Abstract
Objectives
The aim of this study was to investigate the frequency of Stafne bone defect (SBD) and to describe the clinical and radiological characteristics of detected cases.
Methods
A retrospective study was performed using panoramic radiographs from 34 221 patients undergoing dental treatment in the Department of Oral and Maxillofacial Radiology at Erciyes University and Ataturk University, Turkey. After finding an image compatible with SBD in the radiographs, multislice CT (MSCT) on seven patients and cone beam CT (CBCT) on six patients were performed to confirm the diagnosis.
Results
Of the 34 221 patients, 29 (0.08 %) had SBDs, of whom 4 were female (13.8%) and 25 were male (86.2 %). The age range of patients with SBD was 18–77 years (mean age 49.6 years). SBD was found in the lingual molar region in 28 patients and in the lingual canine–premolar region of the mandible in 1 patient. The contour of the concavities on CT images (MSCT and CBCT) was detected. The MSCT revealed glandular tissue within the defects.
Conclusions
According to our results, SBD is an uncommon anomaly. Examination of MSCT images supports the presence of aberrant submandibular glands within these mandibular defects, suggesting that pressure from submandibular gland tissue had caused the SBD, as generally thought. Both CBCT and MSCT can provide adequate support for the detection of SBDs. The CBCT could be suggested as the most suitable non-invasive diagnostic modality for this bony configuration of the mandible since it provides a lower radiation exposure dose than MSCT.
Keywords: bone cyst, computer-assisted three-dimensional imaging, panoramic radiography, cone beam computed tomography, salivary glands
Introduction
Stafne bone defect (SBD) was first described by Stafne in 1942, who reported 35 asymptomatic unilateral radiolucent cavities in the posterior region of the mandible. Lesions were located between the mandibular angle and the third molar, below the inferior dental canal and above the mandibular base.1 Many other terms have been used to describe this entity, including aberrant or ectopic salivary gland; static, latent or idiopathic defect, cavity or cyst; mandibular salivary gland inclusion; lingual mandibular bone cavity, concavity or depression; and Stafne cyst, defect or cavity.2-11
The posterior lingual variant has an incidence of between 0.10% and 0.48% when diagnosed radiologically. However, some cadaver studies have revealed that the incidence of the lesion may be as high as 6.06%. The age range is quite wide, although there is a clear predilection for males in the fifth or sixth decade.3,6,9 When the term SBD is found in the literature, it usually refers to the posterior lingual variant. The anterior lingual variant is seven times less frequent than the posterior and is usually located between the incisor and the premolar areas, above the insertion of the mylohyoid muscle.2,11
When reviewing the literature regarding SBD using the PubMed database (National Library of Medicine), the authors found most of the SBD cases. Although the number of reports continues to accumulate, knowledge about the aetiology and pathogenesis of SBD has been limited and confusing for a long time.3,7-10 Although the radiological features of SBD have been widely reported, the use of cone beam CT (CBCT) for its diagnosis has been rarely reported.11 The aim of this article is to clarify the frequency of this phenomenon by using multislice CT (MSCT) and CBCT to add to our understanding of the aetiology and content of SBDs by MSCT analysis, and to investigate CBCT use as a tool for exploring SBDs.
Materials and methods
A retrospective cohort study was designed consisting of 34 221 panoramic radiographs from patients who presented to the Oral and Maxillofacial Radiology Services at the Ataturk University Dentistry Faculty, Turkey, between January 1996 and January 2010 and the Erciyes University Dentistry Faculty, Turkey, between January 2005 and January 2010. All radiographs were performed by radiography technicians who had a minimum working experience of 5 years using an orthopantomography device with a magnification factor of 1.2. The images were examined by two investigators (one assistant professor and the other a research assistant dentomaxillofacial radiologist at Ataturk University or one associate professor and another research assistant dentomaxillofacial radiologist at Erciyes University) at the same time. To check for the diagnostic reproducibility of the inter-reliability of the two investigators at both centres, 10% of the radiographs assigned to them were randomly examined each day for 3 consecutive days. Examination of results using the Wilcoxon matched-pairs signed-rank test showed no statistically significant differences between the two observers, indicating diagnostic reproducibility. In cases that were detected with only panoramic radiographs, when examiners failed to reach a decisive opinion, they discussed the particular case and either established a consensus and included it in the study or discarded the case. For those patients in whom SBD was suspected and with whom contact was possible, an advanced imaging method [CBCT (NewTom FP QR-DVT 9000, Image Works Verona, Italy) at Ataturk University, MSCT (GE Light Speed 16 Milwakee, WI) at Erciyes University] was taken to confirm the diagnosis.
The age and sex were recorded for all patients and for the cases of SBD, age, sex, laterality, location and, if possible, contour and content were noted as well. The contents of the concavities were examined with respect to the MSCT value (HU).
Location
SBDs can be divided into four topographical variants: (1) lingual anterior mandibular body (incisor–canine–premolar area) above the mylohyoid muscle; (2) posterior to the mandibular angle–first permanent molar area, below the mandibular canal; (3) located to the ascending, lingual mandibular ramus, posterior to the lingual foramen, just below the neck of the condyle; and (4) buccal aspects of the ascending mandibular ramus.9
Contour
According to their relationship to the buccal cortical plane, SBDs can be divided into three types. In Type I, the cavity does not reach the buccal cortex, in Type II, it reaches the buccal cortex without expanding it and in Type III, the buccal osseous cortex is expanded.10
Content
Three categories have also been proposed based on the density value of SBD content. Category F indicates fat density, Category S indicates soft-tissue density and Category G indicates glandular tissue on the inside.
Results
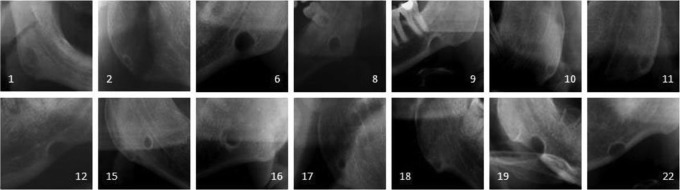
29 (0.08 %) of the 34 221 individuals had SBDs, of whom 4 (0.02%) were female and 25 (0.2%) were male. Anterior Stafne prevalence was found to be 0.003%, whereas posterior Stafne prevalence was 0.081%. The frequency of SBD was higher in persons aged over 40 years than in those aged less than 40 years. Bilateral presentation was not apparent. Of the 29 unilateral cases, 13 (44.8%) were on the left and 16 (55.2%) were on the right side. The gender and age distribution of the study population is presented in Table 1. Figure 1 shows images of the 16 SBD viewed only on panoramic radiographs.
Table 1. Stafne bone defect in a Turkish patient population.
| Number | Stafne bone defect | Prevalence (%) | ||
| Sex | Female | 19 640 | 4 | 0.02 |
| Male | 14 581 | 25 | 0.2 | |
| Age (years) | 4–40 | 19 165 | 6 | 0.03 |
| 41–95 | 15 056 | 23 | 0.15 | |
| Localization | Right | 16 | 0.05 | |
| Left | 13 | 0.04 | ||
| Anterior | 1 | 0.003 | ||
| Posterior | 28 | 0.081 | ||
| Total | 34 221 | 29 | 0.084 |
Figure 1.
The samples of Stafne bone defects detected with only panoramic radiographs
The ages of the patients with SBD ranged from 18 years to 77 years (mean age 49.6 years). 28 defects were located in the angle–molar region of the mandible. Only one case was observed in the premolar area of the right side of the mandible. MSCT and CBCT images demonstrated the defects according to contour and relationship to the buccal cortical plate. Nine cases showed Type I SBDs and four cases showed Type II SBDs. On MSCT images, the tissue within the SBDs showed attenuation value, indicating the presence of glandular tissue. The attenuation did not differ from that of normal submandibular and sublingual gland tissue in all patients (Table 2). Examples of SBD on MSCT and CBCT are shown in Figures 2–6.
Table 2. Details of detected Stafne bone defect cases in present study.
| Patient number | Age (years) | Sex | Laterality | Examination modality | Location | Contour | Outline |
| 1 | 65 | M | R | PR | 2 | N.D. | N.D. |
| 2 | 47 | M | R | PR | 2 | N.D. | N.D. |
| 3 | 33 | M | R | PR+MSCT | 2 | G | II |
| 4 | 64 | F | R | PR+MSCT | 1 | G | I |
| 5 | 42 | M | L | PR+MSCT | 2 | G | I |
| 6 | 61 | M | L | PR | 2 | N.D. | N.D. |
| 7 | 77 | M | R | PR | 2 | N.D. | N.D. |
| 8 | 46 | M | L | PR | 2 | N.D. | N.D. |
| 9 | 65 | M | L | PR | 2 | N.D. | N.D. |
| 10 | 30 | M | L | PR | 2 | N.D. | N.D. |
| 11 | 58 | M | L | PR | 2 | N.D. | N.D. |
| 12 | 51 | M | L | PR | 2 | N.D. | N.D. |
| 13 | 27 | M | L | PR+CBCT | 2 | N.D. | I |
| 14 | 60 | M | R | PR+MSCT | 2 | G | I |
| 15 | 58 | F | R | PR | 2 | N.D. | N.D. |
| 16 | 64 | M | L | PR | 2 | N.D. | N.D. |
| 17 | 56 | F | R | PR | 2 | N.D. | N.D. |
| 18 | 43 | M | R | PR | 2 | N.D. | N.D. |
| 19 | 56 | M | R | PR | 2 | N.D. | N.D. |
| 20 | 49 | M | R | PR+CBCT | 2 | N.D. | II |
| 21 | 39 | M | L | PR+CBCT | 2 | N.D. | I |
| 22 | 52 | M | L | PR | 2 | N.D. | N.D. |
| 23 | 18 | M | R | PR+CBCT | 2 | N.D. | I |
| 24 | 42 | M | L | PR | 2 | N.D. | N.D. |
| 25 | 55 | M | R | PR+MSCT | 2 | G | I |
| 26 | 34 | M | L | PR+MSCT | 2 | G | II |
| 27 | 45 | F | R | PR+MSCT | 2 | G | I |
| 28 | 40 | M | R | PR+CBCT | 2 | N.D. | I |
| 29 | 62 | M | R | PR+CBCT | 2 | N.D. | II |
F, female; M, male; L, left; R, right; G, glandular tissue; N.D., not detected; PR, panoramic radiography; MSCT, multislice CT; CBCT, cone beam CT.
1: the cavity was located to the lingual anterior mandibular body (incisor-canine-premolar area) and above the mylohyoid muscle.
2: the cavity was located to the mandibular angle-first permanent molar area and below the mandibular canal.
I: the cavity did not reach the buccal cortical plate.
II: the cavity reached the buccal cortex, but there was no expansion of the plate.
Figure 2.
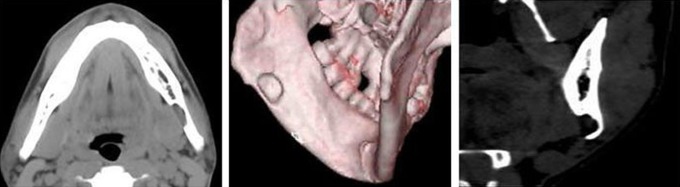
Case 4. Anterior Stafne bone defect. Axial and three-dimensional CT views of the lingual bone defect (arrows) at the premolar region of the mandible. CT value of content was 10 HU (sublingual gland tissue)
Figure 6.
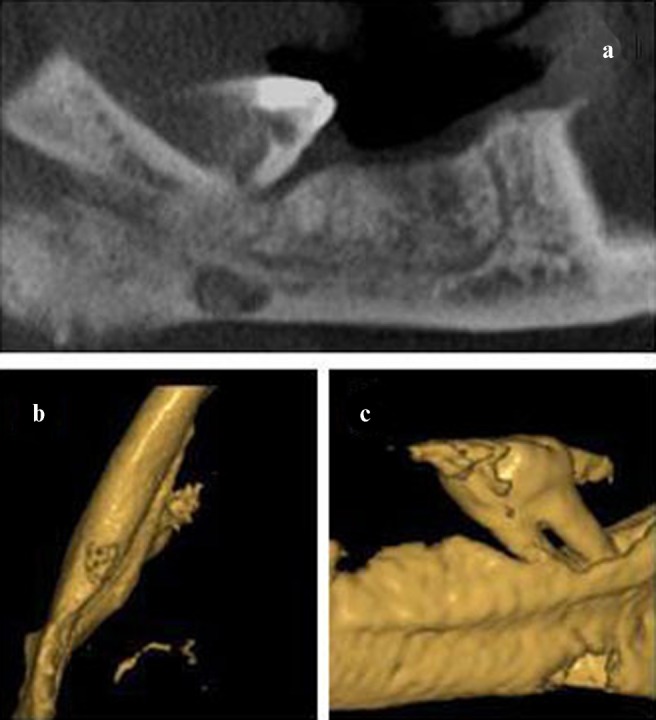
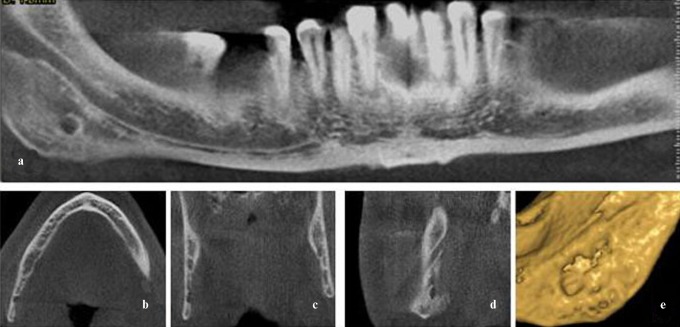
(a) The panoramic view of Case 29 formed on a selected axial section. (b,c) The bone depression (Stafne defect) can be seen in the lingual cortical area
Figure 3.
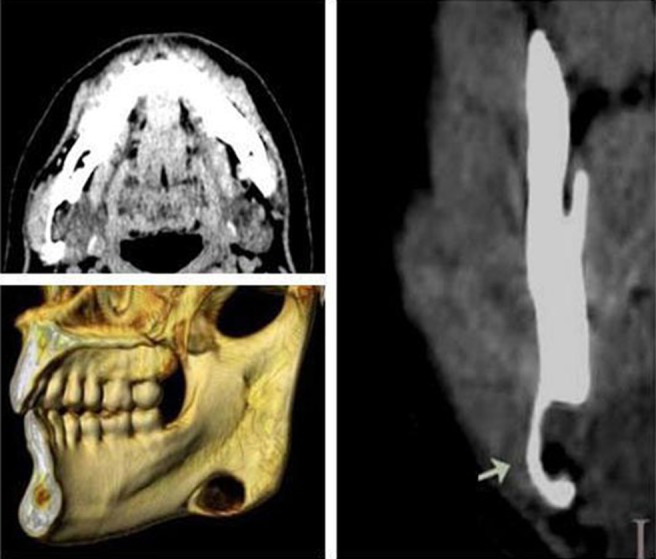
Case 3. Non-contrast-enhanced CT showing submandibular gland tissue (30 HU). Axial, coronal and three-dimensional CT views show typical apperance of Stafne bone cavity near angle of mandible on right side. The bottom of cavity reaches the buccal cortical plate, which is not expanded (arrow)
Figure 4.
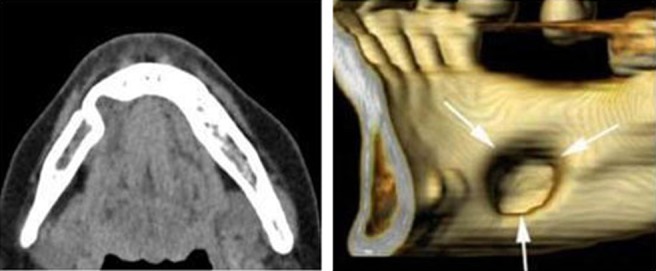
Axial, coronal and three-dimensional CT images of Case 5 showing the Type II class Stafne bone defect in the left region of the mandible of the 42-year-old male. CT value of content does not show fat tissue but attenuation of submandibular gland (40 HU)
Figure 5.
Case 23. (a) Stafne defect situated in the posterior area of the right mandible below the inferior dental canal. (b) Axial, (c) coronal and (d) sagittal cone beam CT images showing the peripheral origin of the defect and the preservation of the lingual cortex. Bottom of defect does not reach buccal cortical plate. (e) Three-dimensional reconstruction
Discussion
As previously stated, the posterior lingual variant has an incidence of between 0.10% and 0.48% when diagnosed radiologically.3,6,9,12,13 This rather large difference in prevalence between studies has been attributed to the difficulty in identifying these entities radiographically. A frequency rate of SBD of 0.08% was determined in this study. Higher incidences were reported in dried mandibles and may be the result of the authors being able to detect the defect in the dried specimens more readily than on a radiograph of the jaw of a living patient.9,14,15 Langlais16 examined 469 dry mandible specimens and reported that 1.3% had either an anterior or a posterior lingual cortical depression. In the study by Harvey and Noble,17 a comparison between the size of the lingual defect and the radiographic appearance indicated clearly that only those cases showing extensive resorption of the lingual cortex had the classic radiographic appearance described by Stafne. Therefore, it is probable that these lesions are more common than published figures indicate.
SBD was described by most authors as static and patient's age on detection was usually in excess of 20 years, most often in the fifth and sixth decades.6,9,15 The 12 SBDs reported by Minowa et al8 were in patients aged 18–64 years (mean age 57 years). Our sample shows a predominance of patients diagnosed between 40 years and 70 years of age. Hansson18 reported a single case of an 11-year-old male; in this case, it was possible to follow the development of the mandibular bone cavity for about 5 years. In our case, the youngest patient with this cavity was 18 years old. SBD also occurs more commonly among males; Philipsen et al,9 in their comprehensive study, showed a 6:1 male-to-female ratio for SBD. Quesada-Gomez et al3 also reported 11 cases of SBD, of which 8 were in males. In the present study, there was also a greater prevalence of males (25:4).
Various theories attempt to explain the aetiopathology of the SBD. Numerous explanations for its aetiology have been proposed, which usually implicate a congenital or embryogenic origin.19 Initially, Stafne1 suggested that formation of SBDs is due to hypoplasia of the mandible during growth and development. The major objection to this theory is that these defects are much more frequently diagnosed in adults than in children, suggesting that the development of these lesions probably occurs later in life, after ossification of the mandible.20 Most subsequent studies have revealed the presence of aberrant submandibular glands within these mandibular defects, suggesting that pressure from submandibular gland tissue had caused the SBD.3,4,9,10 Defenders of this aetiology noted that the submandibular gland is directly related to the posterior variant, while the sublingual gland is related to the anterior variant. They support the idea of a compensatory hypertrophy related to a lymphocytic infiltration and reduced secretory efficiency that increases with age or that there is an increase in salivary gland size as part of general somatic growth. Except for these genetic and embryonic reasons, Lello and Makek21 proposed that SBDs are formed by bone ischaemia. However, they did not suggest any possible cause for the ischaemia. In recent times, Minowa et al7,8,22 suggested that bone erosion due to an acquired vascular lesion is another possible explanation for the formation of SBD of the mandible. According to them, the facial artery and its branches can become tortuous owing to hypertension. Although the submandibular gland, which is composed of soft tissue, can weaken arterial pulses, the mandible is subjected to pressure from the arterial pulses and this pressure acting on the mandible is thought to be the cause of the bone cavity. The incidence of hypertension increases with age and is consistent with the incidence of SBD.
On the basis of these aetiologies, the content of the cavity is critical. Normal or inflamed salivary gland tissue was the most common histological finding3,4,10,23 and is compatible with the result of our reports. In a minority of cases, muscles, fibrous connective tissue, blood vessels, fat or lymphoid tissues have also been reported.8,10 This diversity of tissues could be the result of the removal of soft parts adjacent to the defect. SBD has even been found to be empty. This could be explained by the accidental displacement of tissue during surgical manipulation or intermittent gland herniation.
The diagnosis of SBD is usually easy when it occurs in the posterior region of the mandible. Diagnosis is generally made on routine plain radiographs.2,3,5,10,23 In doubtful posterior cases (including odontogenic cysts and tumour-like lesions) or when the rare anterior type is suspected, additional examinations have to be completed to confirm diagnosis.3,11 CT, MRI and sialography techniques have been used to achieve a final diagnosis of SBD. CT has been reported as a complementary diagnostic procedure for SBDs since other jaw pathologies could be distinguished with this method.3 However, the largest CT series noted that small SBDs may appear to contain only fat or soft tissue.10,23 Segev et al24 stated that diagnosis of SBD with CT is easier than with MRI, but they also mentioned that MRI should be considered to identify the contents of the cavity. Additionally, CT has the disadvantage of high radiation exposure. Owing to these considerations, MRI is suggested as the primary diagnostic procedure for SBDs.2,23,24 The main advantage of MRI is its superior soft-tissue characterization and discrimination. It does not expose the patient to ionizing radiation. However, it is more expensive and artefacts result from the presence of dental materials. Sialography has also been suggested to determine whether glandular tissue exists in the cavity. However, this procedure is rarely performed successfully in anterior salivary gland defects because of the presence of numerous ducts of Rivinus, which have a small diameter.5,25,26 Sialography can be also difficult to perform and uncomfortable for patients, exposing them to ionizing radiation.
CBCT presents with some major advantages compared with MSCT. Firstly, the radiation exposure dose of the patients is relatively low. Secondly, the CBCT machine can be used effectively in a dental clinic but MSCT machine availability is usually limited to hospitals. Thirdly, the level of resolution in CBCT images was reportedly higher than that in MSCT images.27 In the present investigation, no large differences between CBCT and MSCT images were observed regarding the depiction of SBDs. CBCT proved to be at least as accurate as routinely used MSCT in revealing the SBDs. Katz et al11 suggested that CBCT provided detailed information about definitive diagnosis of SBD. It enables both diagnosis and follow-up, particularly through the reformatted images that show radiographic features in fine detail. However, in our study the contents of the concavities were examined with respect to an MSCT value (HU). Although CBCT systems for the oral and maxillofacial region allow measurement of the HU value, the values of CBCT have been reported to differ markedly from the HU values given by a typical MSCT system and they became unstable owing to the effects of adjacent tissues.28 Therefore, the HU values of CBCT were ignored when determining contents of the cavity in the current study.
The radiographic differential diagnosis of SBDs has included benign salivary gland tumours, neurogenic tumours, haemangioma, myxoma, central giant cell lesion, odontogenic cyst, simple bone cyst, ameloblastoma, fibro-osseous lesions, multiple myeloma, eosinophilic granuloma and metastatic disease.9,20,23,29 Therefore, in some cases, more confirmatory diagnostic tools are mandatory.
No treatment is necessary for SBDs in either posterior or anterior variants since these mandibular bone depressions have been shown to be an anatomical rather than a pathological condition.3-5, 9, 20 In general, the management of SBD should be conservative by radiological follow-up. Surgical exploration and biopsy should only be performed when the diagnosis is uncertain or in exceptional cases when an unusually severe pathology (such as pleomorphic adenoma) is suspected.30
In conclusion, diagnosis of SBDs has become incidental because it does not present any clinical symptoms and dentists are more interested in dental pathologies in the examination of radiographs. Panoramic radiographs may ensure a certain amount of information regarding the diagnosis of SBD for experienced practitioners. Since CBCT has low radiation dose and shows fine details and superior features in distinguishing suspicious radiolucent lesions of the mandible, it might be used for diagnosis of SBD cases.
Acknowledgments
We would like to express our sincere gratitude to Ali Caglar Gulluce for his support in proofreading our article.
References
- 1.Stafne EC. Bone cavities situated near the angle of the mandible. J Am Dent Assoc 1942;29:1969–1972 [Google Scholar]
- 2.Sisman Y, Etöz OA, Mavili E, Sahman H, Tarim Ertas E. Anterior Stafne bone defect mimicking a residual cyst: a case report. Dentomaxillofac Radiol 2010;39:124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quesada-Gómez C, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. Stafne bone cavity: a retrospective study of 11 cases. Med Oral Patol Oral Cir Bucal 2006;11:E277–280 [PubMed] [Google Scholar]
- 4.Shimizu M, Osa N, Okamura K, Yoshiura K. CT analysis of the Stafne's bone defects of the mandible. Dentomaxillofac Radiol 2006;35:95–102 [DOI] [PubMed] [Google Scholar]
- 5.de Courten A, Küffer R, Samson J, Lombardi T. Anterior lingual mandibular salivary gland defect (Stafne defect) presenting as a residual cyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:460–464 [DOI] [PubMed] [Google Scholar]
- 6.Correll RW, Jensen JL, Rhyne RR. Lingual cortical mandibular defects: a radiographic incidence study. Oral Surg Oral Med Oral Pathol 1980;50:287–291 [DOI] [PubMed] [Google Scholar]
- 7.Minowa K, Inoue N, Izumiyama Y, Ashikaga Y, Chu B, Maravilla KR, et al. Static bone cavity of the mandible: computed tomography findings with histopathologic correlation. Acta Radiol 2006;47:705–709 [DOI] [PubMed] [Google Scholar]
- 8.Minowa K, Inoue N, Sawamura T, Matsuda A, Totsuka Y, Nakamura M. Evaluation of static bone cavities with CT and MRI. Dentomaxillofac Radiol 2003;32:2–7 [DOI] [PubMed] [Google Scholar]
- 9.Philipsen HP, Takata T, Reichart PA, Sato S, Suei Y. Lingual and buccal mandibular bone depressions: a review based on 583 cases from a world-wide literature survey, including 69 new cases from Japan. Dentomaxillofac Radiol 2002;31:281–290 [DOI] [PubMed] [Google Scholar]
- 10.Ariji E, Fujiwara N, Tabata O, Nakayama E, Kanda S, Shiratsuchi Y, et al. Stafne's bone cavity. Classification based on outline and content determined by computed tomography. Oral Surg Oral Med Oral Pathol 1993;76:375–380 [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Chaushu G, Rotstein I. Stafne's bone cavity in the anterior mandible: a possible diagnostic challenge. J Endod 2001;27:304–307 [DOI] [PubMed] [Google Scholar]
- 12.Oikarinen VJ, Julku M. An orthopantomographic study of developmental mandibular bone defects (Stafne's idiopathic bone cavities). Int J Oral Surg 1974;3:71–76 [DOI] [PubMed] [Google Scholar]
- 13.Karmiol M, Walsh RF. Incidence of static bone defect of the mandible. Oral Surg Oral Med Oral Pathol 1968;26:225–228 [DOI] [PubMed] [Google Scholar]
- 14.Slasky BS, Bar-Ziv J. Lingual mandibular bony defects: CT in the buccolingual plane. J Comput Assist Tomogr 1996;20:439–443 [DOI] [PubMed] [Google Scholar]
- 15.Grellner TJ, Frost DE, Brannon RB. Lingual mandibular bone defect: report of three cases. J Oral Maxillofac Surg 1990;48:288–296 [DOI] [PubMed] [Google Scholar]
- 16.Langlais RP. Anterior and posterior lingual depressions of the mandible. J Oral Surg 1976;34:502–509 [PubMed] [Google Scholar]
- 17.Harvey W, Noble HW. Defects on the lingual surface of the mandible near the angle. Br J Oral Surg 1968;6:75–83 [DOI] [PubMed] [Google Scholar]
- 18.Hansson LG. Development of a lingual mandibular bone cavity in an 11-year-old boy. Oral Surg Oral Med Oral Pathol 1980;49:376–378 [DOI] [PubMed] [Google Scholar]
- 19.Reuter I. An unusual case of Stafne bone cavity with extra-osseous course of the mandibular neurovascular bundle. Dentomaxillofac Radiol 1998;27:189–191 [DOI] [PubMed] [Google Scholar]
- 20.Belmonte-Caro R, Vélez-Gutiérrez MJ, García DeLaVega-Sosa FJ, García-Perla-García A, Infante-Cossío PA, Díaz-Fernández JM, et al. A Stafne's cavity with unusual location in the mandibular anterior area. Med Oral Patol Oral Cir Bucal 2005;10:173–179 [PubMed] [Google Scholar]
- 21.Lello GE, Makek M. Stafne's mandibular lingual cortical defect. Discussion of aetiology. J Maxillofac Surg 1985;13:172–176 [DOI] [PubMed] [Google Scholar]
- 22.Minowa K, Kobayashi I, Matsuda A, Ohmori K, Kurokawa Y, Inoue N, et al. Static bone cavity in the condylar neck and mandibular notch of the mandible. Aust Dent J 2009;54:49–53 [DOI] [PubMed] [Google Scholar]
- 23.Branstetter BF, Weissman JL, Kaplan SB. Imaging of a Stafne bone cavity: what MR adds and why a new name is needed. AJNR Am J Neuroradiol 1999;20:587–589 [PMC free article] [PubMed] [Google Scholar]
- 24.Segev Y, Puterman M, Bodner L. Stafne bone cavity — magnetic resonance imaging. Med Oral Patol Oral Cir Bucal 2006;11:E345–347 [PubMed] [Google Scholar]
- 25.Araiche M, Brode H. Aberrant salivary gland tissue in mandible. Oral Surg Oral Med Oral Pathol 1959;12:727–729 [DOI] [PubMed] [Google Scholar]
- 26.Tominaga K, Kuga Y, Kubota K, Ohba T. Stafne's bone cavity in the anterior mandible: report of a case. Dentomaxillofac Radiol 1990;19:28–30 [DOI] [PubMed] [Google Scholar]
- 27.Okano T, Hirata Y, Sugihara Y, Sakaino R, Tsuchida R, Iwai K, et al. Absorbed and effective doses from cone-beam volumetric imaging for implant planning. Dentomaxillofac Radiol 2009;38:79–85 [DOI] [PubMed] [Google Scholar]
- 28.Yamashina A, Tanimoto K, Sutthiprapaporn P, Hayakawa Y. The reliability of computed tomography (CT) values and dimensional measurements of the oropharyngeal region using cone beam CT: comparison with multidetector CT. Dentomaxillofac Radiol 2008;37:245–251 [DOI] [PubMed] [Google Scholar]
- 29.Drage NA, Renton T, Odell EW. Atypical stafne bone cavity. Clin Radiol Extra 2003;58:51–53 [Google Scholar]
- 30.Simpson W. A stafne's mandibular defect containing a pleomorphic adenoma: report of case. J Oral Surg 1965;23:553–556 [PubMed] [Google Scholar]