In a cohort of men who have sex with men, most of whom were infected with human immunodeficiency virus, men chronically infected with hepatitis B were 2-fold more likely to die a liver-related death than those chronically infected with hepatitis C.
Abstract
Background. It is not known whether chronic hepatitis B (CH-B) or chronic hepatitis C (CH-C) carries a greater risk of liver-related mortality. This study compared rates of liver-related mortality between these 2 groups in the Multicenter AIDS Cohort Study (MACS).
Methods. Six hundred eighty men with CH-B (n = 337) or CH-C (n = 343) at study entry into the MACS were prospectively followed to death, last follow-up visit, or 30 March 2010, whichever came first. Four hundred seventy-two (69.4%) of these men were infected with human immunodeficiency virus type 1 (HIV-1). Causes of death were obtained from death registry matching and death certificates. Liver-related and all-cause mortality rates (MRs) were compared between groups using Poisson regression and adjusted for potential confounders and competing risks.
Results. In 6728 person-years (PYs) of follow-up, there were 293 deaths from all causes (43.5 per 1000 PYs), of which 51 were liver-related (7.6 per 1000 PYs). The all-cause MR was similar between those with CH-B and CH-C; however, the liver-related MR was significantly higher in those with CH-B (9.6 per 1000 PYs; 95% confidence interval [CI], 6.9–13.2) than those with CH-C (5.0 per 1000 PYs; 95% CI, 3.0–8.4). In the HIV-infected subgroup, which had 46 (90.2%) of the liver-related deaths, the liver-related MR remained higher from CH-B after adjusting for potential confounders (incidence rate ratio, 2.2; P = .03) and competing risks (subhazard rate ratio, 2.4; P = .02). Furthermore, among HIV-infected subjects, CD4 cell counts <200 cells/mm3 were associated with a 16.2-fold (95% CI, 6.1–42.8) increased risk of liver-related death compared with CD4 cell counts >350 cell/mm3.
Conclusions. Chronic hepatitis B carries a higher risk of death from liver disease than does CH-C, especially in HIV-infected men with greater immunosuppression.
Both hepatitis B virus (HBV) and hepatitis C virus (HCV) infect the liver, resulting in chronic infection and increasing the risk for end-stage liver disease and death. Chronic HBV infection (CH-B) affects approximately 375 million individuals worldwide, whereas chronic HCV infection (CH-C) is less prevalent, affecting approximately 175 million individuals [1]. Chronic HBV is the leading cause of end-stage liver disease and hepatocellular carcinoma worldwide, which could be a consequence of its higher prevalence compared with CH-C. Alternatively, CH-B may lead to faster liver disease progression and death.
The host factors that affect liver disease progression in both CH-B and CH-C are similar and include age, human immunodeficiency virus (HIV) disease, alcohol use, and genetic variation [2–9]. Human immunodeficienvy virus infection accelerates liver disease progression and increases the risk for liver-related mortality from both CH-B and CH-C [2, 4, 10, 11]. However, to date, neither liver disease progression nor liver-related mortality between CH-B and CH-C has been compared.
A significant barrier to comparing liver-related mortality between these 2 chronic hepatitis infections is the challenge of findings cohorts with similar host characteristics in which subjects are infected with either HBV or HCV but not both. The Multicenter AIDS Cohort Study (MACS), a large, prospectively followed cohort of men who have sex with men, has equivalent numbers of men chronically infected with either HBV or HCV. Thus, in this study, liver-related mortality rates were compared between MACS men who had either CH-B or CH-C.
METHODS
Study Participants and Laboratory Testing
The MACS has prospectively followed men who have sex with men from 4 metropolitan areas in the United States (Baltimore, Maryland; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California) since 1984. The MACS subjects were initially recruited April 1984–March 1985 and subsequently 1987–1991 and 2001–2003. The men are assessed semiannually with repeat interviews and laboratory testing [12]. Participants who were HIV type 1 (HIV) seronegative at study entry have HIV-1 enzyme-linked immunosorbent assay testing at semiannual visits, with positive tests confirmed by Western blot [12]. Mortality data were obtained from death certificates retrieved from periodic death registry searches.
This study included men who had either CH-B or CH-C at entry into the MACS. Tests to determine viral hepatitis status were performed on serum stored at −70°C from all MACS men. Hepatitis B surface antigen (HBsAg) was determined by an enzyme immunoassay (Abbott Laboratories). Hepatitis C virus antibody testing was performed with an enzyme immunoassay (ADVIA Centura HCV assay), and HCV RNA was determined with the COBAS AmpliPrep TaqMan HCV Assay. A participant had CH-B if the HBsAg was positive and had CH-C if the HCV antibody and HCV RNA were positive at study entry. Men who were coinfected with both HBV and HCV or who did not have follow-up were excluded.
Study Design
Men with CH-B or CH-C were followed from study entry to death, last follow-up visit, or 30 March 2010, whichever came first. The primary outcome was liver-related mortality rate (MR). Causes of death were ascertained from death registry matching and death certificates coded per the International Classification of Diseases, Ninth Revision. Any cause of death recorded as hepatitis B, hepatitis C, viral hepatitis, chronic hepatitis, chronic liver disease, portal hypertension, liver cirrhosis, liver failure, hepatic coma, hepatorenal syndrome, or liver cancer was included as liver related.
Data on age, race, injection drug use, alcohol use,HIV status, CD4 cell count, highly active antiretroviral therapy (HAART) use, anti-HBV agent (lamivudine/emtricitabine/tenofovir) use, and anti-HCV therapy (ribavirin and pegylated interferon) use were abstracted for each participant at every visit. Alcohol use was quantified in grams of alcohol consumed per week based on participant response to a questionnaire at each visit. Given the low levels of alcohol use in this cohort, 210 grams per week was defined as heavy alcohol use.
The institutional review boards at each MACS site approved the study, and all participants provided written informed consent for participation in the MACS.
Statistical Analyses
All analyses were performed with Stata version 11.0 (Stata-Corp). The number of person-years (PYs) of follow-up was calculated for each individual. For participants who became HIV-positive during follow-up, the PYs of time accrued before or after HIV seroconversion were classified among the seronegative or seropositive group, respectively. All-cause MRs were calculated for the CH-B and CH-C groups by dividing the number of observed deaths by the PYs of follow-up. Liver-related MRs were calculated similarly. The MRs between the CH-B and CH-C groups were compared based on the Poisson distribution. A multivariable analysis was performed to adjust the incidence rate ratios (IRR) for covariates, including age, race, cumulative alcohol use, CD4 T-cell count, and use of HAART, HBV-active agents, or anti-HCV therapy. All covariates except race were examined as time-varying covariates by partitioning follow-up time into intervals defined according to when these covariates were assessed. Because study participants occasionally missed study visits, time intervals >2 years were censored to ensure validity of the covariates. We also applied this censoring algorithm to follow-up time accrued after a subject's final MACS study visit, which resulted in 5 deaths (1 HCV, 4 HBV) being excluded from the multivariable analysis. A P value < .05 was considered significant.
Prior to 1996, few individuals in MACS were on HAART, but by December 1996 and December 1997, 50% and 65% of HIV–infected individuals, respectively, had initiated HAART [13]. Tenofovir disoproxil fumarate (TDF) was approved for use by the US Food and Drug Administration on 26 October 2001. On the basis of these data, the study period was divided into 3 periods in order to assess the effect of HAART and TDF on liver-related mortality: (1) study entry up to 31 December 1996, the pre-HAART era; (2) 1 January 1997 to 31 December 2001, the pre-TDF/early HAART era; and (3) 1 January 2002 to 30 March 2010, the TDF/current HAART era.
Another consideration is the concept of competing risk of death. The idea is that individuals may die of a cause (competing risk) other than the cause of interest. For this study, subjects may die a non–liver disease death before they have the chance to develop liver disease severe enough to cause a liver-related death. If, by chance, there are more competing causes in 1 group than the other, the risk of liver-related death can be underestimated in the group with more competing causes of death. This is particularly important for data from the pre-HAART era, during which AIDS deaths precluded the occurrence of many liver-related deaths. To account for competing causes of death, a standard method was used in which parallel competing risk models were fit using the method of Fine and Gray, as implemented in Stata 11 [14].
RESULTS
Of the 6972 men enrolled in the MACS, 757 (10.8%) had CH-B and/or CH-C at study entry. Of these, 77 were excluded from this study—15 because they were coinfected with both HBV and HCV and 62 because they did not have any follow-up. Of the remaining 680 men, the distribution of CH-B and CH-C was similar, with 337 in the CH-B group and 343 in the CH-C group. Approximately 70% in both groups were infected with HIV. The CH-B and CH-C groups were similar with respect to alcohol use and nadir and baseline CD4 T-cell count (Table 1). However, HCV-infected individuals were older and more likely to have used injection drugs, and a larger proportion of this group was recruited after 2001. The median follow-up time was 8.5 years (interquartile range (IQR), 5.4–15.9) for the CH-B group and 6.9 years (IQR, 5.1–9.8) for the CH-C group.
Table 1.
Characteristics of Participants by Hepatitis Status
| Variable | Chronic HBV (n = 337) | Chronic HCV (n = 343) |
|---|---|---|
| Age in years, mean (SD)a,* | 32.7 (7.4) | 38.7 (8.8) |
| White, No. (%)* | 260 (77%) | 152 (44%) |
| HIV-1 positive, No. (%)b | 229 (68%) | 243 (71%) |
| Ever used injection drugs, No. (%)c,* | 36 (11%) | 206 (60%) |
| Alcohol per week, median (IQR) (g)d | 22 (2–74) | 11 (0–74) |
| Nadir CD4 count in cells/µL, median (IQR)e | 294 (86–571) | 310 (98–612) |
| Recruited after 2001, No. (%)* | 37 (11%) | 163 (48%) |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
a Age at study entry.
b Includes 43 men who acquired HIV-1 infection during follow up (25 with chronic HBV and 18 with chronic HCV).
c Before or during study.
d Median of each person's median weekly alcohol use over course of study (a median of 210 grams per week was defined as heavy alcohol use).
e CD4 measured in both HIV-1 infected and uninfected individuals.
*P < .05.
These 680 men had 6728 PYs of follow-up during which there were 293 deaths, yielding an all-cause MR of 43.5 per 1000 PYs (Table 2). As expected, the all-cause MRs were significantly higher in individuals infected with HIV (63.1 per 1000 PYs) than in individuals not infected with HIV (8.7 per 1000 PYs) (P < .001). There was, however, no significant difference in all-cause MRs between those with CH-B and those with CH-C (41.2 per 1000 PYs and 46.4 per 1000 PYs, respectively; P = .5).
Table 2.
Mortality Rates by Cause, Hepatitis Status, and HIV-1 Status
| Liver-Related |
All-Cause |
||||||
|---|---|---|---|---|---|---|---|
| Participant Category | PYs | Deaths | Mortality Rate (per 1000 PYs) | IRR (P) | Deaths | Mortality Rate (per 1000 PYs) | IRR (P) |
| All | 6728 | 51 | 7.6 | 293 | 43.5 | ||
| HIV negative | |||||||
| HBV | 1378 | 4 | 2.9 | 2.9 (.33) | 11 | 8.0 | 0.8 (.6) |
| HCV | 1039 | 1 | 1.0 | 1 | 10 | 9.6 | 1 |
| HIV positive | |||||||
| HBV | 2379 | 32 | 13.4 | 2.0 (.03) | 144 | 60.5 | 1.0 (.9) |
| HCV | 1932 | 14 | 7.2 | 1 | 128 | 66.3 | 1 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PY, person-year.
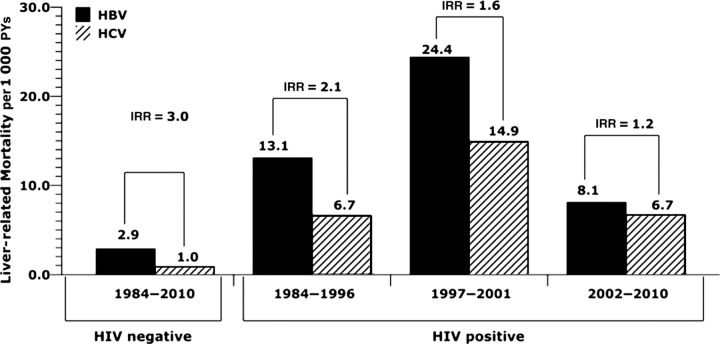
Of the 293 deaths, 51 (17%) were attributable to liver-related causes, yielding an overall liver-related MR of 7.6 per 1000 PYs. Interestingly, 46 (90.2%) of these 51 liver-related deaths occurred in individuals coinfected with HIV (liver-related MR, 10.7 per 1000 PYs vs 2.1 per 1000 PYs in infected and HIV–uninfected individuals, respectively; P < .001). In HIV–infected men, the liver-related MR rose from the pre-HAART era to the early HAART era, but it has dropped since 2002 (Figure 1).
Figure 1.
Time trend in liver-related mortality rates by hepatitis and human immunodeficiency virus type 1 status. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IRR, incidence rate ratio; PY, person-year.
The liver-related MR was significantly higher in men with CH-B than in men with CH-C (9.6 per 1000 PYs vs 5.0 per 1000 PYs, respectively; IRR, 1.9; P = .027). This finding remained true when stratified by HIV status with IRRs of 2.0 (95% CI, 1.1–3.7) and 3.0 (95% CI, .3–26.5) in HIV-infected and HIV-uninfected men, respectively.
Because nearly all of the liver-related deaths occurred in HIV–infected individuals, the multivariable analyses were restricted to this group. In a multivariable analysis adjusting for factors associated with liver-related mortality, CH-B remained significantly associated with an increased risk (IRR, 2.2; P = .03) (Table 3). Notably, more severe immunodeficiency, as represented by lower CD4 cell counts, was independently associated with an increased risk of liver-related mortality (adjusted IRR of 16.2 and 7.0 for those with CD4 cell counts of <200 and 200–350 cells/mm3, respectively, compared with CD4 cell counts >350 cells/mm). Older age was also independently associated with increased liver-related mortality (adjusted IRR per 10 years, 1.6; P = .01). When a competing risk model with the same covariates included in the above multivariable model was fit, CH-B remained associated with an increased risk of liver-related death (subhazard rate ratio, 2.4; 95% CI, 1.14–5.04; P = .02).
Table 3.
Multivariable Analysis of Liver Death in HIV-Infected Individuals
| Variable | IRR | 95% CI | P Value |
|---|---|---|---|
| Hepatitis status | |||
| HCV | 1 | ||
| HBV | 2.2 | 1.1–4.5 | .03 |
| Older age | |||
| Per 10-year increase | 1.6 | 1.1–2.3 | .01 |
| Latest CD 4 count | |||
| >350 cells/mm3 | 1 | ||
| 200–350 cells/mm3 | 7.0 | 2.4–20.1 | <.001 |
| <200 cells/mm3 | 16.2 | 6.1–42.8 | <.001 |
| HAART | |||
| Never | 1 | ||
| Ever | 0.7 | .3–1.6 | .43 |
| Recruitment period | |||
| Before 2001 | 1 | ||
| 2001–2003 | 0.8 | .2–2.3 | .64 |
Adjusted for race and cumulative alcohol use. Age, CD4 cell count, cumulative alcohol use, and highly active antiretroviral therapy use included as time updated covariates.
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IRR, incidence rate ratio.
Because some men were treated for HCV, it was possible that the increased risk of death in those with CH-B was due to men with CH-C clearing HCV with treatment. In the sensitivity analysis excluding the 6 men who had treatment-induced HCV clearance, the association of increased risk of liver-related death with CH-B remained (IRR, 2.3; P = .02).
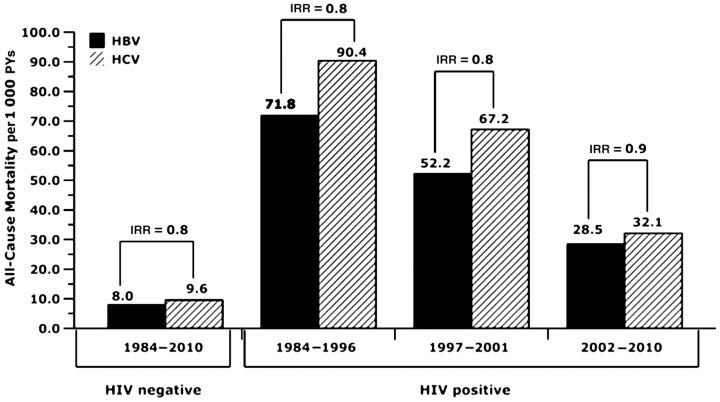
To determine if HAART affected the relative liver-related MRs for CH-B vs CH-C, we compared the risk of liver-related mortality over various time periods. Although liver-related MRs were consistently higher in the HIV/HBV coinfected group than in the HIV/HCV coinfected group, the comparative risk of liver-related mortality associated with CH-B vs CH-C decreased from an IRR of 2.1 to 1.6 to 1.2 across the 3 consecutive time periods (Figure 1), but this trend was not statistically significant. The lower IRR in the most recent time period is due to a decrease in liver-related MRs in those with CH-B because the HCV-associated liver-related MR was identical in the pre-HAART and the current HAART periods at 6.7 per 1000 PYs. However, in those same periods, the HBV-associated liver-related MR dropped from 13.1 per 1000 PYs to 8.1 per 1000 PYs. Although this decline was not statistically significant, it did show a trend toward a reduction in HBV-associated liver-related deaths since the introduction of more effective anti-HBV drugs, such as TDF. In contrast, during these same time periods, all-cause mortality consistently decreased in both HIV-HCV and HIV/HBV coinfected subgroups (Figure 2). To determine whether TDF accounted for the decline in HBV-associated liver-related MR since 2002, we included TDF use as a time-varying covariate in a multivariable analysis restricted to HBV-infected subjects. There was a reduction in liver-related mortality with TDF use (IRR, 0.6; 95% CI, .16–2.3; P = .5); however, it was not statistically significant, likely due to limited power because only 56 subjects had used TDF, with a median use of 1.7 years.
Figure 2.
Time trend in all-cause mortality rates by hepatitis and human immunodeficiency virus type 1 status. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IRR, incidence rate ratio; PY, person-year.
DISCUSSION
This is the first study to clearly demonstrate that the risk of liver-related death is twice as high from CH-B than CH-C in HIV-infected and HIV-uninfected subjects. In the HIV-infected subjects, CH-B remained associated with an increased risk of liver death after accounting for potential confounders, including competing risks of death. Notably, liver-related death was strongly associated with lower CD4 cell counts. Thus, this study emphasizes the need for a more aggressive approach to the prevention, diagnosis, and treatment of CH-B, including increasing vaccination rates among all HBV-susceptible individuals. This is especially true in Asian and African countries where there is a high prevalence of CH-B, HIV infection, and HIV/HBV coinfection.
The biological basis for increased severity of liver disease from CH-B compared with CH-C is not immediately apparent. It is unlikely to be due to differences in virulence of the 2 viruses because neither virus is cytopathic [15, 16]. Because it is the immune response to both viruses that is presumed to induce hepatocyte damage, it is plausible that HBV leads to a more robust T-cell response or a greater induction of cytokines. It is also plausible that the flares of hepatic inflammation, which are common in patients with CH-B, lead to more liver disease [17].
The finding that liver-related mortality was higher among men coinfected with HIV was not surprising, but it was notable that men with CD4 T-cell counts <200 cells/mm3 or between 200–350 cells/mm3 had a 16- and 7-fold increased risk of liver death, respectively, compared with men with CD4 cell counts >350. These data support that in individuals with chronic viral hepatitis, the severity of HIV-related immunosuppression affects the likelihood of liver death, which has been suggested by previous studies [4, 9–11]. These data also support recent trends toward earlier treatment of HIV in HBV-infected subjects [18].
Our finding of an increased risk of liver-related mortality in CH-B was suggested by the Data Collection on Adverse Events of Anti-HIV Drugs study (D:A:D). In this study, of 2482 deaths among 33 308 individuals, the relative risk of liver-related death in those with viral hepatitis compared with those without was 2.37 for subjects with CH-B and 1.67 for those with CH-C [11]. Another study, which compared liver-related mortality in 72 HBV/HIV and 256 HCV/HIV coinfected individuals receiving care at an HIV/hepatitis clinic, found a similar proportion of deaths from liver disease in the 2 groups (15% of HIV/HBV–coinfected individuals vs 13% of HIV/HCV–coinfected individuals) [8]. However, these patients were heterogeneous; thus, the results may have been confounded by increased use of excessive alcohol in the CH-C group compared with the CH-B group (25% vs 13%) and the use of HBV-active agents in the CH-B group. The French Mortality 2000 study, which found liver-related death in 31% of 235 HIV/HCV–coinfected and 22% of 64 HIV/HBV–coinfected individuals, was limited by small numbers of participants with CH-B and lack of adjustment for potential confounding factors, including high rates of excessive alcohol use (62%) in the CH-C group compared with the CH-B group (9%) [7]. In addition, the French study enrolled from clinics throughout France; thus, the HCV- and HBV-infected groups may have been selected from different populations, which was not accounted for. Our study design has clear advantages over these other studies, including a study population that was recruited and prospectively followed in the same manner, with similar numbers of men with CH-B or CH-C, similar rates of alcohol use between groups, and adjustment for potential confounders of liver-related mortality. In addition, this is, to our knowledge, the first study comparing liver-related mortality in CH-B and CH-C groups that accounted for competing causes of death.
Among HIV-1–infected men, the observed increased liver-related MRs after introduction of HAART were present in both HBV- and HCV-infected individuals and are consistent with findings from other cohorts [19, 20]. These increases have been attributed to many factors, including reduction in AIDS-associated mortality, which permitted development of complications from liver disease; increased use of alcohol among HIV-infected persons due to improved health; and hepatotoxicity associated with antiretroviral drugs, which was more frequent in early HAART therapy and is more severe in patients coinfected with hepatitis viruses [19, 21–23]. Of note, alcohol use is unlikely to have played a significant role in liver-related mortality in our study given low levels of alcohol use in the MACS. The more recent downward trend in liver-related MR in our study is consistent with findings from a hospital cohort in Spain [24] and may be related to improvements in care of HIV, including a reduction in the use of earlier antiretroviral drugs, which were associated with more hepatotoxicity [25]. It is, however, interesting to note that although liver-related MRs dropped in the current HAART era compared with the pre-HAART era in the CH-B group, rates remained similar in the CH-C group. These temporal trends suggest a potential beneficial effect of the use of HBV-active agents as part of HAART in HBV-infected individuals.
There are inherent limitations of this study due to its observational nature. It was not possible to ascertain the onset of HBV or HCV infection; thus, we were unable to directly adjust for duration of hepatitis infection. However, it is unlikely that our findings are due to longer duration of CH-B because stratification of liver-related mortality by calendar period (Figure 1) revealed an increased risk of liver-related death from CH-B in all time periods, with the largest difference between 1984 and 1996. Second, although the duration of follow-up in this study was longer for the CH-B group, adjustment for age and period of study recruitment should account for some of this difference. Third, HBV DNA levels were not uniformly available and thus were not taken into account when classifying subjects. However, if we classified people with low or undetectable levels of HBV DNA as being CH-B, the actual risk of liver death from CH-B with high levels of replication would be even greater than we report. Similarly, serial, HBsAg, and HCV RNA testing were not uniformly obtained, so some individuals may have recovered from either infection. Individuals are, however, more likely to spontaneously recover from HBV than HCV. Thus, the chances for misclassification of individuals with acute hepatitis as chronic hepatitis are higher for CH-B than for CH-C, so the risk of liver death from CH-B would be even greater than we have reported if there was misclassification. Fourth, this study was in a cohort of men who have sex with men, the majority of whom were infected with HIV. Further studies are needed to determine whether the results are applicable to women, HIV-uninfected individuals, or to men who are infected with HBV or HCV through other routes.
This study clearly demonstrates that CH-B carries a greater risk of death from liver disease than does CH-C. More emphasis needs to be placed on more-effective global HBV screening and increased efforts for vaccination and treatment of HBV infection worldwide.
Notes
Acknowledgments. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers principal investigators) at the Johns Hopkins Bloomberg School of Public Health Joseph B. Margolick, Lisa P. Jacobson); Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky); University of California, Los Angeles (Roger Detels); and University of Pittsburgh (Charles R. Rinaldo).
Financial support. The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (UO1-AI-35042, UL1-RR025005 [GCRC], UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85:303–15. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 4.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 6.Mai AL, Yim C, O'Rourke K, Heathcote EJ. The interaction of human immunodeficiency virus infection and hepatitis B virus infection in infected homosexual men. J Clin Gastroenterol. 1996;22:299–304. doi: 10.1097/00004836-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004;18:2039–45. doi: 10.1097/00002030-200410210-00008. [DOI] [PubMed] [Google Scholar]
- 9.Puoti M, Spinetti A, Ghezzi A, et al. Mortality for liver disease in patients with HIV infection: a cohort study. J Acquir Immune Defic Syndr. 2000;24:211–7. doi: 10.1097/00126334-200007010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 11.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 12.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 13.Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS. 2001;15:347–55. doi: 10.1097/00002030-200102160-00008. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc. 1999;94:496–509. [Google Scholar]
- 15.Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 16.Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007;112:141–55. doi: 10.1042/CS20060171. [DOI] [PubMed] [Google Scholar]
- 17.Mels GC, Bellati G, Leandro G, et al. Fluctuations in viremia, aminotransferases and IgM antibody to hepatitis B core antigen in chronic hepatitis B patients with disease exacerbations. Liver. 1994;14:175–81. doi: 10.1111/j.1600-0676.1994.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1–4. [PubMed] [Google Scholar]
- 19.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 20.del Amo J, Perez-Hoyos S, Moreno A, et al. Trends in AIDS and mortality in HIV-infected subjects with hemophilia from 1985 to 2003: the competing risks for death between AIDS and liver disease. J Acquir Immune Defic Syndr. 2006;41:624–31. doi: 10.1097/01.qai.0000194232.85336.dc. [DOI] [PubMed] [Google Scholar]
- 21.Kramer JR, Giordano TP, Souchek J, El-Serag HB. Hepatitis C coinfection increases the risk of fulminant hepatic failure in patients with HIV in the HAART era. J Hepatol. 2005;42:309–14. doi: 10.1016/j.jhep.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal E, Poiree M, Pradier C, et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study) AIDS. 2003;17:1803–9. doi: 10.1097/00002030-200308150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 24.Nunez-Fernandez C, Martin-Carbonero L, Valencia ME, et al. Liver complications have reached a plateau as cause of hospital admission and death in HIV patients in Madrid. AIDS Res Hum Retroviruses. 2009;25:383–5. doi: 10.1089/aid.2008.0242. [DOI] [PubMed] [Google Scholar]
- 25.ter Hofstede HJ, de Marie S, Foudraine NA, Danner SA, Brinkman K. Clinical features and risk factors of lactic acidosis following long-term antiretroviral therapy: 4 fatal cases. Int J STD AIDS. 2000;11:611–6. doi: 10.1258/0956462001916498. [DOI] [PubMed] [Google Scholar]