Abstract
Objectives
Although several studies have addressed the relationship between systemic bone mineral status and the severity of periodontitis, there is little knowledge of the relationship between periodontal disease and locally detected bone mineral density. The aim of this study was to compare the mandibular bone mineral density of patients with chronic periodontitis with that of periodontally healthy subjects.
Methods
48 systemically healthy subjects were included in the study and underwent a periodontal examination to determine their status. 24 subjects were periodontally healthy and the other 24 had moderate or severe chronic periodontitis. The mandibular bone mineral density of the subjects was determined by dual energy X-ray absorptiometry. The region of interest on the body of the mandible was independently determined on the dual energy absorptiometry radiographs, and a computer calculated the bone mineral density of these regions.
Results
The mandibular bone mineral density of the subjects with periodontitis was significantly lower than that of the periodontally healthy subjects (p < 0.01). There were significant negative correlations between the mandibular bone mineral density values and parameters related to the amount of periodontal destruction.
Conclusions
Low bone mineral density in the jaw may be associated with chronic periodontitis.
Keywords: periodontitis, bone density, mandible, dual energy X-ray absorptiometry, periodontal diseases
Introduction
Periodontal diseases are characterized by inflammation within the supporting periodontal tissues. The response of periodontal tissues to local bacterial challenge results in bone resorption and loss of periodontal attachment.1 Periodontitis induces the local and systemic release of pro-inflammatory cytokines such as tumour necrosis factor-α, interleukin-1β and interleukin-6,2,3 which are also thought to be associated with osteoporosis.4 Owing to alveolar bone loss being a prominent feature of periodontal disease, disturbances in bone metabolism and a decrease in the bone mineral content of the skeleton, and especially in the jaw, may be an aggravating factor in the case of periodontal disease.5-7 The potential mechanisms by which host factors may influence the onset and progression of periodontal disease, directly or indirectly, include an underlying low bone density in the oral cavity, bone loss as an inflammatory response to infection, genetic susceptibility and exposure to shared risk factors.8
Although the relationship between skeletal bone mineral density (BMD) and periodontitis has been extensively investigated, the results have shown varying degrees of inconsistency.6,9-20 In a review by Wactawski-Wende,8 these inconsistencies were attributed to differences in sample size, the limited control of potentially confounding factors and the definitions of both periodontal disease and osteoporosis. Furthermore, only a few studies have assessed the relationship between local BMD in the jaw and periodontitis, and to our knowledge all were conducted on post-menopausal females. In one study, a significant correlation was found between the alveolar BMD, which was measured using a scale on dental radiographs and periodontal pocket depth and tooth mobility.14 The other study that assessed the BMD of the mandibular cortex using quantitative CT found a negative correlation between periodontal pocket depth and the BMD of the mandibular lingual cortex.5
Likewise, it is not yet known whether the BMD of the jaw is lower in subjects with periodontitis than in their healthy counterparts. The finding that bone mineral content of the jaw is associated with severe periodontitis in young adults suggests that such a difference may very well exist between subjects with and without periodontitis.6 Given that osteoporosis is known to have an effect on the BMD of the jaw, the aim of this study was to compare the mandibular BMD in subjects with and without chronic periodontitis who did not have osteoporosis. The research hypothesis of the present study was that the mandibular BMD in subjects with chronic periodontitis is significantly lower than that of subjects without chronic periodontitis.
Materials and methods
This cross-sectional study was performed as a joint collaboration between the Department of Nuclear Medicine in the Faculty of Medicine and the Department of Periodontology in the Faculty of Dentistry at Süleyman Demirel University. The study protocol was approved by the local ethical committee (date: 5 December 2006; number: 09/10) and was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki as revised in 2002. The inclusive enrolment dates of this study were from January 2007 to June 2009.
The subjects were selected from volunteer subjects attending Süleyman Demirel University, Faculty of Dentistry and Department of Periodontology for periodontal or dental complaints. Subjects with at least 20 natural teeth and all their molars in the mandible except for the third molars were included in the study. All volunteers signed an informed consent form.
The exclusion criteria were having systemic disease; having a diagnosis of osteoporosis or osteopenia or receiving any osteoporosis treatment; being a current or former smoker; medications that may influence bone metabolism; regular alcohol consumption; initial periodontal treatment within the previous 6 months; a history of any surgical periodontal therapy; and antibiotic, anti-inflammatory, immunosuppressive or cytotoxic drug intake within the previous 3 months. Pregnant, lactating or post-menopausal females were also excluded from the study. In addition, the jaws of the subjects were evaluated with oral panoramic radiography and any subjects who had radiolucent or radiopaque lesions which may have influenced the results of mandibular BMD, such as condensing osteitis, osteosclerosis and periapical lesion or cyst in their mandible, were excluded from the study.
The 52 participants who matched the inclusion criteria and consented to the study were referred to the Department of Nuclear Medicine to determine whether or not they had undiagnosed skeletal osteoporosis or osteopenia. The BMD of the femoral neck and trochanter was measured using a dual energy X-ray absorptiometry (DXA) scanner (Norland XR-46; Norland Medical Systems, Fort Atkinson, WI) as a proxy of systemic bone density. Osteoporosis or osteopenia was detected in four subjects according to the World Health Organization’s criteria for diagnosis of osteoporosis and osteopenia, namely BMD >1 standard deviation (SD) below the young adult female reference mean (T-score <−1), and was thus excluded from the study.21
Mandibular bone mineral density evaluation
Mandibular BMD (in grams per centimetre squared) was measured using a DXA scanner (Norland XR-46). BMD measurements were performed on the body of the mandible, which produces greater sensitivity and specificity than the ramus and the symphysis regions, as described by Horner et al.22 The subjects were laid on their right side and their necks were maximally extended to avoid superimposition of the cervical spine. The lateral projection of the DXA scanner provided overlapping images of both sides of the mandible. The mandible was scanned in a rectilinear manner starting from 1 cm above the temporo-mandibular joint through the whole of the mandible on one side. The image of the contralateral side was superimposed. When the two sides of the mandible were not superimposed because of positioning error, the scan was repeated. Once the DXA images had been recorded, the investigator positioned a customized rectangular region of interest (ROI) on the mandibular images on-screen and the computer calculated the BMD of these regions. The ROIs were manipulated to include the maximum available area of the mandibular body on the image and were altered to conform to the shape of the mandible of each subject. Each ROI began at the anterior border of the ramus and was limited anteriorly by the parasymphysis, inferiorly by the cortical border and superiorly by the root apex of the molar teeth.23 The location of the ROI for the measurement of mandibular BMD on a DXA scan is illustrated in Figure 1. The computer software calculated the BMD of the selected region. The DXA scans of the mandible were analysed by one experienced investigator (UŞB), who was blind to the subject’s status to avoid interobserver variation. The reproducibility of the measurement system was assessed by repeating the analysis three times for each image.
Figure 1.
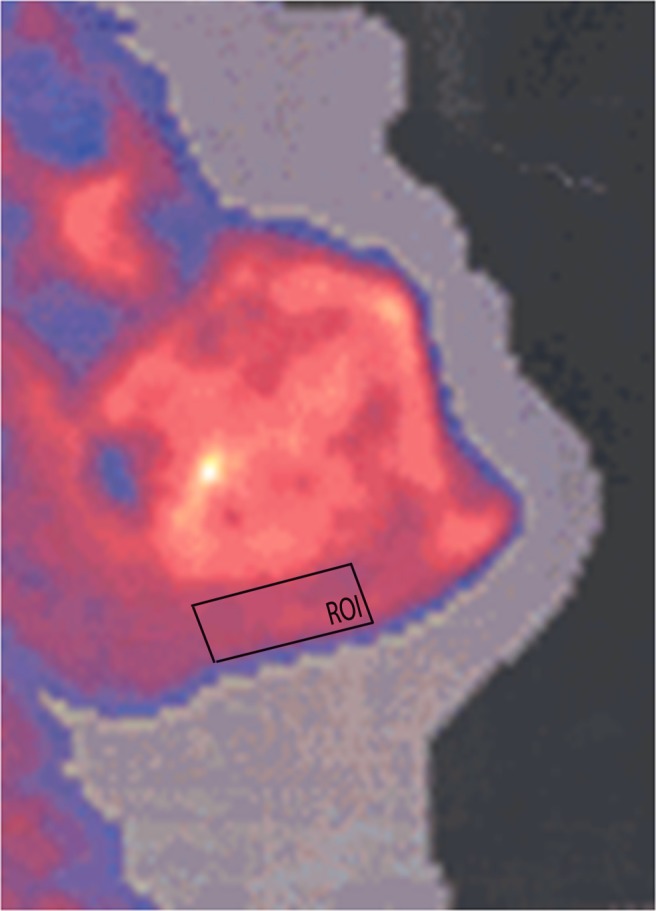
The illustration of the localization of the region of interest and the measurement of mandibular bone mineral density on a dual energy X-ray absorptiometry scan
Periodontal evaluation
All the dental examinations were conducted by one clinician (MÖT). All dental variables were assessed at six different sites (mesiobuccal, mid-buccal, distobuccal, mesiolingual, mid-lingual and distolingual) of each tooth present, excluding the wisdom teeth. Clinical measurements of periodontal parameters included the plaque index (PI),24 gingival index (GI),25 probing pocket depth (PPD), clinical attachment level (CAL) and bleeding on probing (BOP). All assessments were carried out using the Williams periodontal probe (Hu-Friedy, Chicago, IL).
Study groups
After the periodontal measurements were taken and the diagnosis confirmed radiologically using oral panoramic radiography, the subjects were divided into two groups: periodontally healthy and chronic periodontitis. The diagnosis was based on the clinical and radiographic criteria described in the 1999 Consensus Classification of Periodontal Diseases.26
The periodontally healthy group comprised 24 systemically and periodontally healthy (having no loss of periodontal attachment, PPD ≤3 mm and GI <1) subjects (13 female and 11 male, aged between 28 years and 55 years), who were referred to the Department of Periodontology for oral hygiene instruction.
The chronic periodontitis group comprised 24 systemically healthy subjects (12 female, 12 male, aged between 28 years and 56 years), with moderate or severe generalized chronic periodontitis (having loss of clinical attachment ≥4 mm involving at least 30% of sites in their mouths).
Sample size calculation
To determine the sample size required for the study, a pilot study was performed and the SD of BMD values were calculated as 0.38 g cm–2. The sample size of the present study is calculated to detect a difference of 0.30 g cm–2 between the BMD values of the groups at the 0.05 probability level with a power of 80%. Power analysis and sample size estimation were performed using a statistical programme (NCSS/PASS 2008, Dawson Edition; NCSS, Kaysville, UT). The power analysis revealed that the required sample size was a minimum of 24 subjects for each group.
Statistical analysis
A statistics programme (SPSS for Windows version 15.0; SPSS Inc., Chicago, IL) was used for statistical evaluation of the present data. All data were expressed as mean ± SD on a subject basis. The mean ± SD values of CAL and PPD were calculated for both the overall mouth (CAL-t and PPD-t) and the BMD measurement area (left and right mandibular molar teeth) (CAL-m and PPD-m).
The normality of the data distribution was examined using the Kolmogorov–Smirnov test. The differences between the groups were determined using the Mann–Whitney U-test for the non-normally distributed variables (DXA, BOP, CAL, CAL-m and the number of missing teeth) and the independent samples t-test for normally distributed variables (age, PI, GI, PPD and PPD-m). A χ2 test was performed to identify gender differences between the groups. p < 0.01 was considered significant.
Spearman’s correlation coefficient was used to investigate the relationships between mandibular and femoral BMD, age, the number of missing teeth and periodontal clinical parameters.
Results
Subject characteristics
A total of 48 subjects without skeletal osteoporosis or osteopenia (25 female and 23 male) were included in this study. The mean ages of the female and male subjects were 41.82 ± 8.27 years and 42.78 ± 6.08 years, respectively. There was no significant difference between female and male subjects with regards to age and mandibular BMD values (p > 0.01). The characteristics of the study groups are illustrated in Table 1. There were no significant differences between the groups in terms of matching variables (age and gender) (p > 0.01) or the number of missing teeth (p > 0.01).
Table 1. Characteristics of the study groups.
| Study groups | Gender (n) |
Age (years) |
Number of missing teeth |
| Female/male | Mean±SD | Median (min–max) | |
| Periodontitis (n = 24) | 12/12 | 43.58±6.78 | 5 (0–10) |
| Periodontally healthy (n = 24) | 13/11 | 40.83±7.41 | 4 (0–8) |
| Total (n = 48) | 25/23 | 42.20±7.42 | 4 (0–10) |
| p-value | NSa | NSb | NSc |
Max, maximum; min, minimum; NS, not significant (p>0.01).
Normally distributed data are expressed as mean ± standard deviation (SD) and non-normally distributed data are expressed as median (min–max).
aχ2 test.
bIndependent samples t-test.
cMann–Whitney U-test.
Clinical periodontal variables
There were significant differences between the groups regarding the PPD-t, CAL-t, GI and BOP values (p < 0.01) and the CAL-m and PPD-m values (p < 0.01). Table 2 displays the clinical periodontal variables of the study groups.
Table 2. Clinical periodontal parameters and mandibular bone mineral density values of the study groups.
| Study Groups | PI |
GI |
BOP (%) |
PPD-t (mm) |
PPD-m (mm) |
CAL-t (mm) |
CAL-m (mm) |
Mandibular BMD (g cm−2) |
| Mean ± SD | Mean ± SD | Median (min–max) | Mean ± SD | Mean ± SD | Median (min–max) | Median (min–max) | Median (min–max) | |
| Periodontitis n = 24 | 1.77 ± 0.66 | 1.13 ± 0.44 | 67.55 (11.11–100) | 3.39 ± 0.63 | 3.75 ± 0.98 | 3.94 (2.48–6.94) | 4.16 (2.77–7.83) | 0.95 (0.66–1.56) |
| Periodontally healthy n = 24 | 1.52 ± 0.64a | 0.78 ± 0.30a,b | 26.35 (14.28–34.25)b,c | 2.14 ± 0.50a,b | 2.42 ± 0.59a,b | 2.45 (1.20–2.98)b,c | 2.68 (1.03–2.96)b,c | 1.12 (0.71–1.95)b,c |
| Total n = 48 | 1.64 ± 0.65 | 0.96 ± 0.41 | 50.78 (11.11–100) | 2.77 ± 0.85 | 3.08 ± 1.04 | 2.91 (1.20–6.94) | 3.20 (1.03–7.83) | 1.04 (0.66–1.95) |
BMD, bone mineral density; BOP, bleeding on probing; CAL, clinical attachment level; GI, gingival index; m, measurement area; max, maximum; min, minimum; PI, plaque index; PPD, probing pocket depth; SD, standard deviation; t, total.
Normally distributed data are expressed as mean ± standard deviation (SD) and non-normally distributed data are expressed as median (min–max).
aIndependent samples t-test.
bStatistically significant difference between the groups (p < 0.01).
cMann–Whitney U-test.
Mandibular bone mineral density
The mean value of mandibular BMD in the chronic periodontitis group was significantly lower than that of the periodontally healthy group (p < 0.01) (Table 2).
Correlations
In all subjects, mandibular BMD was negatively correlated with age (rho = −0.414, p < 0.01), PPD-t (rho = −0.362, p < 0.01) and CAL-t (rho = −0.364, p < 0.01), and age was positively correlated with PPD-t (rho = 0.395, p < 0.01) and CAL-t (rho = 0.430, p < 0.01). There was a moderate positive correlation between mandibular and femoral BMD (rho = 0.327, p < 0.01).
Discussion
In this study, we evaluated the mandibular BMD in subjects with moderate or severe chronic periodontitis using DXA and compared them with periodontally healthy subjects. Our findings revealed that there was a significant difference between subjects with periodontitis and periodontally healthy subjects regarding mandibular BMD.
To our knowledge, this is the first study designed to evaluate mandibular BMD using DXA in both males and females with periodontitis who were systemically healthy and had no osteopenia or osteoporosis. We tried to compose homogenous study populations with strict inclusion criteria in order to eliminate the confounding factors that affect bone metabolism such as age, osteoporosis and other systemic diseases, menopause, smoking, alcohol consumption and hormone intake. Edentulism affects bone height and density so we were careful to ensure that there was no tooth loss in the region of BMD measurement in the mandible of the subjects. In addition, there were no differences between the groups regarding the number of missing teeth.
DXA is widely accepted as the gold standard for clinical bone mineral measurements.27 The basal area of the mandible posterior to the mental foramen was suggested as the most suitable site for jawbone measurements because of the small inter- and intra-individual variations in anatomical size, shape, bone structure and function.28 The methodology used to measure the mandibular BMD in this study has been used in several investigations.22,23,29,30 The correlation between mandibular BMD and other skeletal sites assessed by various techniques have also been reported.14,15,22,31-34 Similarly, we found significant positive correlation between mandibular and femoral BMD. However, a recent study reported that the BMD of the jaw measured using DXA was not correlated with femoral BMD.30
The association between systemic BMD and periodontal disease has been addressed in a number of studies.6,9-20 While the cross-sectional studies conducted on post-menopausal osteoporotic females and elderly males suggested that there was a correlation between systemic BMD and periodontal disease,9-11, 14, 35 other studies conducted on pre-menopausal females and young male populations did not support such a relationship.6,12,13
A recent longitudinal study conducted on elderly Chinese males suggested that osteoporosis is associated with severe loss of clinical attachment and interproximal gingival recession.18 With the exception of Phipps et al17, the results of other longitudinal studies regarding the relationship between systemic BMD and periodontal disease progression support this finding.19,20 These studies suggest that systemic bone loss arising from uncoupled bone remodelling due to oestrogen deficiency also affects the jawbone and is important for the progression of bone loss in periodontitis.
Von Wowern et al6 suggested that periodontitis in young subjects is a local disorder without systemic alterations of bone mineral status, giving rise to the notion that periodontitis may be associated with the local bone mineral status of the jaw rather than the systemic bone mineral status.
The results of our study support this hypothesis. We found that the mandibular BMD values of the chronic periodontitis group were significantly lower than those of the periodontally healthy group and that there were significant but moderate negative correlations between mandibular BMD and the variables related to periodontal destruction (PPD-t or CAL-t).
Three studies in the literature have evaluated mandibular BMD and the severity of periodontitis.5,14,36 These studies were conducted on post-menopausal females and used panoramic14,36 or dental radiographs5 to measure BMD in the alveolar part of the mandible. Klemetti et al5 found a significant positive correlation between periodontal pocket depth and mandibular BMD and suggested that higher bone density in the periodontally diseased regions may have resulted from a defence reaction of the trabecular portion of the jaw against periodontal destruction. However, similar to our results, Takaishi et al14 detected a significant negative correlation between periodontal attachment levels and mandibular BMD, and Nackaerts et al36 found a significant negative association between alveolar bone height and mandibular BMD. These results suggest that local alveolar bone density influences periodontal bone loss.
The distinctive feature of this study is it was conducted on subjects without skeletal osteoporosis or osteopenia. The results of this studysuggest that local mechanisms may play a role in the decrease of BMD in the jaws of systemically healthy chronic periodontitis patients rather than skeletal disturbances. The same types of cytokines such as interleukin 1-β and tumour necrosis factor-α are implicated as stimulators of bone resorption in periodontitis, rheumatoid arthritis and post-menopausal osteoporosis.37 Although severe periodontitis does not affect skeletal BMD, it may alter the local BMD. The results of the other two studies regarding mandibular BMD and periodontitis severity and the results of our study support this hypothesis.14,36
Previous studies have shown that mandibular cortical thickness has a significant negative correlation with age.24,38 Devlin and Horner24 suggested that the BMD of the mandibular body of females decreases with age. Pluskiewicz et al34 evaluated the mandibular BMD of 36 females and 6 males using DXA and reported that the mandibular BMD of females was lower than that of males. Although the subjects in the current study were younger (22–56 years) than those in other studies,14,22,23,31 we also found a significant negative correlation between age and mandibular BMD. In addition, we found no difference between males and females regarding mandibular BMD. However, we also observed a positive significant moderate correlation between age and CAL. This was not surprising as age is a common risk factor for loss of periodontal attachment and osteoporosis.
This study was limited by its cross-sectional design. In addition, the number of study participants was restricted because subjects with generalized chronic periodontitis who satisfy the strict inclusion criteria of the study are rarely seen.
Conclusion
The BMD of the mandible of subjects with moderate or severe chronic periodontitis was significantly lower than that of periodontally healthy subjects. The results of this study suggest that low BMD in the jaw may be associated with chronic periodontitis. Further longitudinal studies that show the associations between mandibular and skeletal BMD and the severity of periodontal disease and include larger study populations are required.
References
- 1.Schwartz Z, Goultshin J, Dean DD, Boyan BD. Mechanisms of alveolar bone destruction in periodontitis. Periodontol 2000 1997;14:158–172 [DOI] [PubMed] [Google Scholar]
- 2.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res 1991;26:230–242 [DOI] [PubMed] [Google Scholar]
- 3.Page RC, Kornman KS. The pathogenesis of human periodontitis. Periodontol 2000 1997;14:9–11 [DOI] [PubMed] [Google Scholar]
- 4.Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol 2010;54:123–132 [DOI] [PubMed] [Google Scholar]
- 5.Klemetti E, Collen HL, Forss H, Markanen H, Lassila V. Mineral status of skeleton and advanced periodontal disease. J Clin Periodontol 1994;21:184–188 [DOI] [PubMed] [Google Scholar]
- 6.von Wowern N, Westergaard J, Kollerup G. Bone mineral content and bone metabolism in young adults with severe periodontitis. J Clin Periodontol 2001;28:583–588 [DOI] [PubMed] [Google Scholar]
- 7.Geurs NC, Lewis CE, Jeffcoat MK. Osteoporosis and periodontal disease progression. Periodontol 2000 2003;32:105–110 [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol 2001;6:197–208 [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Hollender LG. Relationship between bone mineral density and tooth loss in elderly Japanese women. Dentomaxillofac Radiol 1999;28:219–223 [DOI] [PubMed] [Google Scholar]
- 10.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000;71:1492–1498 [DOI] [PubMed] [Google Scholar]
- 11.Inagaki K, Kurosu Y, Yoshinari N, Noguchi T, Krall EA, Garcia RI. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in Japanese women. Calcif Tissue Int 2005;77:9–14 [DOI] [PubMed] [Google Scholar]
- 12.Elders PJ, Habets LL, Netelenbos JC, Van DerLinden LW, Van DerStelt PF. The relation between periodontitis and systemic bone mass in women between 46–55 years of age. J Clin Periodontol 1992;19:492–496 [DOI] [PubMed] [Google Scholar]
- 13.Hattatoğlu-Sönmez E, Özçakar L, Gökçe-Kutsal Y, Karaağaoğlu E, Demiralp B, Nazlıel-Erverdi H. No alteration in bone mineral density in patients with periodontitis. J Dent Res 2008;87:79–83 [DOI] [PubMed] [Google Scholar]
- 14.Takaishi Y, Okamoto Y, Ikeo T, Morii H, Takeda M, Hide K, et al. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int 2005;16:1875–1882 [DOI] [PubMed] [Google Scholar]
- 15.Corten FG, Van't Hof MA, Buijis WC, Hoppenbrouwers P, Kalk W, Corstens FH. Measurement of mandibular bone density ex vivo and in vivo by dual-energy X-ray absorptiometry. Arch Oral Biol 1993;38:215–219 [DOI] [PubMed] [Google Scholar]
- 16.Brennan-Calanan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Osteoporosis and oral infection: independent risk factors for oral bone loss. J Dent Res 2008;87:323–327 [DOI] [PubMed] [Google Scholar]
- 17.Phipps KR, Chan BKS, Madden TE, Geurs NC, Reddy MS, Lewis CE, et al. Longitudinal study of bone density and periodontal disease in men. J Dent Res 2007;86:1110–1114 [DOI] [PubMed] [Google Scholar]
- 18.Shum I, Leung PC, Kwok A, Corbet EF, Orwoll ES, Phipps KR, et al. Periodontal conditions in elderly men with and without osteoporosis or osteopenia. J Periodontol 2010;81:1396–1402 [DOI] [PubMed] [Google Scholar]
- 19.Payne JB, Reinhart RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999;10:34–40 [DOI] [PubMed] [Google Scholar]
- 20.Yoshihara A, Seida Y, Hanada N, Miyazaki H. A longitudinal study of the relationship between periodontal disease and bone mineral density in community-dwelling older adults. J Clin Periodontol 2004;31:680–684 [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359:1929–1936 [DOI] [PubMed] [Google Scholar]
- 22.Horner K, Devlin H, Alsop CW, Hodgkinson IM, Adams JE. Mandibular bone mineral density as a predictor of skeletal osteoporosis. Br J Radiol 1996;69:1019–1025 [DOI] [PubMed] [Google Scholar]
- 23.Devlin H, Horner K. A study to assess the relative influence of age and edentulousness upon mandibular bone mineral density in female subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:117–121 [DOI] [PubMed] [Google Scholar]
- 24.Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odont Scand 1964;22:121–135 [DOI] [PubMed] [Google Scholar]
- 25.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odont Scand 1963;21:533–551 [DOI] [PubMed] [Google Scholar]
- 26.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1–6 [DOI] [PubMed] [Google Scholar]
- 27.Patel R, Blake GM, Fogelman I. An evaluation of the United Kingdom National Osteoporosis Society position statement on the use of peripheral dual-energy X-ray absorptiometry. Osteoporos Int 2004;15:497–504 [DOI] [PubMed] [Google Scholar]
- 28.von Wowern N. General and oral aspects of osteoporosis: a review. Clin Oral Invest 2001;5:71–82 [DOI] [PubMed] [Google Scholar]
- 29.Buyukkaplan UŞ, Akkaya A, Yıldız M, Bircan A, Aksoy Doğan A, Ozturk O. Mineral status of COPD patients under long-term inhaled corticosteroid therapy. J Prosthodont 2008;17:462–467 [DOI] [PubMed] [Google Scholar]
- 30.Gulsahi A, Paksoy CS, Ozden S, Kucuk NO, Cebeci ARI, Genc Y. Assessment of bone mineral density in the jaws and its relationship to radiomorphometric indices. Dentomaxillofac Radiol 2010;39:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kribbs PJ, Chestnut CH, Ott SM, Kilcoyne RF. Relationships between mandibular and skeletal bone in a population of normal women. J Prost Dent 1990;63:86–89 [DOI] [PubMed] [Google Scholar]
- 32.Klemetti E, Vainio P, Lassila V, Alhawa E. Cortical bone mineral density in the mandible and osteoporosis status in postmenopausal women. Scand J Dent Res 1993;101:219–223 [DOI] [PubMed] [Google Scholar]
- 33.Klemetti E, Vainio P, Lassila V, Alhawa E. Trabecular bone mineral density of mandible and alveolar height in postmenopausal women. Scand J Dent Res 1993;101:166–170 [DOI] [PubMed] [Google Scholar]
- 34.Pluskiewicz W, Tarnawska B, Drozdzowska B. Mandibular bone mineral density measured using dual-energy X-ray absorptiometry: relationship to hip bone mineral density and quantitative ultrasound at calcaneus and hand phalanges. Br J Radiol 2000;73:288–292 [DOI] [PubMed] [Google Scholar]
- 35.Al Habashneh R, Alchalabi H, Khader Y, Hazza’a AM, Odat Z, Johnson GK. Association between periodontal disease and osteoporosis in postmenopausal women in Jordan. J Periodontol 2010;81:1613–1621 [DOI] [PubMed] [Google Scholar]
- 36.Nackaerts O, Gijbels F, Sana AM, Jacobs R. Is there a relation between local bone quality as assessed on panoramic radiographs and alveolar bone level? Clin Oral Invest 2008;12:31–35 [DOI] [PubMed] [Google Scholar]
- 37.Lerner UH. Bone remodeling in post-menopausal osteoporosis. J Dent Res 2006;85:584–595 [DOI] [PubMed] [Google Scholar]
- 38.Ledgerton D, Horner K, Devlin H, Worthington H. Radiomorphometric indices of the mandible in a British female population. Dentomaxillofac Radiol 1999;28:173–181 [DOI] [PubMed] [Google Scholar]


