Abstract
Objectives
The aim of this study was to determine the relative frequencies and clinico-pathological features of odontogenic tumours affecting the jaw bones of patients in the first two decades of life and of rural and periurban African extract.
Methods
Files of patients younger than 20 years of age diagnosed over a period of 26 years with odontogenic tumours were retrieved and analysed for gender, site, tumour size and radiographic appearance.
Results
33% of odontogenic tumours diagnosed in the population sample presented during the first 2 decades of life. Ameloblastoma was the most frequent benign tumour (43%) followed by keratocystic odontogenic tumour (19%) and adenomatoid odontogenic tumour (10%). Four patients (1.6%) presented with ameloblastic carcinoma.
Conclusions
Owing to the unique population demographics of South Africa, odontogenic tumours in the first two decades of life comprise a larger percentage of the total number of cases than in other communities. The frequency of the different odontogenic tumour types generally follows the pattern of those reported in Africa, China and parts of South America. Radiographic examination is indispensable in establishing an accurate diagnosis.
Keywords: odontogenic pathology, paediatric tumours, diagnostic imaging
Introduction
Odontogenic tumours arise from the tooth-forming tissues of the jaws and encompass a spectrum of pathological proliferations ranging from those with a hamartomatous nature and limited growth potential to aggressive neoplasms with a metastatic potential. Their aetiology is unknown and the majority develop without an apparent cause. Most arise within the bones of the jaw (central odontogenic tumours) and less commonly in the surrounding soft tissues (peripheral odontogenic tumours). The majority of central odontogenic tumours are asymptomatic, diagnosed in a late expansive stage and require wide excision with devastating consequences in children for dental development and jaw growth. The most recent World Health Organization (WHO) Classification of Odontogenic Tumours1 is based on the germ cell layer of origin and differs in several aspects from the previous 1992 classification.2 Odontogenic keratocysts and calcifying odontogenic cysts were grouped under “cysts” in the 1992 WHO classification. Their aggressive behaviour served as motivation for the 2005 WHO Working Group for a renaming and regrouping with odontogenic tumours as a “keratocystic odontogenic tumour” and “calcifying cystic odontogenic tumour”, respectively. Only three studies reported in recent literature are based on the 2005 WHO classification.3-5 Although these studies indicate that regional differences exist in the relative frequencies of odontogenic tumours in different population samples, little is known about their occurrence in a paediatric African population sample.
The aim of this study was to determine the relative frequencies and clinicopathological features of odontogenic tumours affecting the jaw bones of patients in the first two decades of life and of rural and peri-urban South African extract.
Materials and methods
Odontogenic tumours located centrally (within the jaw bones) and diagnosed in patients in the first two decades of life between 1982 and 2008 were retrieved from the files of the Departments of Oral Pathology and Maxillofacial and Oral Radiology at the Oral Health Centre of the University of Limpopo, South Africa. The Health Centre serves the rural and peri-urban black population of the northern part of South Africa. The microscopic diagnosis of each tumour was reviewed and reclassified according to the 2005 WHO classification.1 The patient demography, tumour size (measured in centimetres as the longest axis on the excision specimen) and site of occurrence were analysed and compared with findings in the literature.
The literature was retrieved using Scopus and PubMed in English only. For expressing the site of occurrence, the maxilla was divided into left or right, anterior, premolar and molar segments and the mandible into left or right anterior, premolar, molar and ramus segments.
Results
743 odontogenic tumours were diagnosed in the Oral Health Centre of the University of Limpopo during the 26-year period under review. Of these, 254 (33%) occurred in 244 patients in the first 2 decades of life and 250 were benign and 4 malignant. Eight patients presented with more than one keratocystic odontogenic tumour and two patients with two odontogenic fibromas. Table 1 reflects the relative frequency and gender distribution of the patients and Table 2 the site distribution of the tumours. 34% (n = 109) of the total sample of 324 ameloblastomas occurred in the first 2 decades of life. 45% (n = 49) of ameloblastomas were unilocular and of these 19 (39%) were in the anterior region of the jaw bones. 79% of ameloblastomas in the maxilla were unilocular in contrast with the mandible, where 40% (n = 38) were unilocular. The ameloblastomas ranged in size between 5 cm and 12 cm and the majority (n = 20) affected the mandibular molar area and extended into the ramus. All ameloblastomas presented with gross expansion of the cortical plates of the affected jaw bones (Figures 1a,b) and the main complaint of most patients was related to aesthetics. In contrast, almost all keratocystic odontogenic tumours were less than 10 cm in diameter (only one case measured 12 cm) and mandibular lesions failed to show cortical expansion except for one case that extended into the ramus. The majority (72%) exhibited sclerotic borders radiographically and only 12 cases (26%) were multilocular. Most (83%) were associated with impacted teeth in the lesion. Eight patients presented with multiple keratocystic odontogenic tumours (Figure 2). Adenomatoid odontogenic tumours diagnosed in the first 2 decades comprised 76% of the total number of adenoid odontogenic tumours (n = 33) diagnosed in all age groups. The sizes of most were less than 5 cm in diameter. Two cases, both in the maxilla, presented with gross expansion and measured more than 8 cm. The majority were associated with one or more impacted teeth contributing to an erroneous radiological impression of a dentigerous cyst (Figure 3a). Only three cases (two in the maxilla, one in the mandible) were not associated with impacted teeth (Figure 3b). One case showed delicate mural calcifications radiologically. Both complex and compound odontomas occurred in the anterior and posterior regions of the jawbones. In the mandible the compound type was frequent in the molar region (n = 3), whereas in the maxilla the complex type dominated the anterior region (n = 4). The majority of odontogenic myxomas were multilocular (n = 8) with a tennis racquet (n = 5) (Figure 4), soap bubble (n = 2) or honeycomb (n = 1) appearance. Most myxomas (n = 9) displaced adjacent teeth and 50% (n = 6) were associated with root resorption. Odontogenic fibromas showed a predilection for females (n = 9, 90% of cases) and 2 females presented with 2 tumours each. The ameloblastic fibroma, fibro-odontoma and fibrodentinoma group of neoplasms showed an equal gender distribution and uni- or multilocular appearances. The ameloblastic fibro-odontomas and fibrodentinomas were distinguished from the ameloblastic fibromas by the presence of dental hard-tissue deposits, which manifested as radiopacities (Figure 5). 8 out of a total of 16 calcifying cystic odontogenic tumours occurred in the first 2 decades of life. A notable tendency for involvement of the canine-premolar areas of both jaws was noted and the tumours were either well circumscribed unilocular (n = 6), well circumscribed multilocular (n = 1) or poorly circumscribed (n = 1) with (n = 2) or without (n = 6) calcifications on the radiographs. The 4 odontogenic carcinomas (which comprised 17% of odontogenic malignancies in our total sample) were located in the posterior regions of the jaws and showed locally destructive, poorly circumscribed expansive growth characteristics (Figure 6). The calcifying epithelial odontogenic tumours (n = 3) were multilocular (one case showed a honeycomb radiological appearance) and moderately circumscribed with calcifications (Figure 7). All cases were associated with absent teeth. Both cementoblastomas were asymptomatic, firmly attached to the roots of molar teeth and presented as radiopacities surrounded by a radiolucent margin (Figure 8).
Table 1. Gender distribution of odontogenic tumours in children (combined number of tumours in adults and children in parenthesis).
| Female |
Male |
||||
| Tumour type | Total | n | % | n | % |
| Ameloblastoma (324) | 109 | 42 | 39 | 67 | 61 |
| Keratocystic odontogenic tumour (171) | 39a | 16 | 41 | 23 | 59 |
| Adenomatoid odontogenic tumour (33) | 25 | 22 | 88 | 3 | 12 |
| Odontoma (38) | 21 | 8 | 38 | 13 | 62 |
| Odontogenic myxoma (55) | 12 | 8 | 66 | 4 | 34 |
| Ameloblastic fibroma/fibrodentinoma/fibro-odontoma (20) | 11 | 6 | 60 | 5 | 40 |
| Odontogenic fibroma (30) | 10b | 9 | 95 | 1 | 10 |
| Calcifying cystic odontogenic tumour (21) | 8 | 4 | 50 | 4 | 50 |
| Ameloblastic carcinoma (23) | 4 | 1 | 25 | 3 | 75 |
| Calcifying epithelial odontogenic tumour (12) | 3 | 1 | 33 | 2 | 66 |
| Cementoblastoma (16) | 2 | 1 | 50 | 1 | 50 |
| 244 | 118 | 126 | |||
aEight patients presented with more than one tumour
bTwo females presented with two tumours each
Table 2. Site distribution.
| Tumour | Maxilla |
Mandible |
||||||||||
| A | P | M | U | A | P | M | PC | MC | MR | R | U | |
| Ameloblastoma | 8 | 1 | 1 | 4 | 22 | 4 | 2 | 14 | 20 | 6 | 0 | 27 |
| Keratocystic odontogenic tumour | 8 | 3 | 2 | 9 | 4 | 1 | 0 | 6 | 1 | 2 | 1 | 10 |
| Adenomatoid odontogenic tumour | 7 | 4 | 0 | 4 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 4 |
| Odontoma | 8 | 0 | 0 | 6 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 2 |
| Odontogenic myxoma | 2 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Odontogenic fibroma | 0 | 0 | 0 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| Calcifying cystic odontogenic tumour | 1 | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ameloblastic fibroma, fibrodentinoma and fibro-odontoma | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 3 | 1 | 0 | 1 |
| Ameloblastic carcinoma | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Calcifying epithelial odontogenic tumour | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cementoblastoma | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
A, anterior region (incisors and canine); P, premolar region (premolar 1 and 2); M, molar region (Molar 1, 2 and 3); PC, premolar–canine region; MC, molar, premolar and canine; MR, molar and ramus; R, ramus; U, unknown
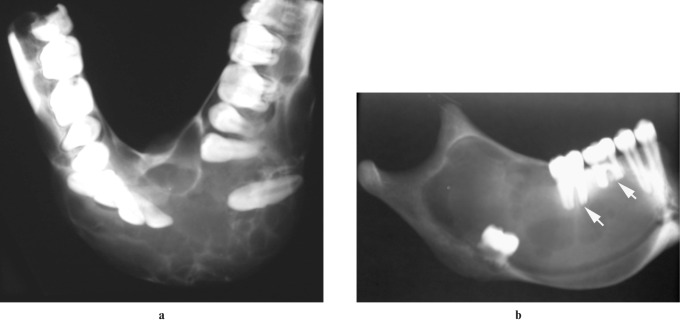
Figure 1.
Radiographs of resection specimen of mandibular ameloblastomas. (a) Extensive cortical expansion and tooth displacement of the multilocular variety. (b) Extension of a unilocular ameloblastoma into the ramus. Note the displacement of the germ of a molar tooth and resorption of the roots of the molars (arrows)
Figure 2.
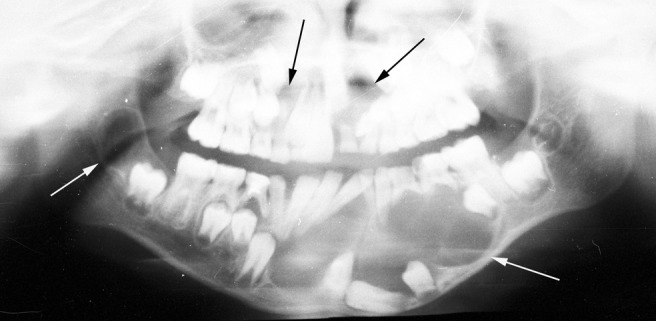
Panoramic radiograph of a 10-year-old patient with multiple keratocystic odontogenic tumours (white and black arrows)
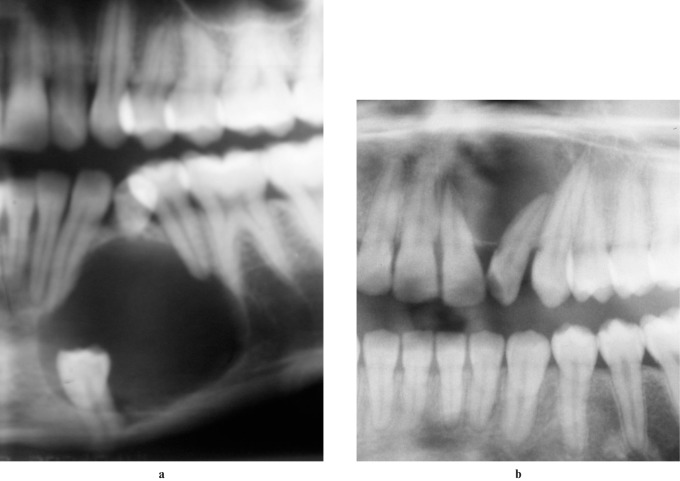
Figure 3.
Radiographs of patients with adenomatoid odontogenic tumours. (a) A cropped panoramic radiograph showing the follicular variety associated with an impacted tooth. (b) A cropped panoramic radiograph of the extrafollicular variety not associated with an impacted tooth. Note the displacement of the lateral incisor and external root resorption of the central incisor
Figure 4.
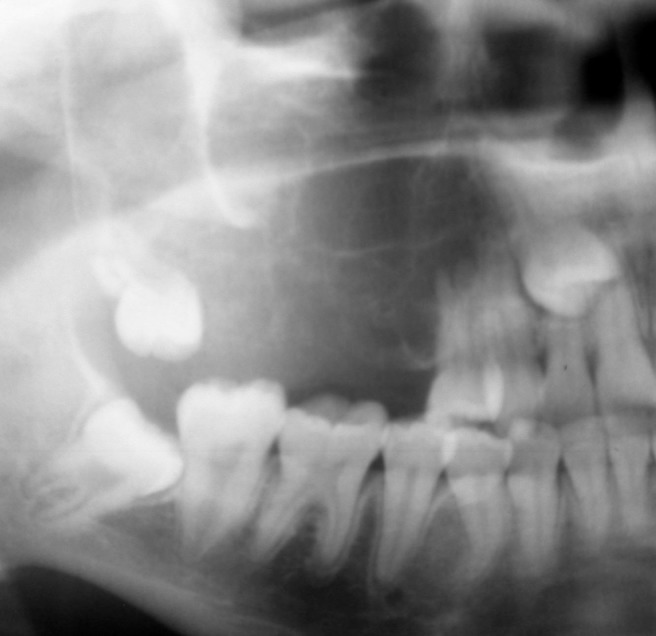
Cropped panoramic radiograph of an odontogenic myxoma in the right posterior maxilla. Note the perpendicular angle between the delicate septa resulting in a tennis racquet appearance
Figure 5.
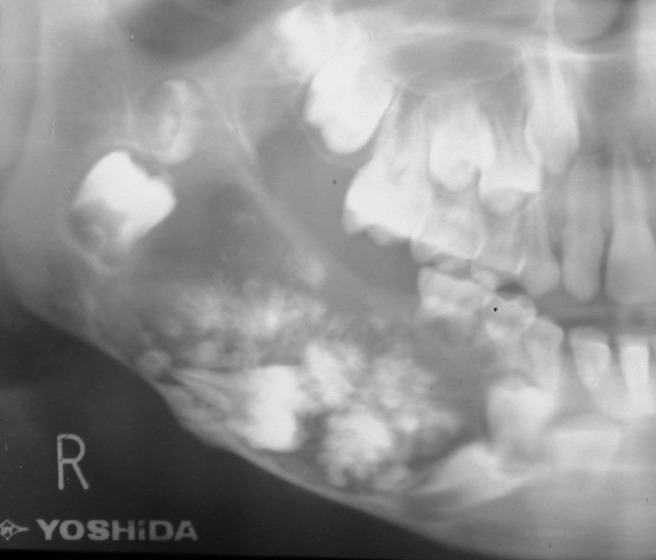
Panoramic radiograph of an ameloblastic fibro-odontoma. Note the coarse calcifications in the lesion and the impacted and displaced molars and premolars
Figure 6.
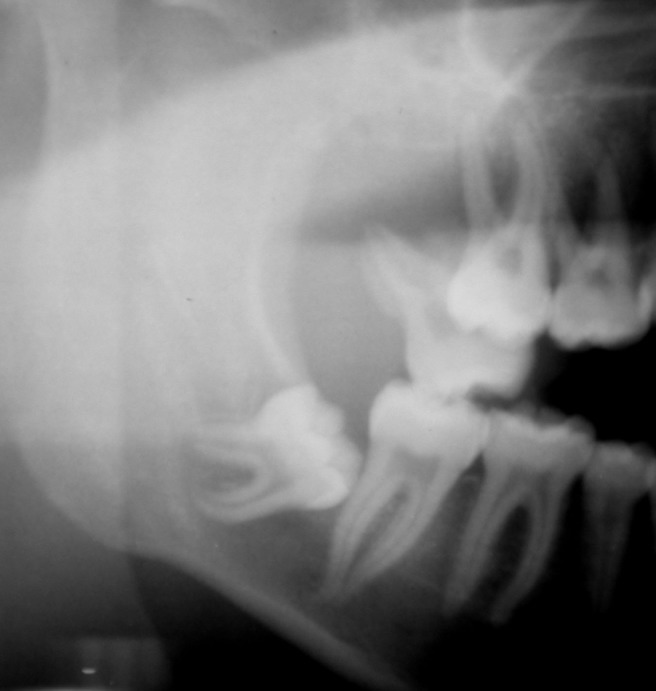
Cropped panoramic radiograph of an ameloblastic carcinoma in the posterior maxilla. Note the poor demarcation and molar tooth displaced from its position in the alveolar bone
Figure 7.
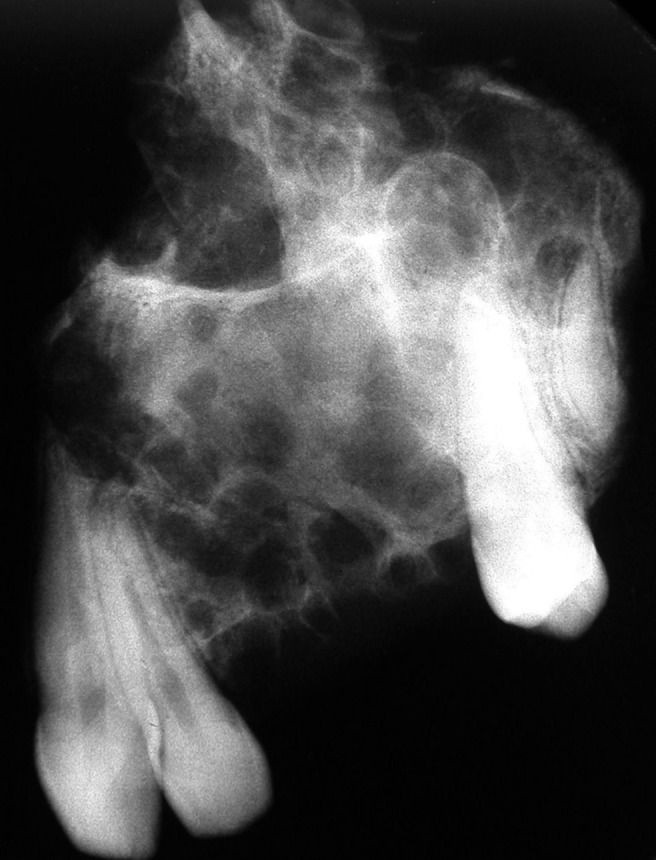
Radiograph of a hemimaxillectomy of a calcifying epithelial odontogenic tumour. Note the moderate circumscription, honeycomb appearance and delicate calcifications in the locules
Figure 8.
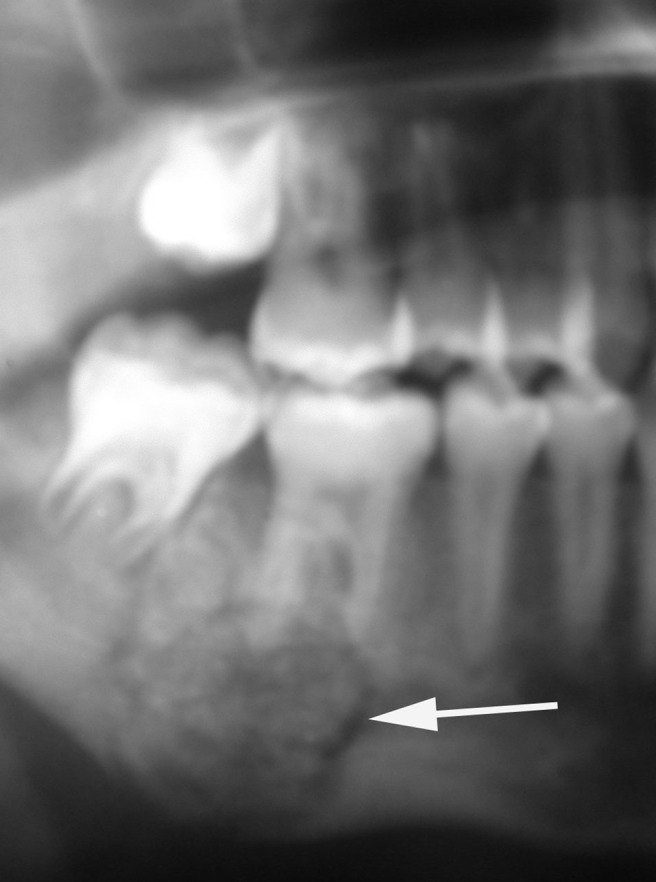
Cropped panoramic radiograph of a cementoblastoma in the posterior mandible. Note the typical radiolucent space surrounding the growth which is firmly attached to the roots of the tooth (white arrow)
Discussion
Over the 26 year period of study, a total of 743 odontogenic tumours were diagnosed in the Oral Health Centre at the University of Limpopo. Of these, 254 (33%) occurred during the first 2 decades of life. In the Chinese study based on the 2005 WHO classification,1 Jing et al3 reported 354 odontogenic tumours in the first 2 decades of life in a total sample of 1642 cases (21.5% of cases). Our higher percentage (33% of cases) is most likely the result of the population demographics of South Africa, where 20.1 million (42.6%) South Africans are below the age of 20 years.6
The most common odontogenic tumour in the first 2 decades of life in our study was ameloblastoma (43% of our sample), with frequencies comparable to reports from Egypt,4 Nigeria,7 Brazil,8 China3 and Sri Lanka.9 Data from Mexico,10 California11 and Argentina12 indicate odontoma to be the most frequently diagnosed odontogenic tumour. This may be the result of differences in dental awareness between the latter regions and Africa as most odontomas are asymptomatic and diagnosed on routine dental radiographs, whereas all patients with ameloblastomas ultimately present for surgical management. However, caution is expressed when comparing studies like these with those based on the new 2005 classification owing to the inclusion of several new entities in the latter. Extension into the mandibular ramus, a uni- or multilocular appearance without calcifications and gross expansion were the hallmarks of all ameloblastomas in our study. The lack of clinical evidence of bony expansion (particularly when located in the body of the mandible) or multiple lesions may place keratocystic odontogenic tumour higher on the list of differential diagnosis than ameloblastoma.
Although not recorded in this study, multiple keratocystic odontogenic tumours may be one of the features of the naevoid basal cell carcinoma syndrome, characterized amongst others by skin, calvarial, rib and vertebral anomalies.13
Unlike ameloblastomas, myxomas have a predilection for the maxilla and affect more females. The majority of odontogenic myxomas in our total sample of odontogenic myxomas diagnosed at all ages (n = 55) presented later than the second decade of life. The cases diagnosed in the first 2 decades (n = 12) comprised a smaller percentage (22%) of our sample of myxomas than in the 2005 Chinese study (39%).3 Our study supports the idea that a tennis racquet radiographic appearance is unique to myxomas.14,15 The honeycomb appearance of one myxoma was shared with a calcifying epithelial odontogenic tumour. Fine calcifications may be indicative of an adenomatoid odontogenic tumour, calcifying epithelial odontogenic tumour, ameloblastic fibrodentinoma or fibro-odontoma. Adenomatoid odontogenic tumours may resemble dentigerous cysts; however, they appear not to affect the molar regions and are more common in the maxilla and in female patients with a male-to-female ratio of 1:8.3. This ratio is higher than in some Asian countries where a male-to-female ratio of up to 1:3.2 has been reported.16 Follicular and extrafollicular types of adenomatoid odontogenic tumours have been described.1 The former is believed to originate from the follicle of an unerupted tooth and is therefore always associated with an impacted tooth. The extrafollicular type of adenomatoid odontogenic tumour probably arises from embryonal odontogenic epithelial remnants outside a developing tooth and is therefore not associated with an impacted tooth. The distinction between the follicular and extrafollicular types may have theoretical value only, as larger extrafollicular types may impact on adjacent tooth follicles not involved in the histogenesis of the tumour, giving a false follicular appearance.
Coarse calcifications may be found in calcifying cystic odontogenic tumours, odontoma and cementoblastoma. In the latter, the calcification is attached to the root of a tooth and surrounded by a radiolucent rim.17 In the 2005 WHO classification, ameloblastic carcinomas are categorized into the primary and secondary intraosseous and secondary peripheral types.1 Both secondary types of ameloblastic carcinomas arise in pre-existing benign ameloblastomas. The 4 ameloblastic carcinomas (out of a total of 23 odontogenic malignancies diagnosed at all ages in our sample) were of the primary intraosseous type as all were located within bone and no microscopic evidence of a pre-existing ameloblastoma could be found with microscopic examination. They were poorly demarcated, infiltrative growths that frequently manifested radiographically with teeth floating in a radiolucent space. Histologically ameloblastic carcinomas combine the features of an ameloblastoma with cytological atypia and increased mitotic activity.1 Although fewer than 60 cases have been reported globally, a surprisingly high number of cases was reported in 1998 in China (6.7% of all odontogenic tumours).18 In a 2005 Chinese study, only 0.3% of odontogenic tumours diagnosed in the first two decades were malignant, indicating that most odontogenic malignancies occurred in adults in their sample.3 Although follow-up of our patients with ameloblastic carcinomas failed owing to poor patient compliance and the remoteness of the region, early diagnosis and 6-month reviews are important in order to limit the potential of local recurrence and metastatic spread.
In conclusion, central odontogenic tumours in the first two decades of life are rare albeit important pathologies that impact on the growth of the jaw bones and tooth development. In the early stages they are asymptomatic and frequently discovered on routine radiographs. At later stages they may give rise to jaw expansion, facial distortion and tooth displacement. Clinical and radiographic assessment provides valuable information on their biological characteristics, involvement of the developing dentition and diagnosis. Their complexity, however, makes biopsy with microscopic examination mandatory for the establishment of a final diagnosis.
References
- 1.Philipsen HP, Reichart PA, Slootweg PJ, et al. Odontogenic tumours. In: Pathology and genetics: head and neck tumours. Lyon: IARC Press, 2005: 283–318 [Google Scholar]
- 2.Kramer IRH, Pindborg JJ, Shear M. Histological typing of odontogenic tumours (2nd edn). Berlin: Springer Verlag, 1992 [Google Scholar]
- 3.Jing W, Xuan M, Lin Y, Wu L, Liu L, Zheng X, et al. Odontogenic tumours: a retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg 2007;36:20–25 [DOI] [PubMed] [Google Scholar]
- 4.Tawfik MA, Zyada MM. Odontogenic tumors in Dakahlia, Egypt: analysis of 82 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e67–e73 [DOI] [PubMed] [Google Scholar]
- 5.Avelar RL, Antunes AA, Santos TDS, Andrade ESDS, Dourado E. Odontogenic tumors: clinical and pathology study of 238 cases. Braz J Otorhinolaryngol 2008; 74:688–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Statistics South Africa. Mid year population estimates, South Africa, 2006. www.statssa.gov.za. [Google Scholar]
- 7.Ajayi OF, Ladeinde AL, Adeyemo WL, Ogunlewe MO. Odontogenic tumors in Nigerian children and adolescence—a retrospective study of 92 cases. World J Surg Oncol 2004;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes AM, Duarte ECB, Pimenta FJ, Souza LN, Santos VR, Mesquita RA, et al. Odontogenic tumors: A study of 340 cases in a Brazilian population. J Oral Pathol Med 2005;34:583–587 [DOI] [PubMed] [Google Scholar]
- 9.Okada H, Yamamoto H, Tilakaratne WM. Odontogenic tumors in Sri Lanka: Analysis of 226 cases. J Oral Maxillofac Surg 2007;65:875–882 [DOI] [PubMed] [Google Scholar]
- 10.Mosqueda-Taylor A, Ledesma-Montes C, Caballero-Sandoval S, Portilla-Robertson J, Ruiz-Godoy Rivera LM, Meneses-Garcia A. Odontogenic tumors in Mexico. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;67:672–675 [DOI] [PubMed] [Google Scholar]
- 11.Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: A study of 1088 cases from northern California and comparisons from other parts of the world. J Oral Maxillofac Surg 2006;64:1343–1352 [DOI] [PubMed] [Google Scholar]
- 12.Guerris M, Piloni MJ, Kezler A. Odontogenic tumors in children and adolescence. A 15-year retrospective study in Argentina. Med Oral Patol Oral Cir Bucal 2007;12:E180–185 [PubMed] [Google Scholar]
- 13.High A, Zedan W. Basal cell nevus syndrome. Curr Opin Oncol 2005;17:160–166 [DOI] [PubMed] [Google Scholar]
- 14.Kaffe I, Naor H, Buchner A. Clinical and radiological features of odontogenic myxomas of the jaws. Dentomaxillofac Radiol 1997;26:299–303 [DOI] [PubMed] [Google Scholar]
- 15.Noffke CEE, Raubenheimer EJ, Chabikuli NJ, Bouckaert MM. Odontogenic myxoma: review of the literature and a report of 30 cases from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101–109 [DOI] [PubMed] [Google Scholar]
- 16.Toida M, Hyodo I, Okuda T, Tatematsu N. Adenomatoid odontogenic tumor: a report of cases and survey of 126 cases in Japan. J Oral Maxillofac Surg 1990;48:404–408 [DOI] [PubMed] [Google Scholar]
- 17.Neville BW, Damm D, Allen CM, Bouquot JE. Oral and maxillofacial pathology (2nd edn). Philadelphia: Saunders, 2002 [Google Scholar]
- 18.Lu Y, Xuan M, Takata T, Wang CE, He Z, Zhou Z, et al. Odontogenic tumors. A demographic study of 759 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:707–714 [DOI] [PubMed] [Google Scholar]