Abstract
Objectives
Mouth breathing causes many serious problems in the paediatric population. It has been maintained that enlarged adenoids are principally responsible for mouth breathing. This study was designed to evaluate whether other mechanical obstacles might predispose the child to mouth breathing.
Methods
67 children with ages ranging from 10 to 15 years were studied and grouped into mouth-breathers and nose-breathers. The children first underwent axial CT scans of the brain for which they were originally referred. In addition, they were subjected to a limited coronal CT examination of the paranasal sinuses. Congenital anatomical variations as well as inflammatory changes were assessed.
Results
87% of mouth-breathing children had hypertrophied adenoids, 77% had maxillary sinusitis, 74% had pneumatized middle concha, 55% had a deviated nasal septum, 55% had hypertrophied inferior conchae, 45% had ethmoidal sinusitis and 23% showed frontal sinusitis. Such changes were significantly less prevalent in nose-breathers. 12.9% of mouth-breathing children did not have adenoids. Of these children, only 3.3% had one or more congenital or inflammatory change whereas the other 9.6% showed a completely normal CT scan signifying the incidence of habitual non-obstructive mouth breathing.
Conclusions
It is clear that adenoids have a dominant role in causing mouth breathing. Yet, we recommend that paediatricians should assess other mechanical obstacles if mouth breathing was not corrected after adenoidectomy. Further research should be performed to test the validity of correction of such factors in improving the quality of life of mouth-breathing children.
Keywords: spiral computed tomography, mouth breathing, congenital anomalies, sinusitis, adenoids
Introduction
Nasal breathing is the primary mode of air intake for humans, and it is essential for a supply of properly cleansed, moistened and warmed air for the lungs. If the primary airway is blocked, mouth breathing becomes obligatory to get air into the lungs.1 Chronic mouth breathing in children leads to pathological adaptations in the postural and morphological characteristics of the stomato-gnathic system.2 Such unfavourable developmental changes predispose the child to many problems, including obstructive sleep apnoea, which is now a growing public concern.3, 4
It has been maintained that enlarged adenoids are principally responsible for obstructive mouth breathing.5, 6 Yet, some children continue to breathe through their mouth even after surgical removal of their adenoids.7 This could be due to habitual mouth breathing and laziness in using the nose despite having an adequate nasal airway, as previously described by Fairchild.8 Alternatively, mechanical obstacles other than adenoids might be present, preventing the child from nasal breathing.
Mechanical obstacles that may contribute to the blockage of the nasal passage and cause the child to shift to mouth breathing include anatomical variations that occur in the nasal cavity and the paranasal region during development, such as Haller cells, agger nasi cells, paradoxical middle turbinate and concha bullosa; as well as inflammatory sinonasal changes, such as recurrent chronic sinusitis and hypertrophy of the inferior nasal concha9, 10. A limited coronal CT scan of the sinuses permits observation of the anatomical disorders11 as well as inflammatory sinonasal disease.12, 13 Spiral CT is a fast scanning method, so it is best suited to examine children who cannot stay motionless for a long time. It also decreases motion artefacts.14
Some studies investigating whether the anatomical variations of the lateral wall of the nose predispose children to chronic sinusitis have been reported.9–11 They mostly found little contribution of the anatomical variations to sinusitis. To the authors' knowledge, the association between the anatomical variations of the nose and mouth breathing have not been previously explored. The ability to recognize the relationship between these variables and mouth breathing in children will help in identifying the nasopharyngeal obstruction that leads to mouth breathing and correcting it.
The aim of this study was to analyse by CT the anatomical and inflammatory changes associated with mouth breathing in 10- to 15-year-old children, with the ultimate goal of treating or removing the abnormalities predisposing the child to mouth breathing, thus giving the child the extensive benefits of early correction of mouth breathing. An additional aim was to determine the relative incidence of obstructive mouth breathing compared with habitual mouth breathing.
Materials and methods
Selection of patients
A prospective study of children undergoing brain CT for indications other than sinusitis was performed. Limited coronal CT examination of the paranasal sinuses was additionally carried out for these children. The study was approved by Ain-Shams ethics committee. The parents were fully informed of the nature of the study and signed a written consent form. Baseline information that included the patient's name, date of birth and detailed medical and dental history, was recorded for each child.
The study population consisted of 67 children (35 girls and 32 boys) aged 10 to 15 years who were referred to El-Abd Scan Center for CT of the brain between April 2006 and March 2008. Patients were excluded if the child had had recent surgery, previous sinus surgery, craniofacial anomalies, facial trauma, nasogastric tubes or facial neoplasms.
Children with a history of systemic disorder, syndromes, acute otitis or tonsillitis, nasal stenosis, choanal atresia, immunodeficiency or cystic fibrosis or those who were taking regular medications were excluded from the study. Children who gave mixed signs and symptoms of their predominant mode of breathing, as well as patients who showed poor co-operation and those whose parents did not approve of the extra CT examination of the paranasal sinuses, were disqualified from entering this study.
Assessment of mode of breathing
History-taking and clinical examination were performed for the children who met the inclusion criteria.
Patient history
The parents of the children answered a questionnaire regarding the child's behaviour while awake (oral respiration, nasal obstruction, oral malodour or hyponasal speech) and during sleep (snoring, frank apnoeas, restless sleep or hypersalivation). Most of the questions were yes or no items. The questionnaire was conducted in a uniform manner by the same examiner to ensure consistency and quality of findings.
Clinical examination
All the patients were then clinically examined by the same examiner. The following clinical tests were performed to aid in the diagnosis of the mode of breathing. The tests were conducted with the patient in a reclined position.
(1) Data on lip posture were obtained by viewing the patient's profile. Presence or absence of lip separation was evaluated while the child was distracted. According to the position of their lips at rest, children were divided into two groups: those who had competent lip closure and those with lip separation.
(2) The degree of maxillary incisor coverage by the upper lip at rest was classified as total coverage, partial coverage or maxillary labial gingiva exposed. Photographs were taken to aid in evaluation.
(3) Nose breathing capability of the child was tested by gently closing the lips together with light pressure of thumb and middle finger for 2–5 min. The child was not informed of the purpose of that act. If the patient stopped breathing, became cyanotic or made attempts to open his or her mouth to breathe, this indicated mouth breathing and the testing was discontinued.
According to the data acquired from the patient's history and clinical examination, children were divided into two groups:
(1) Group A (mouth-breathers): children who demonstrated predominant mouth breathing, diagnosed during clinical examination and confirmed by the parents as being the predominant mode of breathing.
(2) Group B (nose-breathers): paediatric control group who demonstrated predominant nose breathing, diagnosed during clinical examination and supported by the parents as being the predominant mode of breathing.
CT examination
Axial CT scan of the brain
Patient registration information was entered via the diagnostic main console of a spiral CT scanner (Toshiba X/Vision, Toshiba Medical System, Tustin, CA). The children first completed helical axial CT scans of the brain for which they were originally referred. The patients were supine on the CT couch. The scan parameters were 120 kV, 300 mAs, 2 s. The sections were processed to obtain both soft-tissue and bone window images.
Limited coronal CT scan of the paranasal sinuses
The children were then submitted to a limited coronal CT scan of the paranasal sinuses. They were asked to lie prone with their neck extended on the CT couch. Lateral scout views (scanned projection radiographs used for localization) were acquired. Lines perpendicular to the hard palate were defined on the scout view, starting from the posterior margin of the sphenoid sinus to the anterior margin of the frontal sinus.
Helical scanning was carried out to obtain 4 mm slice thickness with 3 mm bed increments. This reduced exposure protocol provided five or six coronal slices. The gantry angulation varied depending on cranial hyperextension. The exposure parameters were 120 kV and 200 mAs, and the scanning time was 1.5 s at a 512×512 matrix. Bone window images were obtained and zoomed to fill the screen.
Assessment of CT scans
The CT scans of children were analysed by an experienced radiologist blinded to the clinical data. Both the dedicated coronal slices and the earlier acquired brain axial cuts were evaluated for anatomical variations and inflammatory changes. CT abnormalities were judged as present or absent, regardless of their unilateral or bilateral location.
Anatomical variations
The following congenital anatomical variations were assessed: pneumatized middle concha, deviated nasal septum, Haller cells, agger nasi cells, Onodi cells, pneumatized superior concha, paradoxical middle concha, large ethmoidal bulla pneumatized nasal septum, and pneumatized uncinate process.
Inflammatory changes
The presence of sinusitis (>2 mm mucous membrane thickening on any sinus wall, opacification, air–fluid levels) was assessed on the maxillary, ethmoidal, frontal and sphenoidal sinuses. The ethmoidal air cells were assessed as a whole without dividing them into anterior, middle and posterior ethmoidal sinuses. Thickened inferior nasal turbinates were also observed. Moreover, hypertrophied adenoids were assessed from the scanogram.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 for windows (SPSS Inc., Chicago, IL). Qualitative data were presented as frequencies, and percentages and the χ2 test was used to compare the groups. Quantitative data are presented as mean and standard deviation (SD) values, and Student's t-test was used to compare the means of the two groups. The significance level was set at P ≤ 0.05.
Results
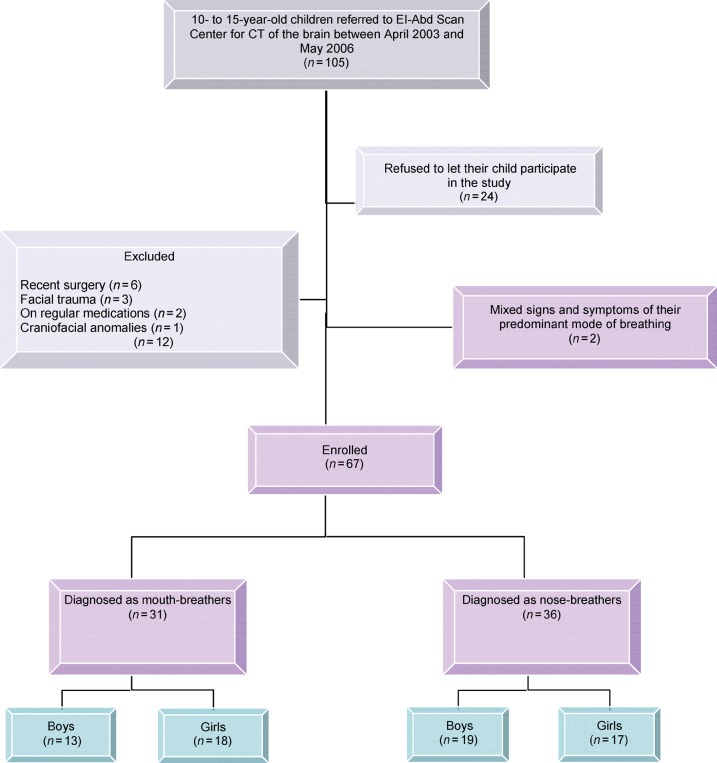
The demographic data of the 105 children screened for this study are shown in Figure 1. Regarding the age of the two groups, the mean age of Group A was 12.62 ± 2.96 years, compared with 13.15 ± 2.43 years for Group B. No significant difference was found between the two groups regarding the age of children (P > 0.05).
Figure 1.
Study profile. Out of the 67 children who participated in this study, 31 were diagnosed as mouth-breathers and 36 as nose-breathers
The prevalence of lip seal in the two groups was assessed. None of the children in Group A (mouth-breathers) showed competent lip seal, whereas 86.1% of the children in Group B had competent lip seal (Figure 2). The difference was highly significant (P ≤ 0.01).
Figure 2.
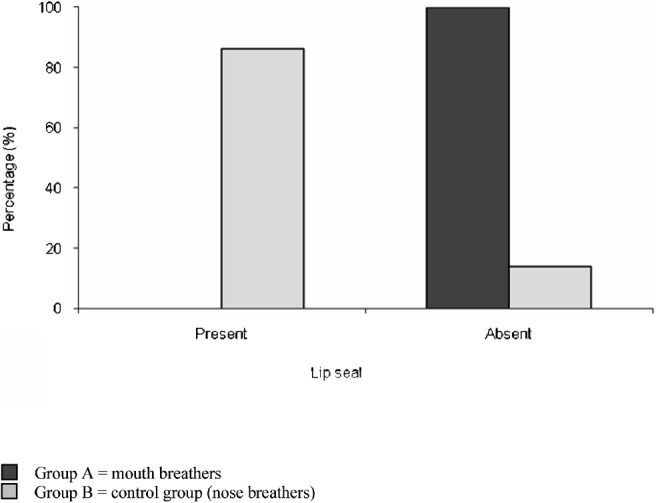
Comparison of both groups as regards lip seal. Incompetent lips were present in 100% of the children in Group A compared with 13.9% of children in Group B. The difference was highly significant (P ≤ 0.01)
Concerning maxillary incisor coverage, Group A showed a higher percentage of cases with partial coverage and maxillary labial gingiva exposure than Group B. In contrast, Group B showed a higher percentage of cases with total coverage. The differences were highly significant (P ≤ 0.01) (Figure 3).
Figure 3.
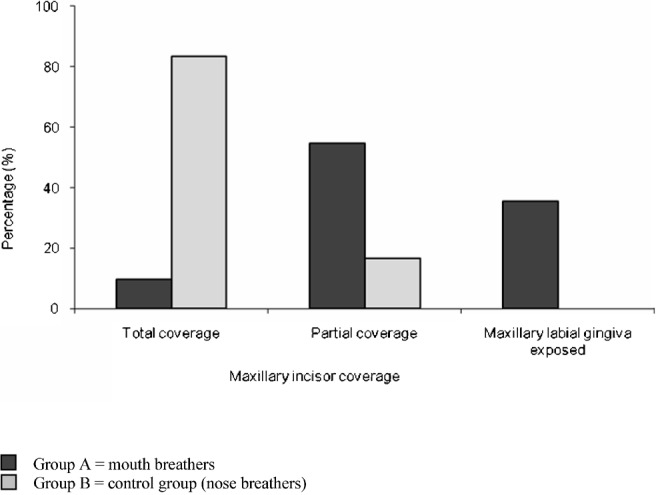
Comparison of both groups as regards maxillary incisor coverage. A higher percentage of cases in Group B showed total coverage of maxillary incisors. The differences were highly significant (P ≤ 0.01)
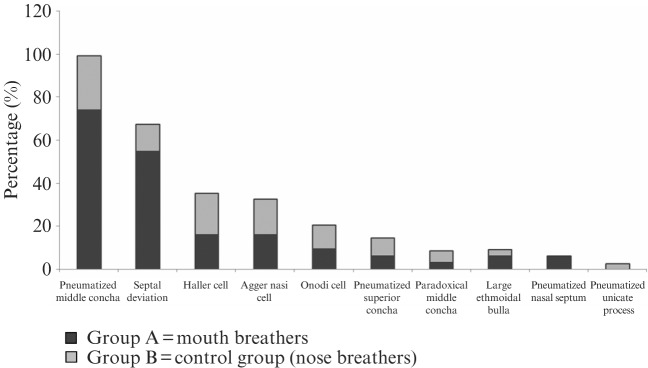
The incidence of anatomical variations in the two groups is presented in Table 1 and Figure 4. A pneumatized middle concha was the most common anatomical variation, followed by septal deviation, Haller cells, agger nasi cells, Onodi cells, pneumatized superior concha, paradoxical middle concha, large ethmoidal bulla, pneumatized nasal septum and, finally, pneumatized uncinate process.
Table 1. The distribution of the anatomical variations and the inflammatory changes among the two groups.
| Group A (n _ 31) |
Group B (n _ 36) |
P-value | ||||
| n | % | n | % | |||
| Anatomical variations | Pneumatized middle concha (concha bullosa) | 23 | 74.2 | 9 | 25.0 | ≤0.05∗ |
| Septal deviation | 17 | 54.8 | 4 | 12.6 | ≤0.05∗ | |
| Haller cell | 5 | 16.1 | 7 | 19.4 | >0.05 | |
| Agger nasi cell | 5 | 16.1 | 6 | 16.6 | >0.05 | |
| Onodi cell | 3 | 9.6 | 4 | 11.2 | >0.05 | |
| Pneumatized superior concha (concha bullosa) | 2 | 6.4 | 3 | 8.4 | >0.05 | |
| Paradoxical middle concha | 1 | 3.2 | 2 | 5.6 | >0.05 | |
| Large ethmoidal bulla | 2 | 6.4 | 1 | 2.8 | >0.05 | |
| Pneumatized nasal septum | 2 | 6.4 | 0 | 0.0 | >0.05 | |
| Pneumatized uncinate process | 0 | 0.0 | 1 | 2.8 | >0.05 | |
| Inflammatory changes | Maxillary sinusitis | 24 | 77.4 | 12 | 33.3 | ≤0.01∗∗ |
| Ethmoidal sinusitis | 14 | 45.1 | 6 | 16.7 | ≤0.05∗ | |
| Sphenoidal sinusitis | 7 | 22.6 | 5 | 13.9 | >0.05 | |
| Frontal sinusitis | 7 | 22.6 | 1 | 2.8 | ≤0.05∗ | |
| Hypertrophied inferior conchae | 17 | 54.8 | 6 | 16.7 | ≤0.01∗∗ | |
| Hypertrophied adenoids | 27 | 87.1 | 11 | 30.5 | ≤0.01∗∗ | |
Group A, mouth-breathers; Group B, control group (nose-breathers)
P > 0.05, not significant; ∗P ≤ 0.05, significant; ∗∗P ≤ 0.01, highly significant
Figure 4.
The distribution of the anatomical abnormalities among the two groups. Group A showed a significantly higher percentage of cases with pneumatized middle concha and septal deviation than Group B. There was no significant difference between the two groups regarding the other anatomical variations
There was no significant difference between mouth-breathers and nose-breathers regarding the anatomical variations except for pneumatized middle concha and septal deviation, the incidences of which were significantly higher in Group A than in Group B (P ≤ 0.05).
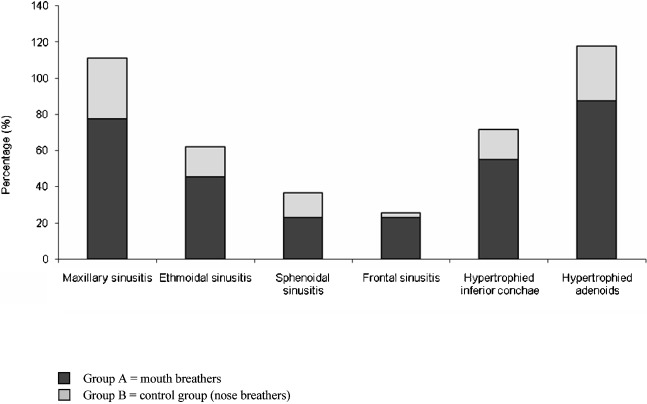
The distribution of the inflammatory changes in the two groups is listed in Table 1 and is presented graphically in Figure 5. Maxillary sinusitis was the most common sinus infection, followed by, in order of decreasing incidence, ethmoidal, sphenoidal and frontal sinusitis.
Figure 5.
The distribution of the inflammatory changes among the two groups. There was a highly significant difference between the two groups regarding maxillary sinusitis, hypertrophied inferior conchae and hypertrophied adenoids as well as a significant difference regarding ethmoid and frontal sinusitis
Mouth-breathers exhibited significantly higher prevelence of inflammatory changes than nose-breathers (P ≤ 0.01 for maxillary sinusitis, hypertrophied inferior concha and hypertrophied adenoids; P ≤ 0.05 for ethmoidal and frontal sinusitis).
Discussion
Mouth breathing involves the intake of air via the oral cavity. In children, mouth breathing was found to be the second most common oral habit after tongue thrust.15 When breathing through the mouth the benefits of nasal breathing are bypassed. Thus, using mouth breathing on a regular basis can cause many serious problems, mainly inadequate facial growth and bone development. Intraorally, mouth breathing affects occlusion and alignment of teeth. It also causes dryness of the oral tissues, compromising gingival health and leading to incisor caries and oral malodour.16 In addition, mouth breathing causes pathological changes in the nasopharyngeal and other respiratory tissues as well as muscle alterations, which influence deglutition, digestion and phonation.17
Mouth breathing is often associated with obstruction or congestion of the upper respiratory tract.6 Therefore, it was thought to be worthwhile to study the CT findings in mouth-breathing children. A prospective study was performed to test the role of the congenital sinonasal anomalies, inflammatory sinus disease, swollen nasal turbinates and enlarged adenoids in inducing nasal obstruction leading to mouth breathing.
31 mouth-breathing and 36 nose-breathing children with ages ranging from 10- to 15-years were included in this study. It would have been useful to study mouth breathing in a younger age group because the rates of the anatomic variations can change from birth to adolescence;18 however, younger children could not be expected to complete both CT scans without sedation. Cotter et al13 reported that the incidence of anatomical variations is higher in the first few years of life than in late childhood, but the difference between the age groups was not significant. Therefore, it is unlikely that the inclusion of younger children would affect the results.
Mouth breathing was primarily diagnosed if the child attempted to open his or her mouth to breathe while the investigator gently closed his or her lips together. Alternatively, Menezes et al19 established the breathing pattern using steam and water time in the mouth tests. The patient's history was taken from the parents using a questionnaire to aid the diagnosis. Children who gave mixed signs and symptoms were excluded from the study so that the CT findings could be directly attributed to the mode of breathing.
CT was used in this study because many authors have already found that CT accurately evaluates the soft tissues and subtle changes in bones and air-filled spaces.14 Conventional radiographs were not used because of the superimposition of the craniofacial skeleton on the area of interest and their limited resolution, which prevents the visualization of delicate structures such as the congenital anomalies found on the lateral wall of the nose, as well as their unacceptable rates of false-positive and false-negative results in diagnosing childhood sinusitis.20
In addition to the brain CT (axial cuts) scan for which the children were originally referred, limited coronal CT scans of the paranasal sinuses that provided five or six coronal slices were used, as performed by McAlister et al.21 The reduced CT protocol allowed adequate visualization of the detailed structures of the nose, sinuses and pharynx while decreasing the exposure dose to the child.
The axial brain CT data were not used to obtain reconstructed coronal slices because routine brain CT starts from the level of the infraorbitomeatal line, which does not cover the maxillary sinus. In addition, for the reconstructed images to be of high quality, a very low pitch should be used, which meant extra radiation dose to the child.
In this study, right and left anatomical and inflammatory changes were not evaluated separately. Conversely, McAlister et al21 evaluated the CT changes as right, left or bilateral. The division of the factors evaluated into right and left would not be beneficial in this study because mouth breathing is not two sided whereas in McAlister et al's study, they were mainly correlating the anatomical variations with sinusitis.
None of the mouth-breathing children showed a competent lip seal, and only 9.7% of them exhibited total lip coverage of the maxillary incisors. The decreased incidence of competent lips in mouth breathers is attributed to the high nasal resistance; hence, the open mouth posture allows for adequate air intake through the mouth.
Lip-apart posture was not absent in nose-breathers. 13.9% of nose-breathers showed incompetent lips and 16.7% showed partial maxillary incisor coverage. Open mouth posture in nose-breathers is probably due to a decreased upper or lower facial height.22 Nose-breathers who maintain a lip-apart posture usually obtain an adaptive posterior oral seal with the tongue against the soft palate.
Analysis of the overall incidence of the anatomical variations in all the children studied, found that pneumatized middle concha was the most commonly found variation (47.8%), followed by septal deviation (31.3%), Haller cells (17.9%), agger nasi cells (16.4%), Onodi cells (10.4%), pneumatized superior concha (7.5%), paradoxical middle concha (4.5%), large ethmoidal bulla (4.5%), pneumatized nasal septum (3%) and pneumatized uncinate process (1.5%).
Similarly, Sivasli et al23 evaluated 47 children with coronal and axial CT and found that a pneumatized middle concha was the most common anatomical variation, followed by pneumatization of the superior concha, Haller cells and agger nasi cells. Conversely, Jun Kim et al24 found that agger nasi cells were the most common variation. In addition, Eryilmaz et al25 found the most common anatomical variation in 44 children to be septal deviation, followed by concha bullosa and agger nasi cells. The causes of these differences are attributed to race, age and regional differences.
A pneumatized middle concha (concha bullosa) can be described as an air-filled swelling of the middle turbinate (Figure 6). It is caused by pneumatization of the middle turbinate by ethmoidal air cells. It was the most commonly found anatomical variation and was present in 47.8% of all the children in the study. The results concerning concha bullosa in children are quite diverse and range from 8% to 58%.25 The incidence of pneumatized middle concha was significantly higher in mouth-breathers (74.2%) than in nose-breathers (25%). The possible explanation is that pneumatized middle concha increases nasal resistance, causing the child to change to mouth breathing.
Figure 6.
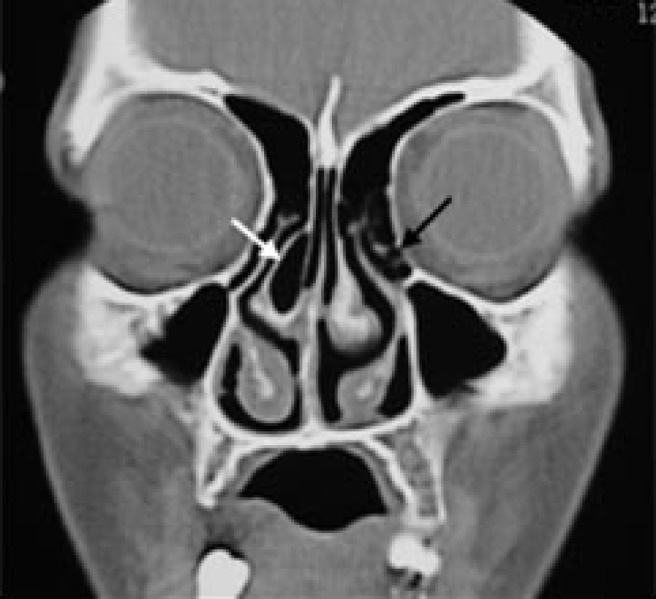
Coronal CT of a mouth-breathing child showing a pneumatized right middle concha (white arrow) as well as left Haller cells (black arrow) and bilateral hypertrophied inferior turbinates more evident on the right side. The maxillary and frontal sinuses are clear
Septal deviation was the second most common anatomical variation. It was found in 31.34% of the children (Figure 7). The frequency of septal deviation in children has been variably reported to be 13%9 and 46%.26 Jun Kim et al24 found the incidence of septal deviation in children with persistent symptoms of sinusitis to be 44.3%. The prevalence of septal deviation has been reported to increase with age due to secondary growth and/or external trauma.24 Mouth-breathing children showed a significantly higher incidence of septal deviations than nose-breathers. This could be because a deviated septum has a larger surface area than a straight septum and thus may increase nasal resistance.
Figure 7.
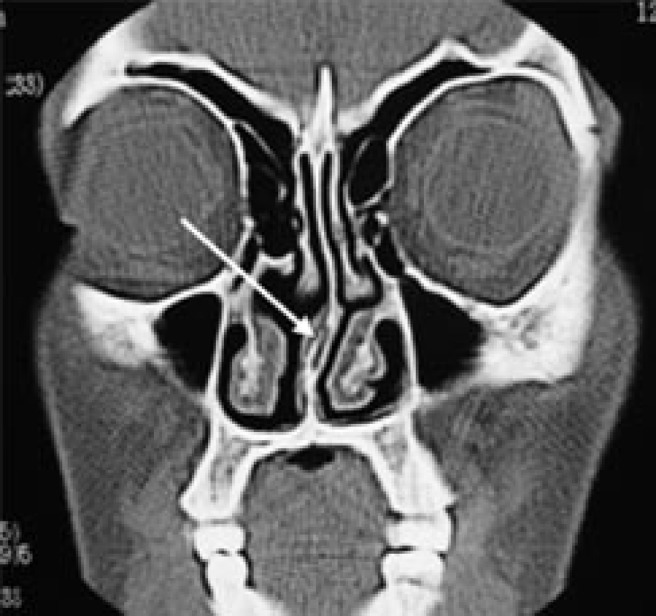
Coronal CT of a mouth-breathing child showing a deviated and pneumatized nasal septum (arrow) in addition to thickened inferior turbinates
Haller cells (infraorbital ethmoid) are ethmoidal cells with an infraorbital extension (Figure 6). In the present study, Haller cells were found in 17.9% of the children. The frequency in children has been reported to be between 5.3% and 18%.23 However, in Jun Kim et al's study24 their incidence rate was 34.5%. There was no significant difference between mouth-breathers and nose-breathers regarding the prevalence of Haller cells.
Agger nasi cells (supraorbital ethmoid) (Figure 8) are far anterior ethmoidal air cells.27 They expand sufficiently to encroach upon the medial aspect of the frontal sinus floor, narrowing or obstructing the nasofrontal duct; thus, they may cause frontal sinusitis. 16.4% of the children in the study had agger nasi cells. The frequency has been reported to be as high as 98.5% in adults,28 but only 15% in children.23 No significant difference was found between mouth-breathers and nose-breathers regarding the incidence of agger nasi cells.
Figure 8.
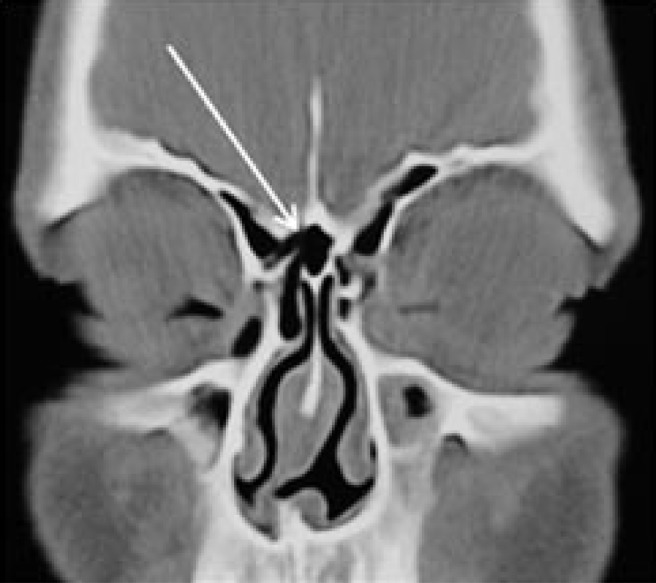
Coronal CT of a mouth-breathing child showing an agger nasi cell (beneath the arrow) with absence of frontal sinusitis. The left maxillary sinusitis is noted
Onodi cells or sphenoethmoid cells are the extensive lateral pneumatization of the posterior ethmoidal air cells beyond the sphenoid sinus.23 They are located posterior to all the posterior ethmoid cells crossing the anterosuperior portion of the sphenoid sinus. It has been reported that CT scans have limitations in demonstrating Onodi cells because on CT they are frequently misconceived as sphenoid sinuses,24 so the actual frequency of Onodi cells can be ascertained only through surgical dissection.29 Although the exact prevalence of Onodi cells is unclear, they were discovered in 6% of children by Sivasli et al23 and in 9.8% of children by Jun Kim et al.24 Onodi cells were found in 10.4% of children in this study. There was no statistically significant difference between mouth-breathers and nose-breathers regarding Onodi cells.
Pneumatization of the superior concha was found in 7.5% of the children in this study. However, Sivasli et al23 encountered this anatomical variation in almost one-third of their paediatric patients. The superior turbinate has been known to involute with age,30 so the increased incidence of pneumatized superior concha in Sivasli et al's study might be attributed to the younger age of the children scanned. Pneumatized superior concha is unlikely to cause mouth breathing. This is in agreement with the findings of this study. No significant difference was found between the two groups regarding the rate of pneumatized superior concha.
Paradoxical middle turbinate is a middle concha that turns towards the lateral nasal wall, and its curved portion is positioned adjacent to the nasal septum. As a result, buckling of the mucus may occur, and paranasal sinusitis can develop. Paradoxical middle concha was encountered in 4.48% of the children. The previously reported incidences of paradoxical middle concha range from 4.4% to 10%.23,31 Paradoxical middle turbinate might compress the middle nasal meatus, blocking the ethmoid infundibulum and causing mucosal hyperaemia and inflammation. Yet, there was no significant difference in this study between mouth-breathers and nose-breathers regarding the incidence of paradoxical middle concha.
A large ethmoidal bulla (bullosa ethmoidalis) is an inferiorly placed ethmoidal air cell (Figure 9). Large ethmoidal bulla is a rare variation, and was found in 4% of the cases. There was no significant difference between mouth-breathers and nose-breathers regarding bullosa ethmoidalis.
Figure 9.
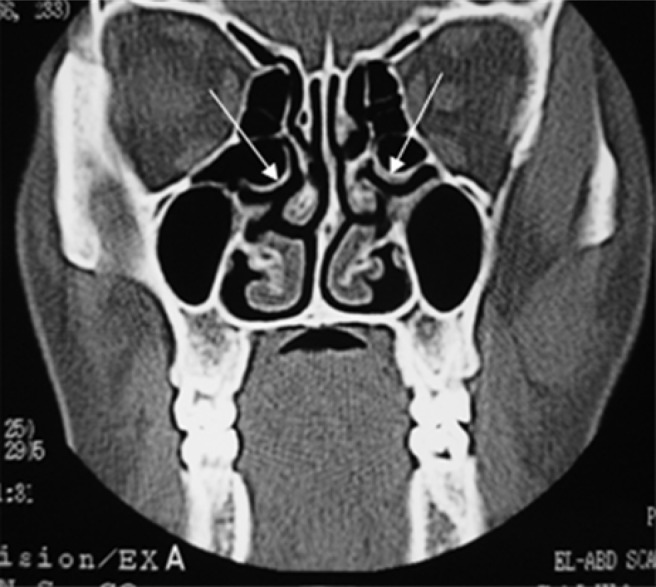
Coronal CT of a nose-breather showing bilateral bulla ethmoidalis (arrows) with no evidence of maxillary or ethmoid sinusitis
Pneumatized nasal septum (Figure 7) was found in 3% of the children in this study. Midilli et al11 reported its incidence to be 6%. There was no significant difference between mouth-breathers and nose-breathers regarding the relative incidence of pneumatized nasal septum.
The uncinate process is regarded as the descending portion of the first ethmoturbinal ridge.11 Uncinate process pneumatization was the least frequent anatomical variation (1.49%). The previously reported incidences of this variation in the paediatric population ranged between 0% and 2.5%.10,28 There was no significant difference between the two groups regarding uncinate process pneumatization.
Maxillary sinusitis was the most common sinus infection in the children (53.7%), followed by ethmoidal (29.9%), sphenoidal (17.9%) and frontal sinusitis (11.94%). This is in accordance with Sivasli et al23 and Jun Kim et al.24 However, Eryilmaz et al25 found that the most commonly involved sinuses were anterior ethmoid and maxillary sinuses followed by posterior ethmoid, sphenoid and frontal sinuses.
Tantimongkolsuk et al32 found that, in 100 paediatric patients clinically diagnosed with sinusitis, all paranasal sinus radiographs were abnormal, with the maxillary sinus being the most commonly involved sinus (99%) followed by the ethmoid sinus (91%). The majority of patients had involvement of more than one sinus.
The maxillary sinus is a pneumatic cavity which drains into the nasal cavity by a narrow osteomeatal unit. For the maxillary sinus, the mucociliary activity must drain against gravity. The obstruction of the outflow causes sinusitis.14 The overall incidence of maxillary sinusitis in this study was 53.7% (Figure 10). In children, the incidence of maxillary sinusitis has been reported to range between 51% and 89%.24 The incidence of maxillary sinusitis was significantly higher in mouth-breathers (64.5%) than in nose-breathers (28%).
Figure 10.
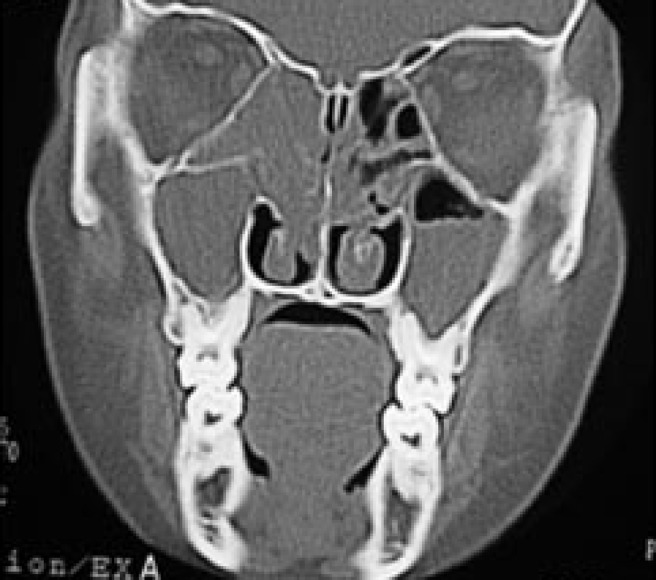
Coronal CT of a mouth-breathing child showing bilateral maxillary and ethmoidal sinusitis more pronounced on the right side.
The overall incidence of ethmoid sinusitis in this study was 29.9% (Figure 10). Jun Kim et al24 reported an incidence of 91.1% for anterior ethmoid sinusitis and 68.1% for posterior ethmoid sinusitis. The excessively high incidence rate in that study was because their sample was 113 children with persistent sinusitis resistant to medical therapy. In the present study, mouth-breathers showed a significantly higher incidence of ethmoid sinusitis (45.1%) than nose-breathers (16.7%).
Of the 67 patients examined, 12 sphenoid sinuses were associated with soft tissues in the sinus. These soft tissues may be extensions of infection from the ethmoid air cells or solitary inflammatory changes. Associated or isolated diseases of the sphenoid sinus can cause severe complications such as optic nerve involvement.33 Sphenoid sinusitis in children is reported to range between 13% and 37%.24 The overall incidence of sphenoid sinusitis in this study was 17.9%. There was no significant difference between the two study groups regarding the incidence of sphenoid sinusitis.
The reported incidence of frontal sinusitis fluctuates between 2% and 63%.24 Frontal sinusitis occurred in 11.9% of the children in this study. Frontal sinusitis was significantly more widespread in mouth-breathers (22.6%) than in nose-breathers (2.8%). This might be because of the rarity of frontal sinusitis in nose-breathers. Only one child in Group B had frontal sinusitis.
The overall incidence of sinusitis was generally higher in mouth-breathers than in nose-breathers (Table 1). The differences were statistically significant except for sphenoidal sinusitis. Three reasons for the high incidence of sinusitis in mouth-breathers can be assigned. First, inflammatory sinus disease offers a mechanical obstacle against the airflow through the nasal passage. Second, sinusitis is usually associated with blockage of the osteomeatal unit, which poses a second barrier facing the airflow. Third, the newly developed unified airway concept34 explains that the respiratory system functions as an integrated unit and that diffuse inflammation often affects the mucosal surfaces of the nose, sinuses, middle ear and tracheobroncheal tree simultaneously.
Inferior turbinate hypertrophy was found in 33% of the children in this study. Similarly, Jun Kim et al24 found the incidence of inferior turbinate hypertrophy to be 31%. It was found that inferior turbinate hypertrophy was more prevalent in mouth-breathers than in nose-breathers (P ≤ 0.01) in the present study. This is probably because hypertrophied nasal concha increase the surface area of the nose, increasing the resistance to nasal respiratory airflow and forcing the child to switch to mouth breathing.
The incidence of adenoid hypertrophy in the present study was 88.1% in mouth-breathers compared with 31% in nose-breathers. This difference was highly significant (P ≤ 0.01). The very high incidence of hypertrophied adenoids emphasizes the leading role of adenoids as a causative agent in mouth breathing. It is not a surprise that Weider et al35 found that upper airway relief, most commonly by performing only an adenoidectomy, can lead to normalization of occlusion in children who were obligate mouth-breathers.
The association between mouth-breathing and the anatomical variables can be easily attributed to the anatomical variables i.e. such anomalies, because they were present from the start, caused mouth breathing and not the other way round. This is not the case regarding the inflammatory changes. It is difficult to tell whether sinusitis, for example, predisposes the child to switch to mouth breathing or whether mouth breathing introduces cold, dry, unprepared air that insults the tissues through which it passes, leading to inflammation. Most probably, a mouth-breathing child enters a vicious cycle, causing more sinusitis, which in turn leads to more obstruction and increased mouth breathing, and so on. Thus, the significant inflammatory changes seen in the scans of mouth-breathing children might be the outcome of mouth breathing and not their cause.
Of the 12.9% of mouth-breathing children who did not have adenoids, only 3.3% had one or more congenital or inflammatory change, whereas the other 9.6% showed a completely normal CT scan. This underlines the leading role of adenoids in causing mouth breathing. It is not possible to tell for sure whether mouth breathing in the few children who did not have adenoids, but had another CT finding, is caused by the congenital or inflammatory factor present or whether these children are just habitual mouth-breathers with a coincidental CT finding.
Among the mouth-breathing children in this study, 9.6% demonstrated normal CT findings representing the incidence of habitual non-obstructive mouth breathing. Likewise, Warren et al36 reported that approximately 12% of their sample were habitual mouth-breathers despite having an adequate nasal airway. Vig and Zajac37 also suggest that oral respiration is extremely common in children and not necessarily related to nasal airway impairment. Thus, mouth breathing may be the result of habit, with or without any impairment of the upper airway.
In conclusion, in order of the most important to the least, hypertrophied adenoids, maxillary sinusitis, pneumatized middle conchae, deviated nasal septum, hypertrophied inferior conchae, and ethmoidal and frontal sinusitis have a role in mouth breathing. It is clear that adenoids have a dominant role in causing mouth breathing. Yet, it is recommend that the paediatrician should assess other mechanical obstacles if mouth breathing was not corrected after adenoidectomy. Further research should be performed to test the validity of correction of such factors in improving the quality of life of mouth-breathing children.
Acknowledgments
We are most grateful to the El-Abd Scan Center for performing the CT scans needed for this study and to Dr Hany Halim El-Abd for interpreting the CT data.
References
- 1.Kenna MA. Nelson text book of pediatrics, 16th edn. Philadelphia, PA: WB Saunders, 2000 [Google Scholar]
- 2.Cattoni DM, Fernandes FD, Di Francesco RC, Latorre Mdo R. Characteristics of the stomatognathic system of mouth breathing children: anthroposcopic approach. Pro Fono 2007;19:347. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein Y, Wexler D, Berger G, Nachmany A, Shapiro-Feinberg M, Ophir D. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. Arch Otolaryngol Head Neck Surg 2000;126:593–600 [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Davidson TM. Home sleep testing in the diagnosis and treatment of sleep disordered breathing. Otolaryngol Clin North Am 2007;40:761–784 [DOI] [PubMed] [Google Scholar]
- 5.Peltomäki T. The effect of mode of breathing on craniofacial growth: revisited. Eur J Orthod 2007;29:426–429 [DOI] [PubMed] [Google Scholar]
- 6.Constantine J, George P, Ekonomides J, Dratsa J. The effect of hypertrophic adenoids and tonsils on the development of posterior cross bite and oral habits. J Clinic Pediat Dent 1994;18:197–201 [PubMed] [Google Scholar]
- 7.Goldstein NA, Fatima M, Campbell TF, Rosenfeld RM. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch Otolaryngol Head Neck Surg 2002;128:770–775 [DOI] [PubMed] [Google Scholar]
- 8.Fairchild RC. A pediatrician views the tonsil and adenoid problem. Am J Orthod 1968;54:491–494 [DOI] [PubMed] [Google Scholar]
- 9.April MM, Zinreich SJ, Baroody FM, Naclerio RM. Coronal CT scan abnormalities in children with chronic sinusitis. Laryngoscope 1993;103:985–990 [DOI] [PubMed] [Google Scholar]
- 10.Lusk RP, McAlister B, Fouley A. Anatomic variation in pediatric chronic sinusitis. A CT study. Otolaryngol Clin North Am 1996;29:75–91 [PubMed] [Google Scholar]
- 11.Midilli R, Aladag G, Erginoz E, Karci B, Savas R. Anatomic variations of the paranasal sinuses detected by computed tomography and the relationship between variations and sex. Kulak Burun Bogaz Ihtis Derg 2005;14:49. [PubMed] [Google Scholar]
- 12.Eggesbø HB. Radiological imaging of inflammatory lesions in the nasal cavity and paranasal sinuses. Eur Radiol 2006;16:872–888 [DOI] [PubMed] [Google Scholar]
- 13.Cotter CS, Stringer S, Rust KR, Mancuso A. The role of computed tomography scans in evaluating sinus disease in pediatric patients. Int J Pediatr Otorhinolaryngol 1999;50:63–68 [DOI] [PubMed] [Google Scholar]
- 14.Perrella A, Rocha S, Cavalcanti M. Quantitative analyses of maxillary sinus using computed tomography. J Appl Oral Sci 2003;11:229–233 [DOI] [PubMed] [Google Scholar]
- 15.Kharbanda OP, Sidhu SS, Sundaram KR, Shukla DK. Oral habits in school going children of Delhi: a prevalence study. J Indian Soc Pedo Prev Dent 2003;21:120–124 [PubMed] [Google Scholar]
- 16.Kanehira T, Takehara J, Takahashi D, Honda O, Morita M. Prevalence of oral malodor and the relationship with habitual mouth breathing in children. J Clin Pediat Dent 2004;28:285–288 [DOI] [PubMed] [Google Scholar]
- 17.Rubin RM. Mode of respiration and facial growth. Am J Orthod 1980;78:504–510 [DOI] [PubMed] [Google Scholar]
- 18.Zeifer B. Pediatric sinonasal imaging: normal anatomy and inflammatory disease. Neuroimaging Clin North Am 2000;10:137–159 [PubMed] [Google Scholar]
- 19.Menezes VA, Leal RB, Moura MM, Granville-Garcia AF. Influence of socio-economic and demographic factors in determining breathing patterns: a pilot study. Rev Bras Otorrinolaringol (Engl Ed) 2007;73:826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton D. Textbook of radiology and imaging, 7th edn. Edinburgh: Elsevier Science, 2003, pp 1519–1530. [Google Scholar]
- 21.McAlister WH, Lusk R, Muntz HR. Comparison of plain radiographs and coronal CT scans in infants and children with recurrent sinusitis. AJR Am J Roentgenol 1989;153:1259–1264 [DOI] [PubMed] [Google Scholar]
- 22.Keall CL, Vig PS. An improved technique for the simultaneous measurement of nasal and oral respiration. Am J Orthod 1986;9:207–212 [DOI] [PubMed] [Google Scholar]
- 23.Sivasli E, Şirikçi A, Bayazýt YA, Gümüsburun E, Erbagci H, Bayram M, et al. Anatomic variations of the paranasal sinus area in pediatric patients with chronic sinusitis. Surg Radiol Anat 2002;24:400–405 [DOI] [PubMed] [Google Scholar]
- 24.Jun Kim H, Jung Cho M, Lee JW, Tae Kim Y, Kahng H, Sung Kim H, et al. The relationship between anatomic variations of paranasal sinuses and chronic sinusitis in children. Acta Otolaryngol 2006;126:1067–1072 [DOI] [PubMed] [Google Scholar]
- 25.Eryilmaz A, Gocer C, Dursun E, Korkmaz H, Akmansu H, Boynuergi S. The incidence of anatomic variations and sinus opacities in pediatric patients with chronic sino-nasal symptoms. Kulak Burun Bogaz Ihtis Derg 2004;13:116. [PubMed] [Google Scholar]
- 26.van derVeken PJ, Clement PA, Buisseret T, Desprechins B, Kaufman L, Derde MP. CT-scan study of the incidence of sinus involvement and nasal anatomic variations in 196 children. Rhinology 1990;28:177–184 [PubMed] [Google Scholar]
- 27.Haaga JR, Lanzieri CF, Gilkeson RC. Computed tomography and magnetic resonance imaging of the whole body, 4th edn. St. Louis: Mosby Inc., 2003, pp 555–561. [Google Scholar]
- 28.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope 1991;101:56–64 [DOI] [PubMed] [Google Scholar]
- 29.Driben JS, Bolger WE, Robles HA, Cable B, Zinreich SJ. The reliability of computerized tomographic detection of the Onodi (sphenoethmoid) cell. Am J Rhinol 1998;12:105–111 [DOI] [PubMed] [Google Scholar]
- 30.Wolf G, Anderhuber W, Kuhn F. Development of the paranasal sinuses in children: implications for paranasal sinuses surgery. Ann Otol Rhinol Laryngol 1993;102:705–711 [DOI] [PubMed] [Google Scholar]
- 31.Lusk R, Muntz H. Endoscopic sinus surgery in children with chronic sinusitis: a pilot study. Laryngoscope 1990;100:654–658 [DOI] [PubMed] [Google Scholar]
- 32.Tantimongkolsuk C, Pornrattanarungsee S, Chiewvit P, Visitsunthorn N, Ungkanont K, Vichyanond P. Pediatric sinusitis: symptom profiles with associated atopic conditions. J Med Assoc Thai 2005;88:149–155 [PubMed] [Google Scholar]
- 33.Delano MC, Fun FY, Zinreich SJ. Relationship to the optic nerve to the posterior paranasal sinuses: a CT anatomic study. Am J Neuroradiol 1996;17:669–675 [PMC free article] [PubMed] [Google Scholar]
- 34.Krouse JH. The unified airway conceptual framework. Otolaryngol Clin North Am 2008;41:257–266 [DOI] [PubMed] [Google Scholar]
- 35.Weider DJ, Baker GL, Salvatoriello FW. Dental malocclusion and upper airway obstruction, an otolaryngologist's perspective. Int J Pediat Otorhinolaryngol 2003;67:323–331 [DOI] [PubMed] [Google Scholar]
- 36.Warren DW, Hairfield WM, Seaton D, Morr KE, Smith LR. The relationship between nasal airway size and nasal oral breathing. Am J Orthod Dentofacial Orthop 1988;93:289–293 [DOI] [PubMed] [Google Scholar]
- 37.Vig PS, Zajac DJ. Age and gender effects on nasal respiratory function in normal subjects. Cleft Palate Craniofacial J 1993;30:279–284 [DOI] [PubMed] [Google Scholar]