Abstract
Objective
The aim of this study was to investigate the usefulness of texture analysis in the characterization of oral cancers involving the buccal mucosa and to assess its effectiveness in differentiating between the various grades of the tumour.
Methods
Contrast enhanced CT examination was carried out in 21 patients with carcinoma of the buccal mucosa who had consented to retrospective analysis during a research study that was approved by the institutional review board. Two regions of interest (ROIs) were created, one at the site of the lesion and the other at the contralateral normal side. Texture analysis measures of fractal dimension (FD), lacunarity and grey level co-occurrence matrix (GLCM) were computed for each ROI. The numeric data from the two ROIs were compared and were correlated with the tumour grade as confirmed by biopsy.
Results
The difference between the mean FD and GLCM parameters of the lesion vs the normal ROI were statistically significant (p < 0.05); no significant difference was observed between the three grades of tumour for any of the parameters (p > 0.05).
Conclusion
Texture analysis on CT images is a potential method in the characterization of oral cancers involving the buccal mucosa and deserves further investigation as a predictor of tumour aggression.
Keywords: fractals, oral cancer, X-ray computed tomography, diagnosis, tumour staging
Introduction
Oral cancer is one of the major global health problems, with an alarmingly high incidence in the developing world particularly. It is the eighth most common cancer in males worldwide, and in south central Asia it ranks among the three most common types of cancers affecting the body.1 In India, the age-standardized incidence rate of cancers of the oral cavity remains as high as 12.6 per 100 000 of population.2 Despite many advances in the treatment of these cancers, there is a high mortality rate with an overall 5-year survival rate of 20–43%.3 Besides this, those patients treated for oral cancers are left with severe aesthetic and functional compromises leading to significant morbidity affecting their psychological and physical welfare. The major factors contributing to this scenario are delay in diagnosis and high recurrence rates owing to inadequate patient treatment protocol. The current therapeutic decision making is based on tumour staging with clinical examination, visual assessment of radiological images and conventional histopathological tumour grading. Patients in the early stages are treated with either radiation therapy or surgical resection, while those in advanced stages require a combination of these including chemotherapy. Any inadequacy in the assessing and staging of the tumour may reflect as treatment failure and cause further deterioration of patients' health. This underscores the need for a more quantitative and reproducible approach which can be used as an adjuvant tool to effectively assess tumours and their behaviour before the initiation of therapy.
Various imaging modalities are used to evaluate the oral cavity cancers including plain film radiography (intraoral radiography, panoramic radiography), nuclear medicine scintigraphy, ultrasonography, CT, MRI and positron emission tomography combined with CT (PET/CT). Use of conventional radiographs, scintigraphy and ultrasonography in the evaluation of primary tumours is highly restricted owing to their inherent limitations in accuracy. Although MRI and PET/CT are promising in the accurate assessment of oral cancers, cost and availability limit their routine use. CT is the most commonly used imaging modality and provides high-resolution images especially when a contrast agent is used to highlight the vascularity of the examined tissues. The characteristics commonly used to describe the tumours are size, extent of involvement and bone invasion. However, no information is sought on the heterogeneity, texture/surface irregularity and complexity of the lesions, which may be important for the extraction of diagnostic information from CT images. In recent years, texture analysis techniques have been applied for different imaging modalities to distinguish normal and abnormal tissues in the body4-9 and also to characterize lung tumours as aggressive or non-aggressive.10 Studies have also demonstrated use of the method to classify salivary gland pathologies as benign or malignant on ultrasonographic images.11-13 Such an analysis is concerned with the study of the variation in intensity of acquired image pixel values. The ability to extract such useful, otherwise hidden information through digitally processed images can be an important tool for oncologists to support an accurate diagnosis. Additionally, the information could be used to determine the biological behaviour of tumours, thereby assisting in the stratification of patients in a cost-effective manner. However, there remains a dearth of literature on the use of such a novel technique in the characterization of cancers of the oral cavity. Further, it is worth exploring the effectiveness of texture examination on CT images which are routinely acquired for oral cancer assessment and treatment planning.
This study was attempted with the following aims and objectives: (a) to investigate the usefulness of texture analysis as a parameter for numerical expression in the characterization of oral cancers involving the buccal mucosa and (b) to establish whether the texture analysed with the use of different parameters could be used to discriminate among the various grades of oral cancers (grade I—well differentiated, grade II—moderately differentiated and grade III—poorly differentiated) as confirmed by biopsy.
Materials and methods
Ethical aspects
All procedures in this study were conducted in full accordance with the ethical principles and received approval from the institutional review board of the Government Dental College and Research Institute, Bangalore, India.
Image acquisition
21 patients (12 males and 9 females) aged 30–73 years with carcinoma involving the buccal mucosa underwent high-resolution quantitative contrast enhanced CT (Siemens Emotion; Siemens Healthcare, Munich, Germany) examination for tumour staging. The slice thickness was 2 mm; table speed 2 mm s−1; scans began 12–16 s after the injection of iopramide contrast agent with iodine concentration of 370 mg ml−1 and were acquired at an anatomical level containing the largest transverse dimension of the cancer lesions of the head and neck. A standard algorithm was used for image reconstruction and the images were 512 × 512 pixels. The image data were transferred with a dynamic sequence in digital imaging and communication in medicine (DICOM) format to a computer with a processor speed of 1.86 GHz and a 3 GB system memory. One image which appeared with the maximum effect of the contrast was chosen for further analysis.
Image analysis
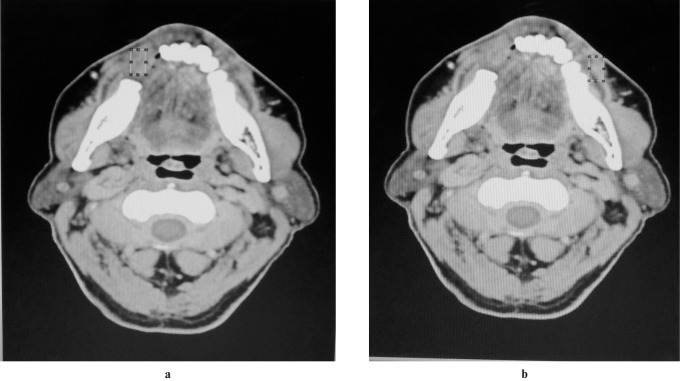
The ImageJ 1.43 program (US National Institute of Health, Bethesda, MA, http://rsb.info.nih.gov/nih-image ) was used for image processing and analysis. All the acquired images were first converted to 8 bit pixels. Two regions of interest (ROIs) were chosen, one at the site of the lesion and the other at the contralateral normal side (Figure 1).
Figure 1.
(a) Region of interest (ROI) extraction of lesion. (b) ROI extraction of contralateral normal area
The choice of the ROI was carried out according to the following criteria:
Its shape was a rectangle.
Its position was determined manually under the supervision of an expert radiologist.
The ROI always excluded the bone and air spaces.
Its size was arbitrarily selected as 30 × 15 pixels. As the analysis was restricted to buccal mucosa, a larger size of ROI particularly in the normal area would have resulted in inclusion of air and/or bone, which would have drastically altered the analysis, while a smaller area would not have sufficiently covered the area, particularly in the region of the lesion.
The ROIs were cropped and each of these greyscale images were subjected to three methods of texture analysis—two model-based feature methods and one statistically-based feature extraction method. The former included fractal dimension (FD) and lacunarity with the differential box counting method, and the latter included grey level co-occurrence matrix (GLCM) texture analysis (Figure 2). For the GLCM analysis, the default case 0° with one separating pixel was adopted as the tissue regions were stochastic without a visible repletion pattern.
Figure 2.
Texture analysis of CT image with the software
In the study, greyscale images were used for analysis rather than binary counterparts as the former are considered richer in information which is deemed necessary for image analysis.14 No filtering was used as the noise was subtle and smoothing the image would have resulted in blurring out some of the tissue characteristics (i.e. reducing pixel variation in the ROI) which are essential in the operation of the applied texture measures.
The numerical data for various parameters of texture from the lesional and normal ROIs were compared. Further, the data from the lesional ROI were correlated with the tumour grade as confirmed by biopsy: grade I—well differentiated; grade II—moderately differentiated; grade III—poorly differentiated. Statistical analysis was carried out with SPSS version 15.0 (SPPS Inc., Chicago, IL).
Concepts of texture analysis
FD is a mathematical descriptor which provides a structure's geometrical complexity. FD is based on the hypothesis that spatial patterns are self similar, i.e. they repeat themselves among many scales and exhibit a certain hierarchical dependency when they are simultaneously analysed in different scales. This hierarchical dependency may provide valuable information to characterize such patterns. There are many algorithms to estimate the fractal dimensions of an image. One of the most applied is the box-counting algorithm15 which was used in the study. According to this algorithm, an image is systematically covered with grids. Each one is composed by adjacent boxes of size n, where n is the corresponding scaling factor and theoretically takes a value from 0 to infinity. However, in practice, for a 512 × 512 mm CT image with a resolution of 8–16 bits per pixel, the optimal n range is between 2 pixels and 7 pixels.10 Then, for each successive grid, the number of boxes of a particular size which are needed to cover the image are counted.
Lacunarity can be defined as a complimentary measure of fractal dimension or the deviation of a geometric structure from its translational invariance. It allows the viewer to distinguish spatial patterns through the analysis of their gap distribution in different scales. The higher the lacunarity of a spatial pattern, the higher the variability of its gaps in an image and the more heterogeneous its texture will be. There are numerous algorithms to calculate the lacunarity of an image. Among them, two have been commonly used: gliding-box and differential box-counting. In the present study, the latter was used.
The GLCM proposed by Haralick et al16 represents the joint probability of certain sets of pixels having a certain grey level value. It calculates how many times a pixel with a particular grey level occurs jointly with another pixel having a different grey value. This matrix is used to extract the second order statistical texture features. Haralick et al suggested 14 features describing the two-dimensional probability density function. In this research, four popular features were used: angular second moment (ASM), contrast, inverse difference moment (IDM) and entropy. ASM is a way to measure disorders in an image through summing the square of all pixels, with higher values indicating that pixels are very similar. Contrast, also known as inertia, is the measurement of intensity contrast or local variations between the image pixels, giving lower values for uniform texture. IDM is related to the contrast of the texture and is also called the homogeneity. Entropy is a measurement of randomness in the image, with higher entropy values indicating complex or random texture.
Results
The comparison of various parameters of texture analysis between the lesion and normal group is presented in Table 1. The Mann–Whitney U-test was used here as the data did not follow normality. Higher mean FD, ASM, contrast and IDM were recorded in the lesion group than in the normal group. The differences in these parameters between the two groups were found to be statistically significant (p < 0.01). Higher mean entropy was recorded in the normal group than in the lesion group and the difference was significant (p = 0.002). However, there was no difference in the mean lacunarity between the lesion and normal groups (p = 0.521).
Table 1. Comparison of various parameters of texture analysis between the lesion and normal group.
| Parameter | Group | Mean | SD | Median | Minimum | Maximum | Za | p-value |
| FD | Lesion | 0.712 | 0.097 | 0.711 | 0.489 | 0.876 | −3.258 | 0.001b |
| Normal | 0.612 | 0.086 | 0.594 | 0.485 | 0.831 | |||
| ASM | Lesion | 0.0047 | 0.0035 | 0.0030 | 0.0010 | 0.0150 | −2.891 | 0.004b |
| Normal | 0.0024 | 0.0009 | 0.0020 | 0.0010 | 0.0050 | |||
| Contrast | Lesion | 1511.86 | 375.51 | 1467.22 | 728.25 | 2292.26 | −2.327 | 0.020b |
| Normal | 1187.14 | 427.04 | 1254.48 | 400.75 | 1900.73 | |||
| IDM | Lesion | 0.268 | 0.121 | 0.256 | 0.053 | 0.587 | −3.082 | 0.002b |
| Normal | 0.167 | 0.060 | 0.145 | 0.082 | 0.291 | |||
| Entropy | Lesion | 5.81 | 0.55 | 5.89 | 4.66 | 6.68 | −3.056 | 0.002b |
| Normal | 6.26 | 0.28 | 6.36 | 5.68 | 6.64 | |||
| Lacunarity | Lesion | 0.0193 | 0.0165 | 0.0117 | 0.0001 | 0.0590 | −0.641 | 0.521 |
| Normal | 0.0195 | 0.0225 | 0.0104 | 0.0001 | 0.0888 |
ASM, angular second moment; FD, fractional dimension; IDM, inverse difference; SD, standard deviation.
aZ score quantifies the distance that a given data point is from the mean of a data set.
bDenotes significant difference.
Table 2 presents the texture parameters of lesional ROIs assessed for every tumour grade. As the data pertaining to lesion group alone followed normality, the analysis of variance test was used. No significant difference was observed between the three grades of tumour for any of the parameters (p > 0.05).
Table 2. Comparison of various parameters of texture analysis between different tumour stages in the lesion group.
| Parameter | Tumour grade | Mean | SD | Median | Minimum | Maximum | F | p-value |
| ASM | I | 0.0054 | 0.0033 | 0.0050 | 0.0010 | 0.0110 | 0.996 | 0.389 |
| II | 0.0034 | 0.0018 | 0.0030 | 0.0020 | 0.0070 | |||
| III | 0.0058 | 0.0054 | 0.0030 | 0.0020 | 0.0150 | |||
| Contrast | I | 1359.05 | 302.35 | 1417.97 | 728.25 | 1716.30 | 1.290 | 0.299 |
| II | 1555.98 | 399.08 | 1645.32 | 954.27 | 1999.94 | |||
| III | 1685.78 | 421.84 | 1768.30 | 1181.51 | 2292.26 | |||
| IDM | I | 0.282 | 0.135 | 0.293 | 0.053 | 0.417 | 0.720 | 0.500 |
| II | 0.229 | 0.067 | 0.231 | 0.134 | 0.339 | |||
| III | 0.307 | 0.167 | 0.242 | 0.176 | 0.587 | |||
| Entropy | I | 5.66 | 0.61 | 5.64 | 4.90 | 6.68 | 1.071 | 0.363 |
| II | 6.03 | 0.36 | 6.06 | 5.40 | 6.41 | |||
| III | 5.70 | 0.69 | 5.89 | 4.66 | 6.33 | |||
| FD | I | 0.713 | 0.130 | 0.751 | 0.489 | 0.838 | 0.123 | 0.885 |
| II | 0.701 | 0.080 | 0.692 | 0.624 | 0.876 | |||
| III | 0.729 | 0.079 | 0.747 | 0.630 | 0.816 | |||
| Lacunarity | I | 0.023 | 0.016 | 0.019 | 0.004 | 0.053 | 0.822 | 0.455 |
| II | 0.013 | 0.010 | 0.011 | 0.002 | 0.029 | |||
| III | 0.023 | 0.025 | 0.012 | 0.000 | 0.059 |
ASM, angular second moment; F, ratio of mean sum of squares to error sum of squares; FD, fractional dimension; IDM, inverse difference; SD, standard deviation.
Discussion
Texture analysis is a mathematical technique for quantification of complex structures by studying the variation in the intensity of image element (pixel) values acquired under certain conditions. Numerous studies have employed diverse measures of texture analysis to characterize lesions in the various regions of the body and to distinguish them from the normal tissues.3-9 Investigators commonly use the measurement of FD and have consistently found higher values in the region of malignancy. Similar results were obtained in our study with pathological tissues have a higher FD when compared with the normal tissues, implying a greater complexity and surface roughness of the tumours. Also, the second order statistical texture features extracted with the GLCM, namely contrast, was found to be effective for pattern recognition between the normal and the pathological regions. The parameter presented higher mean values for the lesion ROI when compared with the normal ROI, indicating irregularity in the tumour region. The presumed reason for such heterogeneity could be that in the tumours, owing to chaotic angiogenesis, the general shape of the blood vessels is altered and deformed, becoming very rough and resulting in increased texture characteristics. Thus, when a contrast agent is injected, there is enhanced intensity of blood vessels in the images and corresponding texture values.
Although the parameters ASM, IDM and entropy are useful in texture characterization, the results are controversial; ideally, lower values of ASM and IDM and higher values of entropy represent randomness of the structure. However, in the study, the mean values of these parameters behaved unexpectedly, thereby representing less randomness of the tumour area. This discrepancy could be owing to either the sensitivity of the parameters to minor variations in the noise and intensity of acquired images when compared with the other parameters used in the study or the inherent limitations of the software used. Furthermore, the slice thickness of the acquired CT images may have also influenced the values. Nevertheless, the authors believe that the results are debatable and require further confirmation.
As the treatment of oral cancers is largely based on assessment with scalpel biopsy, we attempted to compare the texture characteristics on the CT images with the histopathological tumour grading. This comparison revealed that none of the parameters of texture analysis could effectively discriminate between the grades of the tumour. It is well known that the histopathological analysis is critically dependant on the biopsy taken from the most representative area of the lesion, which in turn relies greatly on the skill and experience of the surgeon. Furthermore, as pointed out by Pentenero et al,17 histopathological features can differ in different parts of the lesion, and biopsy samples may not always represent the most aggressive area of the whole lesion. Besides, the histopathological grading by the pathologists is highly subjective with intra- and interobserver variability and has low reproducibility.18,19 In the present study, the inconsistent correlation of texture analysis and tumour grading could be influenced by the possibility that the incisional biopsy did not actually represent the most aggressive area of the tumour. Thus, it would have been most ideal to compare the texture characteristics with a better and more definitive marker of aggression such as fludeoxyglucose PET or molecular markers of cancers in the biopsied tissues. However, limited availability and high cost hindered their use in this study. These issues should to be adequately addressed in future studies by correlating texture characteristics on CT images with completely excised tumour mass and analysed with better predictors of tumour aggression than conventional histopathology of incisional biopsy.
This study is a stepping stone in texture analysis of CT images of oral cancers. Future research in the use of this novel technique for cancers of buccal mucosa and other sites of the oral cavity may provide an understanding of the practicality of its use in treatment planning of oral cancers. Also, confirmation of these results in a larger sample size can make available cut-off values which can differentiate between the lesion and normal areas.
In conclusion, FD and GLCM texture analysis methods are useful in the characterization of buccal mucosa cancers, but their role in the discrimination of tumour grades requires further exploration. Because this technique is non-invasive and cost-effective it may prove as a promising adjuvant tool in oral cancer workup, particularly in resource-limited developing countries. Nonetheless, use of this technique is still at infancy and future studies are essential to regard or disregard its use in the diagnosis and treatment planning of oral malignancies.
Acknowledgments
This research was awarded first prize in the IADMFR Maxillofacial Imaging Research Award competition in 2011 at the 18th International Congress of Dentomaxillofacial Radiology, Hiroshima, Japan. The authors wish to acknowledge the staff and postgraduates of the Department of Radiotherapy, Victoria Hospital, Bangalore, India, Dr Kallur KG, Department of Nuclear Medicine, and Dr Shilpa, Department of Pathology, Bangalore Institute of Oncology, India, for their immense support during the study.
References
- 1.Steward BW, Kleihues P. World Cancer Report. Lyon, France: WHO International Agency for Research on Cancer, 2003 [Google Scholar]
- 2.World Health Organization Health Situation in the South-East Asia Region 1998-2000. New Delhi, India: WHO Regional Office for South-East Asia, 2002 [Google Scholar]
- 3.Rao DN, Shroff PD, Chattopadhyay G, Dinshaw KA. Survival analysis of 5595 head and neck cancers—results of conventional treatment in a high-risk population. Br J Cancer 1998;77:1514–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir AH, Hanmandlu M, Tandon SN. Texture analysis of CT images. IEEE Eng Med Biol Mag 1995;14:781–786 [Google Scholar]
- 5.Mihara N, Kuriyama K, Kido S, Kuroda C, Johkoh T, Naito H, et al. The usefulness of fractal geometry for the diagnosis of small peripheral lung tumors. [In Japanese.] Nihon Igaku Hoshasen Gakkai Zasshi 1998;58:148–151 [PubMed] [Google Scholar]
- 6.Penn AI, Bolinger L, Schnall MD, Loew MH. Discrimination of MR images of breast masses with fractal-interpolation function models. Acad Radiol 1999;6:156–163 [DOI] [PubMed] [Google Scholar]
- 7.Lee WL, Chen YC, Hsieh KS. Ultrasonic liver tissues classification by fractal feature vector based on M-band wavelet transform. IEEE Trans Med Imaging 2003;22:382–392 [DOI] [PubMed] [Google Scholar]
- 8.Kido S, Kuriyama K, Higashiyama M, Kasugai T, Kuroda C. Fractal analysis of internal and peripheral textures of small peripheral bronchogenic carcinomas in thin-section computed tomography: comparison of bronchioloalveolar cell carcinomas with nonbronchioloalveolar cell carcinomas. J Comput Assist Tomogr 2003;27:56–61 [DOI] [PubMed] [Google Scholar]
- 9.Petkovska I, Shah SK, McNitt-Gray MF, Goldin JG, Brown MS, Kim HJ, et al. Pulmonary nodule characterization: a comparison of conventional with quantitative and visual semiquantitative analyses using contrast enhancement maps. Eur J Radiol 2006;59:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Kadi OS, Watson D. Texture analysis of aggressive and nonaggressive lung tumor CE CT images. IEEE Trans Biomed Eng 2008;55:1822–1830 [DOI] [PubMed] [Google Scholar]
- 11.Yoshiura K, Miwa K, Yuasa K, Tokumori K, Kanda S, Higuchi Y, et al. Ultrasonographic texture characterization of salivary and neck masses using two-dimensional gray-scale clustering. Dentomaxillofac Radiol 1997;26:332–336 [DOI] [PubMed] [Google Scholar]
- 12.Yonetsu K, Ohki M, Kumazawa S, Eida S, Sumi M, Nakamura T. Parotid tumors: differentiation of benign and malignant tumors with quantitative sonographic analyses. Ultrasound Med Biol 2004;30:567–574 [DOI] [PubMed] [Google Scholar]
- 13.Chikui T, Tokumori K, Yoshiura K, Oobu K, Nakamura S, Nakamura K. Sonographic texture characterization of salivary gland tumors by fractal analyses. Ultrasound Med Biol 2005;31:1297–1304 [DOI] [PubMed] [Google Scholar]
- 14.Sarkar N, Chaudhuri BB. An efficient differential box-counting approach to compute fractal dimension of image. IEEE Trans Syst Man Cyber n 1994;24:115–120 [Google Scholar]
- 15.Filho MB, Sobreira F. Accuracy of lacunarity algorithms in texture classification of high spatial resolution images from urban areas. Int Arch Photogramm Remote Sensing Spatial Inform Sci 2008 . XXXVII Part B3b:417–422 [Google Scholar]
- 16.Haralick RM, Shanmugan K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern 1973;3:610–621 [Google Scholar]
- 17.Pentenero M, Carrozzo M, Pagano M, Galliano D, Broccoletti R, Scully C, et al. Oral mucosal dysplastic lesions and early squamous cell carcinomas: underdiagnosis from incisional biopsy. Oral Dis 2003;9:68–72 [DOI] [PubMed] [Google Scholar]
- 18.Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol 1985;14:698–708 [DOI] [PubMed] [Google Scholar]
- 19.Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:188–191 [DOI] [PubMed] [Google Scholar]