soluble antioxidants that show great bioavailability and that may be common components of the human diet to reduce the genotoxic effect induced by the radiation about to be applied.
Obviously, even if the test detects increased MN frequency and, therefore, increased chromosomal damage in patients, the real biological consequences with regards to the risk of later lesion still needs to be established.21,22 At present, it is widely believed that an increase in the level or number of chromosomal alterations may indicate an increased risk of cancer7,12 although the results of several epidemiological studies suggest that this is not necessarily the case.32,33
Amifostine is the most commonly used radioprotector in radiotherapy.34 However, the fact that it is a sulfhydryl compound with a high level of toxicity means that it should not be used in radiological protection.35 The results of this study suggest that several non-toxic compounds might be used instead. For example, the protective effect of RO against possible damage from X-rays in radiodiagnostic explorations should be mentioned, although we have already described how it can protect against the damage induced by gamma radiation36 and even against the damage induced by ultraviolet radiation.37
The possibility of using such compounds before carrying out diagnostic explorations with ionizing radiation and of increasing the levels of antioxidants in the organism of PEW in an attempt to reduce the harmful effect of radiation represents a new strategy for reducing the biological damage to ALARA levels at the present time.
In conclusion, the use of antioxidant, non-toxic substances that form part of the normal human diet may offer protection against the biological damage induced by ionizing radiation. Administering them to patients before radiological exploration should not depend on the level of radiation to be applied or on the biological damage expected.
Acknowledgments
This report was supported by a grant from the National Spanish R&D Programme CENIT of the Spanish Ministry of Science and Technology denominated SENIFOOD.
References
- 1.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 2001 Report. Hereditary Effects of Radiation. United Nations Publications, Vol II, Sales Nos.E00IX. Available from: http://www.unscear.org/docs/reports/2001/2001Annex_pages%208-160.pdf. [Google Scholar]
- 2.Alcaraz M, Martínez-Beneyto Y, Pérez L, Jódar S, Velasco E, Canteras M. The status of Spain's dental practices following the European Union directive concerning radiological installations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:476–482 [DOI] [PubMed] [Google Scholar]
- 3.Alcaraz M. Dental radiology in Spain (1st edn) Murcia: Spanish Council of Nuclear Safety-Service of Publications of the University of Murcia, 2008. (in Spanish) [Google Scholar]
- 4.Prasad KN. Rationale for using multiple antioxidants in protecting humans against low doses of ionizing radiation. Br J Radiol 2005;78:485–492 [DOI] [PubMed] [Google Scholar]
- 5.Castillo J, Benavente-García O, Lorente J, Alcaraz M, Redondo A, Ortuño A, et al. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (procyanidins) from grape seeds (Vitis vinifera): Comparative study versus other phenolic and organic compounds. J Agr Food Chem 2000;48:1738–1745 [DOI] [PubMed] [Google Scholar]
- 6.Benavente-García O, Castillo J, Lorente J, Alcaraz M. Radioprotective effects in vivo of phenolics extracted from Olea europaea L. leaves against X-ray-induced chromosomal damage: comparative study versus several flavonoids and sulfur-containing compounds. J Med Food 2002;5:125–135 [DOI] [PubMed] [Google Scholar]
- 7.Fenech M, Morley A. Measurement of micronuclei in lymphocytes. Mutation Res 1985;147:29–36 [DOI] [PubMed] [Google Scholar]
- 8.International Atomic Energy Agency Cytogenetic analysis for radiation dose assessment. A manual. Techical Reports Series no 405 Vienna, Austria: IAEA, 2001 [Google Scholar]
- 9.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc 2007;2:1084–1104 [DOI] [PubMed] [Google Scholar]
- 10.Sarma L, Kesavan PC. Protective effects of vitamin C and E against γ-ray-induced chromosomal damage in mouse. Int J Radiat Biol 1993;63:759–764 [DOI] [PubMed] [Google Scholar]
- 11.Feinendegen LE. Significance of basic and clinical research in radiation medicine: challenges for the future. Br J Radiol 2005;Supplement 27:185–195 [Google Scholar]
- 12.Schmid W. The micronucleus test. Mutation Res 1975;31:9–15 [DOI] [PubMed] [Google Scholar]
- 13.Baliga MS, Jagetia GC, Venkatesh P, Reddy R, Ullor JN. Radioprotective effect of abana, a polyherbal drug following total body irradiation. Br J Radiol 2004;77:1027–1035 [DOI] [PubMed] [Google Scholar]
- 14.Prasad KN, Cole WC, Haase GM. Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol 2004;77:97–99 [DOI] [PubMed] [Google Scholar]
- 15.Sarma L, Devasagayan TPA, Mohan H, Mittal JP, Kesavan PC. Mechanism of protection by buthionine sulphoximine against γ ray-induced micronuclei in polychromaytic erythrocytes of mouse bone marrow. Int J Radiat Biol 1996;69:633–643 [DOI] [PubMed] [Google Scholar]
- 16.Abraham SK, Sarma L, Kesavan PC. Protective effects of chorogenic acid, curcumin and β-carotene against γ-radiation-induced in vivo chromosomal damage. Mutat Res 1993;303:109–112 [DOI] [PubMed] [Google Scholar]
- 17.Castillo J, Benavente-García O, Del Baño MJ, Lorente J, Alcaraz M, Dato MJ. Radioprotective effects against chromosomal damage induced in human lymphocytes by gamma-rays as a function of polymerization grade of grape seed extracts. J Med Food 2001;4:117–123 [DOI] [PubMed] [Google Scholar]
- 18.Del Baño MJ, Castillo J, Benavente-García O, Lorente J, Martín-Gil R, Acevedo C, et al. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J Agr Food Chem 2006;54:2064–2068 [DOI] [PubMed] [Google Scholar]
- 19.Popova L, Kishkilova D, Hadjidekova VB, Hristova RP, Atanasova P, Hadjidekova VV, et al. Micronucleus test in buccal epithelium cells from patients subjected to panoramic radiography. Dentomaxillofac Radiol 2007;36:168–171 [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira EMM, Meireles JRC, Lopes MA, Junqueira VC, Gomes-Filho IS, Trindade S, et al. Genotoxic effects of X-rays on keratinised mucosa cells during panoramic dental radiography. Dentomaxillofac Radiol 2008;37:398–403 [DOI] [PubMed] [Google Scholar]
- 21.Navarro JL, Alcaraz M, Gómez-Moraga A, Vicente V, Canteras M. Absence of chromosomic and genotoxic damage from the radiation dose administered in scintigraphic examinations. Rev Esp Med Nucl 2004;23:174–182 [DOI] [PubMed] [Google Scholar]
- 22.Alcaraz M, Gómez-Moraga A, Dato MJ, Navarro JL, Canteras M. Efecto genotóxico inducido por la exposición a rayos X durante exploraciones complejas de radiodiagnóstico médico. Oncología 2002;25:159–168 (in Spanish). [Google Scholar]
- 23.Serna A, Alcaraz M, Navarro JL, Acevedo C, Vicente V, Canteras M. Biological dosimetry and Bayesian analysis of chromosomal damage in thyroid cancer patients. Radiat Prot Dosimetry 2007;19:1–9 [DOI] [PubMed] [Google Scholar]
- 24.Castillo J, Alcaraz M, Benavente-García O. Antioxidant and radioprotective effects of olive leaf extract. In: Preedy VR, Watson RR. Olives and olive oil in health and disease prevention. Oxford: Academic Press, 2010, pp 951–958 [Google Scholar]
- 25.Benavente-García O, Castillo J, Lorente J, Alcaraz M, Yánez J, Martínez C, et al. Antiproliferative activity of several phenolic compounds against melanoma cell lines. Agroo Food Industries Hi-tech 2005;16:30–34 [Google Scholar]
- 26.Benavente-Garcia O, Castillo J, Marín F, Ortuño A, del Rio JA. Uses and properties of citrus flavonoids. J Agr Food Chem 1997;45:4505–4515 [Google Scholar]
- 27.Martínez-Conesa C, Vicente V, Yáñez MJ, Alcaraz M, Canteras M, Benavente-García O, et al. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agr Food Chem 2005;53:6791–6797 [DOI] [PubMed] [Google Scholar]
- 28.Yáñez J, Vicente V, Alcaraz M, Castillo J, Benavente-García O, Canteras M, et al. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: relationship between structure and activity. Nutr Cancer 2004;49:191–199 [DOI] [PubMed] [Google Scholar]
- 29.Benavente-García O, Castillo J, Alcaraz M, Vicente V, del Rio JA, Ortuño A. Beneficial action of citrus flavonoids on multiple cancer-related biological pathways. Current Cancer Drug Targets 2007;7:3325–3334 [DOI] [PubMed] [Google Scholar]
- 30.Castillo J, Piedad M, Marchante C, del Baño MJ, Lorente J, Benavente García O, et al. Citroflavonoids and Flavon-3-ols. High radioprotective capacity and prospects for health. Nutrafoods 2002;1:27–39 [Google Scholar]
- 31.Alcaraz M, Acevedo C, Castillo J, Benavente-García O, Armero D, Vicente V, et al. Liposoluble antioxidants provide an effective radioprotective barrier. Br J Radiol 2009;82:605–609 [DOI] [PubMed] [Google Scholar]
- 32.Hall P, Boice JD, Berg G. Leukaemia incidence after iodine-131 exposure. Lancet 1992;340:1–4 [DOI] [PubMed] [Google Scholar]
- 33.Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, et al. Cancer mortality following treatment for adult hyprthyroidism. JAMA 1998;280:347–355 [DOI] [PubMed] [Google Scholar]
- 34.Koukourakis MT. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs 2002;13:181–209 [DOI] [PubMed] [Google Scholar]
- 35.Kouvaris JR, Koloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 2007;12:738–47 [DOI] [PubMed] [Google Scholar]
- 36.Del Baño MJ, Castillo J, Benavente-García O, Lorente J, Martín-Gil R, Acevedo C, et al. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by γ-rays. J Agric Food Chem 2006;54:2064–2068 [DOI] [PubMed] [Google Scholar]
- 37.Sánchez Campillo M, Gabaldon JA, Castillo J, Benavente-García O, Del Baño MJ, Alcaraz M, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol 2009;47:386–392 [DOI] [PubMed] [Google Scholar]