Abstract
Objectives
The purpose of this study was to determine whether bony changes in temporomandibular joint (TMJ) osteoarthritis (OA) is correlated with pain and other clinical signs and symptoms.
Methods
Clinical data and cone beam CT (CBCT) images of 30 patients with TMJ OA were analysed. The criteria of Koyama et al (Koyama J, Nishiyama H, Hayashi T. Follow-up study of condylar bony changes using helical computed tomography in patients with temporomandibular disorder. Dentomaxillofac Radiol 2007; 36: 472–477.) and Ahmad et al [Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 844–860.] were used to classify the condyles observed on the CBCT. Clinical measures included self-reported pain, mandibular range of motion, TMJ sound, pain on palpation of the TMJ and masticatory muscles, and pain on jaw function. Generalized linear modelling was used to correlate the clinical and radiographic findings and Spearman's rho was used to correlate the two classification systems.
Results
There was poor correlation between the maximum condyle change and pain rating (Koyama: r2 = 0.1443, p = 0.3995; Ahmad: r2 = 0.0273, p = 0.9490), maximum mouth opening (Koyama: r2 = 0.2910, p = 0.0629; Ahmad: r2 = 0.2626, p = 0.0951), protrusion (Koyama: r2 = 0.0875, p = 0.7001; Ahmad: r2 = 0.1658, p = 0.3612), right lateral motion (Koyama: r2 = 0.0394, p = 0.9093; Ahmad: r2 = 0.0866, p = 0.6877) and left lateral motion (Koyama: r2 = 0.0943, p = 0.6494; Ahmad: r2 = 0.1704, p = 0.3236). Strong correlation was observed between Koyama et al's and Ahmad et al's classifications for average (r = 0.9216, p < 0.001) and maximum (r = 0.7694; p < 0.0001) bony change.
Conclusions
There was poor correlation between condylar changes (as observed on CBCT images), pain and other clinical signs and symptoms in TMJ OA.
Keywords: cone beam computed tomography, temporomandibular joint, osteoarthritis, pain
Introduction
Temporomandibular joint (TMJ) imaging is very challenging because the bony components are small and superimpositions from the base of the skull often result in a lack of clear delineation of the joint.1,2 Different imaging modalities have been used for diagnosing TMJ osteoarthritis (OA). Problems such as superimpositions, high radiation dose and long scanning time present severe limitations. These disadvantages have led to an increase in popularity of the use of cone beam CT (CBCT) for TMJ imaging. It is a fairly new imaging modality that can produce images of high diagnostic quality using a lower radiation dose than medical CT.2
Temporomandibular disorders (TMD) are disorders affecting the TMJ, masticatory muscles and/or associated structures.3 Osteoarthritis of the TMJ, also known as degenerative joint disease (DJD),4 is an age-related disorder characterized by the destruction of the articular surfaces of the mandibular condyle and glenoid fossa often brought about by increased loading of the joint.4 Continuous loading results in resorption of the subarticular bone. TMJ OA is characterized by a gradual progressive destruction of articular tissues. With advanced degeneration, the subchondral cortical layer is lost and erosion and other radiographic signs of OA appear.5-7 Often, TMJ OA is at an advanced stage by the time it is perceived clinically and/or radiographically.8 Previous studies attempting to correlate pain intensity levels with the quality of bony changes in TMJ OA using different imaging modalities were equivocal.9-11
The aim of this study is to determine whether condylar changes in TMJ OA (based on CBCT images) is correlated with pain and other clinical signs and symptoms.
Materials and methods
This study received approval from the Institutional Review Board of the University of North Carolina at Chapel Hill. CBCT images and clinical records of patients with TMJ OA who sought treatment at the University of North Carolina at Chapel Hill Orofacial Pain Clinic from January 2007 to August 2008 were reviewed in this study. Inclusion criteria for this study included meeting the research diagnostic criteria (RDC) for TMD (RDC/TMD): Group IIIb osteoarthritis of the TMJ, defined by the presence of arthralgia and either TMJ crepitations or CBCT bony changes including erosion, sclerosis, flattening of joint surfaces or osteophyte formation.12 Exclusion criteria included a history of TMJ surgery, condylar fracture, jaw trauma and polyarthritis (such as rheumatoid arthritis, gout arthritis and psoriatic arthritis). Subjects with missing data were also excluded.
A detailed history taking and clinical assessment was performed on all subjects by an orofacial pain specialist. Self-reported average pain intensity level in the past week was rated on a 0 to 10 verbal rating scale where “0” was no pain and “10” was the worst pain possible. Clinical assessments included mandibular range of motion (maximum mouth opening, right and left lateral range of motion and protrusion), TMJ pain on palpation and on jaw functions, and the presence or absence of TMJ crepitations.
The CBCT images were taken with Galileos (Sirona Dental Systems Inc., Bersheim, Germany) with voltage set at 85 kV and current at 7 mA. The effective dose was approximately 70 μSv13 and the field of view was 6 inches.14 Reconstructed three-dimensional data were saved in a proprietary data format file and multiplanar images were exported in digital imaging and communications in medicine (DICOM) format files. Invivo Dental (Anatomage, Inc. San Jose, CA) software was used to view the images that were selected for export to DICOM media. Images were viewed in the axial, coronal and sagittal planes in the software's multiplanar reformatted view. Corrected axis cross-sections of the joint were also viewed. All images were interpreted by 3 oral and maxillofacial radiologists who had more than 20 years of experience and routinely interpreted TMJ CBCT images. A Lenovo (Lenovo, Morrisville, NC) T60p monitor with 1024 × 768 resolution was used. Based on the CBCT images, the type of condylar bony change was classified using both Koyama et al's classification15 and the image analysis criteria developed recently by Ahmad et al.16 If there was doubt about which classification should be assigned, the volume was revisited with the radiologist who initially interpreted the image until an agreement was reached.
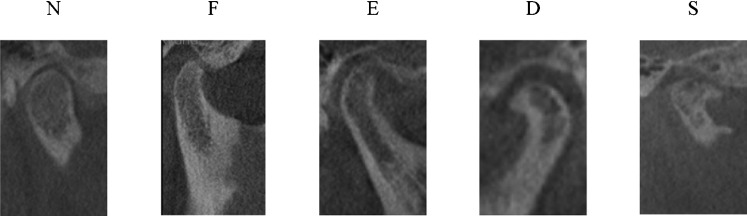
The criteria for determination of the type of condylar bony changes according to Koyama et al15 (Figure 1) are as follows: N (no proliferation or thickening on the cortical surface of the condyle displaying typical morphology) or normal; F (flattened contour at the anteroposterior and/or posterosuperior portions of the condyle) or flattening; E (proliferation or partial hypodense change with or without roughening of the cortical surface of the condyle) or erosion; D (condyle has a deformed contour shaped like a beak, without proliferation or partial hypodense change on the condylar surface) or deformity, marginal proliferation and osteophyte; and S (type D accompanied by type E) erosion, deformity, osteophyte and marginal proliferation. Glenoid fossa changes were classified as “positive” in the presence of flattening, erosion and/or sclerosis, or “negative” when the glenoid fossa appeared normal. The criteria for image analysis developed recently by Ahmad et al16 (Figure 2) are as follows: A (no OA) normal relative size of the condylar head, no subcortical sclerosis or surface flattening, and no deformation due to subcortical cyst, surface erosion, osteophyte or generalized sclerosis; B (indeterminate for OA) normal relative size of the condylar head, subcortical sclerosis with/without articular surface flattening or articular surface flattening with/without subcortical sclerosis, and no deformation due to subcortical cyst, surface erosion, osteophytes or generalized sclerosis; and C (OA) deformation due to subcortical cyst, surface erosion, osteophyte or generalized sclerosis.
Figure 1.
Sample images of condylar bony changes classified according to Koyama et al's15 criteria. Images are from subjects in this study. N, normal; F, flattening; E, erosion; D, deformity, marginal proliferation and osteophyte; S, erosion, deformity, osteophyte and marginal proliferation
Figure 2.
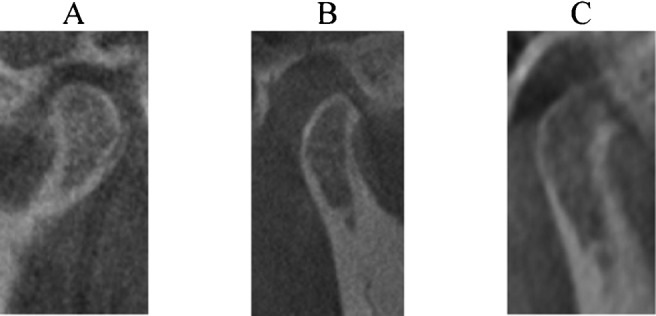
Sample images of condylar bony changes classified according to Ahmad et al's16 classification. Images are from subjects in this study. A, no osteoarthritis; B, indeterminate for osteoarthritis; C, osteoarthritis
All data were entered into Excel 2007 (Microsoft, Redmond, WA) and SAS version 9.1 (SAS Institute Inc., Cary, NC) was used for statistical testing. Only the maximum bony change of the condyle was used as covariate. The generalized linear modelling procedure analysed the correlation between the maximum condyle change with verbal pain rating and the mandibular ranges of motion. Spearman's rho correlation was used to correlate the average and maximum condyle and glenoid fossa changes for both the Koyama et al15 and Ahmad et al16 classifications.
To determine interexaminer reliability in assigning a classification, nine subjects were randomly selected and their CBCT interpretations were reviewed by a second observer. The observer was given a visual instruction sheet with images of bony changes taken from the subjects in this study to serve as a guide in designating a classification. To establish intraexaminer reliability, another nine subjects were randomly selected 2 weeks after the initial review. These radiological reports were reviewed under the same standardized conditions.
Results
A total of 30 patients (26 female and 4 male) fulfilled the inclusion and exclusion criteria. The mean age of the patients was 41 years [range 16–71 years, standard deviation (SD) 19 years]. The mean self-reported pain rating was 5.7 (range 2–8, SD 1.8). Mean maximum opening was 46.7 mm (range 30–66 mm, SD 10.1 mm), mean protrusion was 6.7 mm (range 4–11 mm, SD 2.0 mm), mean right lateral movement was 8.5 mm (range 4–12 mm, SD 2.1 mm) and left lateral motion was 9.0 mm (range 1–14 mm, SD 2.4 mm). TMJ crepitation was present in only the right TMJ of 6 patients (20%) and only in the left TMJ of 8 patients (27%). 5 patients (17%) had bilateral TMJ crepitations. Strong inter- (kappa coefficient 0.77–1, p < 0.001) and intraexaminer (kappa coefficient 0.72–1, p < 0.02) agreements were observed on all variables. Perfect agreement was obtained for interexaminer analysis of the right and left glenoid fossa and for intraexaminer analysis of the right glenoid fossa.
There was poor correlation between maximum condyle bony change and verbal pain rating (Koyama r2 = 0.1443, p = 0.3995; Ahmad r2 = 0.0273, p = 0.9490) (Table 1). No statistically significant correlation was observed between maximum condyle change and maximum opening (Koyama r2 = 0.2910, p = 0.0629; Ahmad r2 = 0.2626, p = 0.0951). Correlation of maximum condyle change and protrusion was very weak (Koyama r2 = 0.0874, p = 0.7001; Ahmad r2 = 0.1658, p = 0.3612). Likewise, the correlation with right (Koyama r2 = 0.0393, p = 0.9093; Ahmad r2 = 0.0866, p = 0.6877) and left lateral range of motion (Koyama r2 = 0.0943, p = 0.6494; Ahmad r2 = 0.1704, p = 0.3236) was poor.
Table 1. Correlation of maximum bony change of the right and left condyles with pain rating and mandibular range of motion.
| Koyama's classification |
Ahmad's classification |
|||
| r2 | p-value | r2 | p-value | |
| Pain rating | 0.1443 | 0.3995 | 0.0273 | 0.9490 |
| Mouth opening | 0.2910 | 0.0629 | 0.2626 | 0.0951 |
| Protrusion | 0.0874 | 0.7001 | 0.1658 | 0.3612 |
| Right lateral | 0.0393 | 0.9093 | 0.0866 | 0.6877 |
| movement | ||||
| Left lateral | 0.0943 | 0.6494 | 0.1704 | 0.3236 |
| movement | ||||
r2, square of correlation coefficient.
Strong correlation was observed between Koyama's and Ahmad's classifications, as shown in Table 2. There was a statistically significantly high correlation of the average and maximum changes for the condyle and glenoid fossa for both classifications.
Table 2. Correlation between Koyama et al's15 and Ahmad et al's16 classifications based on the average and maximum changes for condyle and glenoid fossa (Spearman's correlation coefficient).
| Ahmad classification | Koyama classification |
|||
| Ave condylar changes | Max condylar changes | Ave glenoid fossa changes | Max glenoid fossa changes | |
| Ave condylar changes | 0.9341 | |||
| Max condylar changes | 0.7694 | |||
| Ave glenoid fossa changes | 0.8308 | |||
| Max glenoid fossa changes | 0.7754 | |||
Ave, average; Max, maximum.
p < 0.0001.
Discussion
The results in this study are consistent with previous studies of TMJ OA in that the radiographic findings correlated poorly or not at all with the clinical signs and symptoms.9-11 One of the reasons for the lack of correlation is related to the multidimensional experience of pain. Pain is defined by the International Association for the Study of Pain as a sensory and emotional experience.17 The sensory discriminative dimension of pain is elicited from verbal pain intensity rating. However, the cognitive-motivational and evaluative dimensions are better derived from instruments such as the McGill Pain Questionnaire and the Gracely Box Scale.18 These data, if acquired, may or may not reveal additional correlation(s) between pain and bony changes in TMJ OA. Future prospective studies should utilize multidimensional instruments to measure pain including the cognitive, motivational and evaluative components instead of just the sensory discriminative aspect.19 Secondly, masticatory muscle pain often accompanies TMJ OA, and patients are unable to distinguish pain of masticatory origin from pain of TMJ origin owing to the proximity of the structures. Masticatory myalgia is thus a potential confounder which is probably difficult if not impossible to eliminate. Also, the pain intensity reported could be influenced by the presence and level of expression of certain inflammatory mediators in the synovial fluid.20 Future studies should determine such information from joint fluid analysis. Degenerative changes that are not evident on radiographs may also play a significant role in joint pain.21 Finally, other mitigating factors include elevated psychological distress,22 which is a hallmark feature of chronic TMD, and oral parafunctional habits such as bruxism.23
While some patients with radiographically normal TMJs complain of pain, other patients with radiographic evidence of DJD may not experience any pain.24 Prediction of radiographic findings from clinical signs and symptoms is typically challenging because these associations are not well founded.9,25-27 Patients may experience symptoms for months before bony changes are evident on radiographs. In the early stages of TMJ OA, radiographs may appear normal and may not be helpful in validating the diagnosis.28 Radiographic changes such as flattening, osteophytes, cystic formation and decreased articular space typically appear in the later stages of the disease.29 Some joints may present with radiographic evidence of DJD as a consequence of remodelling when clinically the condition has stabilized. The course of the degenerative change seems to burn-out with time.30 However, the remodelling that has taken place in the condyle and fossa remains.31
The study by Wiese et al25 did not find any association between degenerative bony changes in TMJ tomograms and any pain-related variables. They explained that this non-association may be due to the difference in the onset of pain and detectable radiographic bony changes, because radiographs do not depict ongoing processes but the effect of a previous process. This is highly applicable to our cross-sectional study. Prospective cohorts will be able to capture pain intensity levels and radiographic records of the disease process at different time points instead of a single measure.
We used two classification systems in this study in order to verify that our results can be duplicated by another classification system. Both Koyama's and Ahmad's criteria yielded no correlation between maximum condyle change and verbal pain rating as well as mandibular ranges of motion. To our knowledge, this is the first study that has compared the bony changes of osteoarthritic TMJ based on two different classification systems. The finding that both classification systems when correlated for average and maximum bony changes yielded very significant correlations may suggest that Ahmad's criteria may be the criteria of choice since it is simpler than Koyama's criteria and is based on the RDC/TMD.12
In conclusion, the results of this study showed that while a high correlation existed between Koyama's nd Ahmad's classification on average and maximum condylar bony changes in TMJ OA, both classification schemes revealed poor or no correlation between pain intensity and mandibular ranges of motion with maximum condylar bony change. Factors mitigating this lack of correlation warrant further investigation.
Acknowledgments
We are grateful to Dr Wisam Al Rawi, Dr Ceib Phillips and Dr Riten Mitra for their invaluable assistance in this study.
References
- 1.Ludlow JB, Davies KL, Tyndall DA. Temporomandibular joint imaging: a comparative study of diagnostic accuracy for the detection of bone change with biplanar multidirectional tomography and panoramic images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:735–743 [DOI] [PubMed] [Google Scholar]
- 2.Tsiklakis K, Syriopoulos K, Stamatakis HC. Radiographic examination of the temporomandibular joint using cone beam computed tomography. Dentomaxillofac Radiol 2004;33:196–201 [DOI] [PubMed] [Google Scholar]
- 3.de Leeuw R. (ed). Orofacial pain. Guidelines for assessment, diagnosis, and management (4th edn) Hanover Park, IL: Quintessence Publishing Co, Inc, 2008 [Google Scholar]
- 4.Okeson JP. The clinical management of temporomandibular disorders and occlusion (6th edn) New York, NY: Mosby Elsevier, 2008 [Google Scholar]
- 5.Stegenga B, de Bont LG, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg 1989;47:249–256 [DOI] [PubMed] [Google Scholar]
- 6.Stegenga B, de Bont LG, Boering G, van Willigen JD. Tissue responses to degenerative changes in the temporomandibular joint: a review. J Oral Maxillofac Surg 1991;49:1079–1088 [DOI] [PubMed] [Google Scholar]
- 7.de Bont LG, Stegenga B. Pathology of temporomandibular joint internal derangement and osteoarthrosis. Int J Oral Maxillofac Surg 1993;22:71–74 [DOI] [PubMed] [Google Scholar]
- 8.Israel HA, Saed-Nejad F, Ratcliffe A. Early diagnosis of osteoarthrosis of the temporomandibular joint: correlation between arthroscopic diagnosis and keratan sulphate levels in the synovial fluid. J Oral Maxillofac Surg 1991;49:708–711; discussion 12 [DOI] [PubMed] [Google Scholar]
- 9.Ohlmann B, Rammelsberg P, Henschel V, Kress B, Gabbert O, Schmitter M. Prediction of TMJ arthralgia according to clinical diagnosis and MRI findings. Int J Prosthodont 2006;19:333–338 [PubMed] [Google Scholar]
- 10.Crow HC, Parks E, Campbell JH, Stucki DS, Daggy J. The utility of panoramic radiography in temporomandibular joint assessment. Dentomaxillofac Radiol 2005;34:91–95 [DOI] [PubMed] [Google Scholar]
- 11.Larheim TA. Current trends in temporomandibular joint imaging. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:555–576 [DOI] [PubMed] [Google Scholar]
- 12.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992;6:301–355 [PubMed] [Google Scholar]
- 13.Valentin J. (ed) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007; 37: 1–332 [DOI] [PubMed] [Google Scholar]
- 14.Ludlow JB, Ivanovic M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:106–114 [DOI] [PubMed] [Google Scholar]
- 15.Koyama J, Nishiyama H, Hayashi T. Follow-up study of condylar bony changes using helical computed tomography in patients with temporomandibular disorder. Dentomaxillofac Radiol 2007;36:472–477 [DOI] [PubMed] [Google Scholar]
- 16.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:844–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain 2008;137:473–477 [DOI] [PubMed] [Google Scholar]
- 18.Gracely RH. Measuring pain in the clinic. Anesth Prog 1990;37:88–92 [PMC free article] [PubMed] [Google Scholar]
- 19.Jamison RN, Rudy TE, Penzien DB, Mosley TH., Jr Cognitive-behavioral classifications of chronic pain: replication and extension of empirically derived patient profiles. Pain 1994;57:277–292 [DOI] [PubMed] [Google Scholar]
- 20.Kaneyama K, Segami N, Yoshimura H, Honjo M, Demura N. Increased levels of soluble cytokine receptors in the synovial fluid of temporomandibular joint disorders in relation to joint effusion on magnetic resonance images. J Oral Maxillofac Surg 2010;68:1088–1093 [DOI] [PubMed] [Google Scholar]
- 21.Kurita H, Kojima Y, Nakatsuka A, Koike T, Kobayashi H, Kurashina K. Relationship between temporomandibular joint (TMJ)-related pain and morphological changes of the TMJ condyle in patients with temporomandibular disorders. Dentomaxillofac Radiol 2004;33:329–333 [DOI] [PubMed] [Google Scholar]
- 22.Dworkin SF. Somatization, distress and chronic pain. Qual Life Res 1994;3:S77–83 [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane TV, Gray RJM, Kincey J, Worthington HV. Factors associated with the temporomandibular disorder, pain dysfunction syndrome (PDS): Manchester case-control study. Oral Dis 2001;7:321–330 [DOI] [PubMed] [Google Scholar]
- 24.Brooks SL, Brand JW, Gibbs SJ, Hollender L, Lurie AG, Omnell KA, et al. Imaging of the temporomandibular joint: a position paper of the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:609–618 [DOI] [PubMed] [Google Scholar]
- 25.Wiese M, Svensson P, Bakke M, List T, Hintze H, Petersson A, et al. Association between temporomandibular joint symptoms, signs, and clinical diagnosis using the RDC/TMD and radiographic findings in temporomandibular joint tomograms. J Orofac Pain 2008;22:239–251 [PubMed] [Google Scholar]
- 26.Kopp S, Rockler B. Relationship between clinical and radiographic findings in patients with mandibular pain or dysfunction. Acta Radiol Diagn (Stockh) 1979;20:465–477 [DOI] [PubMed] [Google Scholar]
- 27.Hansson LG, Hansson T, Petersson A. A comparison between clinical and radiologic findings in 259 temporomandibular joint patients. J Prosthet Dent 1983;50:89–94 [DOI] [PubMed] [Google Scholar]
- 28.Alexiou K, Stamatakis H, Tsiklakis K. Evaluation of the severity of temporomandibular joint osteoarthritic changes related to age using cone beam computed tomography. Dentomaxillofac Radiol 2009;38:141–147 [DOI] [PubMed] [Google Scholar]
- 29.Clark GT. Treatment of myogenous pain and dysfunction. Laskin DM, Greene CS, Hylander WL. (eds). Temporomandibular disorders: an evidence-based approach to diagnosis and treatment. Hanover Park, IL: Quintessence Publishing Co., 2006, pp 483–500 [Google Scholar]
- 30.De Leeuw R, Boering G, Stegenga B, De Bont LG. Symptoms of temporomandibular joint osteoarthrosis and internal derangement 30 years after non-surgical treatment. J Craniomand Prac 1995;13:81–88 [DOI] [PubMed] [Google Scholar]
- 31.Pertes R, Gross S. Disorders of the temporomandibular joints. Clinical management of temporomandibular disorders and orofacial pain. Carol Stream, IL: Quintessence Publishing Co., 1995, pp 69–89 [Google Scholar]