SUMMARY
The development of T lymphocytes in the thymus and the function of mature T cells in adaptive immune responses are choreographed by antigen receptors, co-stimulatory molecules, adhesion molecules, cytokines and chemokines. These extrinsic stimuli are coupled to a diverse network of signal transduction pathways that control the transcriptional and metabolic programs that determine T cell function. At the core of T lymphocyte signal transduction is the regulated metabolism of inositol phospholipids and the production of two key lipid second messengers: polyunsaturated diacylglycerols (DAG) and phosphatidylinositol (3,4,5) triphosphate (PI-(3,4,5)-P3). The object of the present review is to discuss facts, controversies and unresolved issues about DAG and PI-(3,4,5)-P3 production in T lymphocytes and to discuss some of the serine/threonine kinases that control unique aspects of T lymphocyte biology and coordinate T cell participation in adaptive immune responses.
Keywords: Serine/threonine kinase; diacylglycerol; PI-(3,4,5)-P3; T lymphocyte; PI3-kinase; signal transduction
Diacylglycerol metabolism in T cells
Multiple species of DAG are produced as intermediate metabolites in phospholipid re-synthesis pathways and consequently quiescent T lymphocytes (T cells) constitutively express high levels of these DAG species prior to immune activation. Antigen receptors can induce further production of polyunsaturated DAG via phospholipase C gamma (PLCγ) mediated hydrolysis of PI-(4,5)-P2 but it has been a challenge to understand how immune activation triggers specific DAG signaling without triggering a huge increase in intracellular DAG concentration [1]. Some insight as to how this happens has come from studies using eGFP-tagged high affinity DAG binding domains to visualize DAG localization in living cells [2, 3]. DAG can thus bind with high affinity to proteins that contain a conserved cysteine-rich domain (CRD) (H-X12-C-X2-C-X13/14-C-X2-C-X4-H-X2-C-X7-C) and eGFP-tagged CRD domains can be used to monitor intracellular DAG distribution. In T lymphocytes, DAG binding proteins include the Ras guanyl releasing protein (GRP) family of nucleotide exchange factors (which activate Ras GTPases); α2- and β2-chimaerins (which are Rac-specific GTPase activating proteins) and serine/threonine kinases of the Protein Kinase C (PKC) and Protein Kinase D (PKD) families. DAG kinases (DGKs), which catalyze the phosphorylation of DAG to phosphatidic acid and thereby terminate DAG signaling, also contain CRD domains that can weakly interact with DAG, potentially bringing the lipid closer to the catalytic domain for phosphorylation.
The CRDs of these proteins do not have identical selectivity for different species of DAG and accordingly they have the potential to monitor the intracellular distribution of different pools of this lipid [4]. For example, the CRDs of PKD and PKCθ act as sensors of plasma membrane DAG whereas the CRD domain of RasGRP1 appears more responsive to DAG localised at Golgi membranes [2, 3]. To date there have only been a limited number of studies that have used these CRDs to visualise DAG pools in T cells but these have been informative. For example they have revealed that gradients of DAG can be formed at the T cell plasma membrane to create localized increases in DAG [3]. In particular, the immunological synapse, a structure formed at the contact point between T cells and antigen primed Antigen Presenting Cells (APC) provides a focus for DAG signalling.
T cells are activated physiologically when their T cell antigen receptor (TCR) binds cognate antigen/major histocompatibility complex (MHC) molecules on the surface of APCs. The contact point between the T cell and the APC organizes into a highly ordered structure where receptors and signaling molecules segregate into distinct zones known as supramolecular activation clusters or SMACs. In stable immune synapses, these zones are arranged in concentric areas, with the TCR accumulating in the central or cSMAC, whereas integrins such as LFA-1 are segregated into a peripheral or pSMAC [5, 6]. The concept that the immune synapse has a role in DAG mediated signal transduction stems from experiments designed to visualise DAG metabolism by following the intracellular distribution of an eGFP-tagged CRD domain from Protein Kinase D [3]. These studies revealed that the high basal levels of DAG in quiescent T cells are uniformly distributed around the plasma membrane. However, following T cell activation there is a change in the intracellular distribution of DAG that creates a focused concentration of this lipid at the centre of the immunological synapse. The mechanisms that create this DAG gradient are not understood but must reflect either a localized increase in DAG production at this site or a localized increase in the DAG half-life. Here it is relevant that the adapter proteins Linker for Activation of T cells (LAT) and Src Homology 2 (SH2) domain-containing Leukocyte Phosphoprotein of 76 kDa (SLP76), which recruit PLCγ to the plasma membrane, are localized at the immune synapse in activated T cells [7, 8]. It is thus probable that there are localized increases in DAG production at the T cell/APC contact zone. However, plasma membrane levels of DAG in T cells are also tightly regulated by DGKα and ζ [9-13]. In addition, antigen receptor triggering induces p56lck-mediated tyrosine phosphorylation of DGKα, which regulates its plasma membrane localization [14]. Thus the mechanisms that determine intracellular accumulation of DAG at the immune synapse would thus almost certainly include a localized inactivation/exclusion of DGKs.
Is the formation of DAG gradients in activated T cells sufficient to explain how the TCR triggers DAG signaling cascades? Possibly, but one other critical insight is that DAG accessibility to CRD domains within proteins is also regulated. For example, an isolated CRD domain from Protein Kinase D localizes to the plasma membrane in quiescent T cells whereas full length PKD is cytosolic. Accordingly, there is sufficient DAG at the plasma membrane of unstimulated lymphocytes to recruit PKD but in the inactive wild-type enzyme the CRD is inaccessible or ‘masked’, thereby preventing DAG binding and membrane translocation [3]. This observation is also true for PKCθ. The activation of DAG binding proteins must therefore involve some initial conformational change that unmasks the CRD. This change could results from protein phosphorylation and/or binding of other molecules. For example, PKCα and PKCβ both have calcium-binding domains and increases in intracellular calcium and calcium binding could be the trigger to unmask the DAG binding domain [15, 16]. Ras GRPs and PKDs can be phosphorylated by PKCs and this could also key trigger for a conformational change.
As discussed in detail below, DAG activates several key signalling pathways that play important roles in controlling T cell development, activation responses and effector functions. However regulating the duration and amplitude of DAG signaling is also important for normal T cell development and function. For example, hypo-responsiveness in anergic T cells is linked to increased expression of DGKα and reduced DAG levels, resulting in reduced RasGRP1 activity and impaired activation of downstream Ras/Erk signalling [10, 11]. In contrast, DGKζ has a key role in limiting TCR signalling and T activation [9]. Additional reduction of DGK activity in T cells, as recently observed in DGKα/ζ double knockout mice, further enhances DAG signaling, causing defective T cell development and development of thymic lymphomas [12].
DAG and Protein Kinase Cs in T cells
T lymphocytes express multiple PKC isoforms including PKCs α, βI, βII which are regulated by calcium, diacylglycerol (DAG) and phospholipids; PKCs δ, ε, η and θ)which are regulated by DAG and phospholipids. PKC activity is also regulated by a series of trans and autophosphoylation events and one key step is phosphorylation of a conserved T-loop motif within the catalytic domain that acts as a priming signal to enable newly synthesized PKCs to achieve catalytic maturity via a series of subsequent autophosphorylation steps [17, 18]. The proposed priming kinase for PKC is Phosphoinositide-dependent protein kinase-1 (PDK1) [19] although there may be alternate priming kinases as PDK1-null cells retain residual PKC T-loop phosphorylation and appear to have normal PKC functions [20, 21]. Mature phosphorylated PKCs then need only diacylglycerol, calcium, and phospholipid binding to achieve full catalytic function. There has been some controversy about whether the phosphorylation of the conserved T-loop motifs in the PKCs is constitutive or regulated by immune activation [22]. There are thus many studies showing that PKCs expressed in quiescent lymphocytes are constitutively phosphorylated on their T loop sites (e.g. Thr 538 for PKCθ and Thr505 in PKCδ) [22, 23] and others that report that TCR triggering can further increase this phosphorylation[24]. To date, no one has addressed this issue quantitatively and considered the stoichiometry of PKC T-loop phosphorylation under different conditions. However, it is important to consider that the key role for T-loop phosphorylation in PKCs is to stabilize protein expression and in the absence of T-loop phosphorylation PKCs are unstable and protein levels are strikingly reduced [25, 26]. If there was no basal phosphorylation of PKCs on their T-loop sites it would not be possible for quiescent T cells to express high levels of different PKC isoforms. The high basal levels of PKCs in quiescent T cells is thus strong evidence that T-loop phosphoryation must occur prior to immune activation. This does not exclude that there is some further modulation of PKC T-loop phosphorylation in activated T cells but its relevance is unclear. Hence, it should also be emphasized that PKC T-loop phosphorylation is not a sufficient marker for PKC activation. Nor are measurements of in vitro catalytic activity useful because the in vivo catalytic function of T-loop phosphorylated PKCs is determined locally at membranes or on protein scaffolds by a combination of DAG, phosphatidylserine and calcium signals. The isolation of PKCs in detergent lysates for measurements of in vitro activity thus destroys the membrane microenvironment that is so important for PKC activity and function. Assays of PKC function need to quantify the phosphorylation of specific PKC substrates and not simply extrapolate from non-quantitative measurements of PKC phosphorylation.
A key observation that drew the attention of immunologists to PKCs was the discovery that PKCθ is selectively polarized in the T cell plasma membrane in the contact zone formed between a T cell and APC [27]. The selective localisation of PKCθ to the immunological synapse is mediated by a combination of DAG binding, changes in the T cell cytoskeleton and the formation of protein complexes between PKCθ and adaptor proteins [28-32]. PKCθ has many important roles in T cells but is not obligatory for many facets of T cell biology [33, 34]. PKCθ is thus not required for antigen driven T cell activation and proliferation in vivo nor for Th1 immune responses. However PKCθ is essential for Th2 immune responses, for optimal differentiation of effector cytotoxic T cells and in regulating the function of Th17 cells. In this context, it is clear that other PKC isoforms are as important as PKCθ for T immune function. PKCα is thus required for optimal T cell-dependent IFNγ production and controls T-dependent B cell responses [35]. PKCδ also has an essential role in effector cytotoxic T lymphocytes (CTL), where it localizes to secretory lysosomes and is required for antigen receptor-induced polarisation and exocytosis of the lytic granules that deliver the ‘lethal’ hit needed to kill target cells [36, 37].
One of the current big challenges in understanding the actions of PKCs in T cells is the identification of direct substrates for these kinases that explain their immunogical functions. Recently several substrates for PKCθ have been identified that afford some insights as to why this kinase is important. For example, PKCθ phosphorylates the scaffolding protein CARMA1 (Caspase recruitment domain (CARD) containing protein 11) [38], which induces its binding to Bcl10/IKKγ, promoting the assembly of the IKK signalling complex and activation of NFκB . PKCθ also controls the transcriptional program of effector T cells via its substrate NR2F6, a transcriptional repressor that modulates IL-17 expression, to control autoimmune responses [39]. Importantly, the role of PKCθ in T cells is not limited to direct effects on gene transcription but includes control of T cell adhesion/migration. Phosphorylation of the Rap guanine nucleotide exchange protein RapGEF2 by PKCθ thus triggers activation of the guanine nucleotide binding protein Rap1 and hence activates the integrin LFA-1 [40]. This adhesion molecule is crucial for T cell interactions with APCs and also controls T cell transmigration across endothelial membranes and T cell entry into secondary lymphoid tissues [41, 42]. The ability of PKCθ to control cellular Rap-1 activity will therefore impact on the duration of T cell/APC contacts and control T cell migration and positioning in secondary lymphoid organs. This could explain why PKCθ is essential to ensure oscillations in cell migration that allow repeated transient interactions between primary T cells and APCs [30]. PKC enzymes can also phosphorylate the β chain of LFA-1 on S745 and T758, sites that regulate the interaction of LFA-1 with cytoskeletal proteins, adhesion to ICAM-1 and outside-in signalling [43-45]. Thus, DAG/PKC signalling may regulate T cell adhesion and migration through multiple different mechanisms.
DAG and Protein Kinase D in T cells
One abundant subset of DAG binding proteins in lymphocytes is the Protein kinase D (PKD) family. There are three members of this family, PKD1, PKD2, and PKD3, all of which have a highly conserved N-terminal regulatory domain containing two cysteine-rich diacylglycerol (DAG) binding domains and an autoinhibitory pleckstrin homology (PH) domain [46, 47]. Most cells typically express two PKD isoforms either the combination of PKD1 and PKD3 or, as in lymphocytes, the combination of PKD2 and PKD3. The selective advantage of these different isoforms is not known and generally they appear have common substrate specificities and are coordinately activated. There is the potential for PKD isoforms to differentially localize in lymphocytes as they do in endothelial cells and other cell lineages but this has not been explored.
PKD activation requires binding of DAG to the its N-terminus but also requires phosphorylation of key conserved serine residues within the PKD activation loop (Ser744/Ser748 in murine PKD1) [48, 49]. These sites are substrates for most classical and novel PKCs including for PKCα, δ, ε, η, and θ. Not surprisingly, elimination of a single PKC isoform rarely prevents phosphorylation and activation of Protein kinase D and elimination of the activity of multiple PKCs is required. PKC regulation of PKD is thus a classic example of how the functions of different PKC isoforms can be redundant. The exact role of Protein Kinase D in primary T cells is currently under investigation. However, one conserved function for this family of kinases is to control gene transcription via the regulation of class II histone deacetylases (HDACs) [50-53]. Acetylation/deacetylation of nucleosomal histones regulates chromatin accessibility and is a fundamental mechanism that controls gene expression, but class II HDACs can also control gene activity by directly interacting with various transcription factors (including those of the MEF2 family) to repress their transcriptional activity [54]. The suppressive effects of class II HDACs on gene transcription are relieved by stimulatory signals that lead to the inducible phosphorylation of serine residues within the N-termini of these HDACs. Phosphorylation at these regulatory sites by PKD promotes the redistribution of class II HDACs to the cytosol, thus disrupting repressive interactions between HDACs and histones and/or transcription factors and allowing gene transcription to occur [54]. PKDs thus act as a critical link between antigen receptors and epigenetic control of chromatin structure and gene expression. As discussed, lymphocytes can express multiple isoforms of PKD and it is relevant that in the context of HDAC regulation they appear redundant. For example, the avian DT40 B cell line co-expresses PKD1 and PKD3 and the loss of HDAC phosphorylation only occurs following deletion of the entire cellular PKD protein pool and not when either PKD1 alone or PKD3 alone are deleted [53].
DAG and Ras/MAP kinases in T cells
One major role for DAG signalling in T cells is to couple the TCR to p21ras guanine nucleotide binding proteins [55, 56]. In quiescent T lymphocytes, Ras GTPases exist predominantly in an inactive GDP bound state but switch rapidly to an active GTP bound form following engagement of the TCR [57]. In their active GTP bound state Ras proteins bind to the serine threonine kinase Raf-1 and activate a mitogen activated protein kinase cascade that phosphorylates and actives Erk1 and Erk2. DAG controls Ras activity by regulating the intracellular localisation and PKC-mediated phosphorylation of RasGRPs, guanine nucleotide exchange proteins for Ras [58-63]. It should be emphasised that activation of Erk1/2 is not restricted to antigen receptor signalling but is a response that is also induced by cytokines such as Interleukin2 (IL-2) and Interleukin15 (IL-15) or via triggering of the chemokine receptors such as the CXCR4 or CCR7. These common γc chain cytokines do not activate Ras/MAP kinases via DAG signalling but instead recruit the Ras guanine nucleotide exchange protein SOS (Son of Sevenless) to the plasma membrane via regulating the localisation of two adaptors Grb2 and Shc.
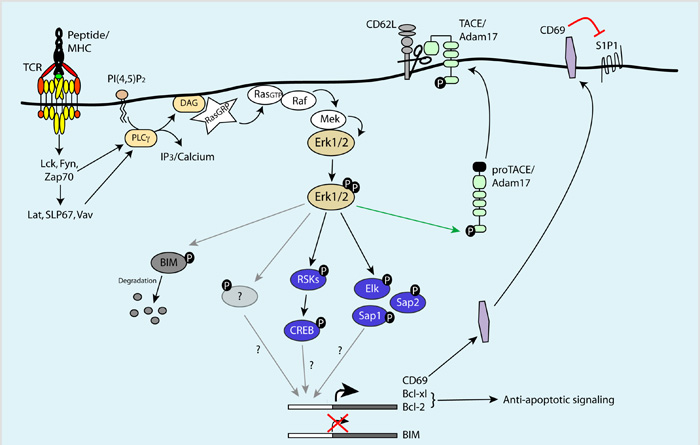
It was established several years ago that the Erk1/2 play a crucial role in T cell activation, in particular they are essential for antigen receptor control of thymocyte positive selection and in regulating the survival of CD8 effector T cells [64, 65]. The prototypical role for Erks in lymphocytes is to control gene transcription [66]. For example, Erk1/ kinases activate the ternary complex factor (TCF) subfamily of ETS-domain transcription factors (Elk-1, SAP-1 and Net/SAP-2), which are known to be essential for thymocyte positive selection [67]. The mechanism underpinning the role of Erks in T cell survival most likely reflects a role for Erk signalling in regulating the transcription of Bcl-2 family members Bcl-2, Bcl-x(L) and Bim [65], however the transcription factors involved in regulating their expression have not been conclusively identified. Importantly, Erk kinases can regulate the activity of other transcription factors in addition to those of the TCF family, including CREB. Unlike TCFs, which are directly phosphorylated and activated by Erks, CREB is probably regulated indirectly via p90 RSKs, which are downstream substrates of Erk kinases that regulate diverse cellular processes [68]. The role of CREB in regulating TCR-induced transcriptional programs is not fully understood but it is interesting to note that in developing B cells, Erk1/2-dependent cell survival and proliferation is mediated by specific transcriptional programs that are co-ordinately regulated by both CREB and Elk-1 transcription factors [69].
It should be emphasised that the importance of Erks for T cells cannot be solely ascribed to their role as regulators of gene transcription; rather it reflects the involvement of these kinases in multifaceted processes. For example, Erk kinases can phosphorylate newly synthesised Bim molecules, inducing their degradation and preventing the formation of pro-apoptotic Bim/Mcl-1 and Bim/Bcl-x(L) complexes, which may play a key role in mediating Erk-dependent T cell survival [70, 71]. Another relatively newly recognized function for Erk1/2 is to phosphorylate disintegrin and metalloprotease (ADAM)17, also known as Tumor necrosis factor (TNF)-converting enzyme (TACE), and control it’s trafficking to the plasma membrane [72]. This Erk/ADAM17 pathway regulates the proteolytic cleavage and secretion of TNFalpha, a key proinflammatory cytokine [73] and also controls cell surface expression of CD62L/L-Selectin, an adhesion molecule required for T lymphocyte entry into secondary lymphoid tissues [74]. CD62L is expressed at high levels on naïve T cells but is acutely down-regulated during immune activation following ADAM17-dependent proteolytic cleavage of CD62L from the T cell membrane [74]. This endoproteolytic cleavage of CD62L allows dynamic turnover of the molecule and is essential for normal T cell recirculation and anti-viral effector function [75]. The ability of the TCR to control ADAM17 activity and thus regulate CD62L expression via Erk signaling is only one of the complex processes that redirects the trafficking pattern of immune activated T cells. One other input of activated Erks to this process is to induce cell surface expression of CD69, which can form a complex with the sphingosine 1-phosphate receptor-1 (S1P1) to negatively regulate its function [76]. SIP1 responds to S1P gradients that are established by S1P lyase activity to control lymphocyte egress from secondary lymphoid organs [77-79]. Increased expression of CD69 in response to Erk signaling thus promotes retention of activated T cells in lymphoid tissues.
PI-(3,4,5)-P3 metabolism in T cells
In T lymphocytes, phosphatidylinositol (3,4,5) triphosphate (PI-(3,4,5)-P3) is generated by the phosphorylation of the 3′-OH position of the inositol ring of phosphatidylinositol (4,5) biphosphate by class I phosphoinositide 3-kinases (PI3Ks). Class 1 PI3Ks comprise a p110 catalytic subunit and an adapter regulatory subunit. Four p110 isoforms exist (α,β,γ,δ) and three adapter subunits, p85α, p85β and p55γ [80]. These different isoforms function in distinct pathways in T cells. For example, the p110δ PI3K subunit produces the PI(3,4,5)P3 that is generated in response to TCR triggering whereas the p110γ PI3K subunit mediates chemokine receptor signaling [81, 82]. The activity of PI3Ks, and hence cellular levels of PI-(3,4,5)-P3, is balanced by the activity of lipid phosphatases, particularly PTEN (phosphatase and tensin homologue deleted on chromosome 10) a lipid phosphatase with specificity for the 3′ position of PI(3,4,5)P3 [83].
One of the first experiments to implicate PI3Ks in T cell immune responses showed that ligation of the co-stimulatory molecule CD28 recruited PI3K to the plasma membrane via binding of the src-homology (SH) 2 domain of the p85 adapter to a phosphorylated tyrosine residue in the CD28 cytoplasmic tail [84]. This observation has resulted in the misconception that PI3K is solely and selectively activated by CD28. However, recruitment of PI3K to a receptor complex does not equate to enzyme activation and before making conclusions about the ability of an receptor to activate PI3K it is necessary to understand whether triggering of that receptor can induce PI(3,4,5)P3 generation in T cells. Here confocal imaging based assays using eGFP-tagged high affinity PI(3,4,5)P3 binding domains (pleckstrin homology (PH) domains) have been a sensitive and quantitative tool to track inositol lipid metabolism as T cells respond to a specific immune stimulus. T cells are seen to produce PI-(3,4,5)-P3 within seconds of contacting an antigen pulsed APC and to sustain this response for 6-9 hours if they maintain contact with the APC. In the immediate response to antigen/APC engagement there is an initial accumulation of PI-(3,4,5)-P3 in the immune synapse but this soon changes so that in the sustained T cell activation response PI-(3,4,5)-P3 is uniformly distributed around the T cell plasma membrane in a prolonged dynamic process that requires constant PI3K activity and TCR engagement [85-87].
The initial trigger for PI-(3,4,5)-P3 accumulation is mediated by the antigen receptor and does not need any co-stimulation [85]. There is a requirement for both antigen receptor and CD28 co-stimulatory signals in order for T cells to sustain PI(3,4,5)P3 levels but strikingly this does not require tyrosine phosphorylation of CD28 on its Y170 residue originally identified as the PI3K binding site [88]. Importantly, disruption of TCR/antigen/MHC interactions or downstream signalling at any time causes loss of PI-(3,4,5)-P3 [85, 87]. CD28 signaling is thus not sufficient to induce PI-(3,4,5)-P3 production but it can promote sustained PI-(3,4,5)-P3 generation in TCR activated T cells [88].
It is not known how TCR signalling induces PI-(3,4,5)-P3 accumulation. Possible mechanisms include p85-SH2-mediated binding to phosphorylated tyrosine residues in adapters such as LAT (Linker of Activated T cells) or direct activation of the p110δ catalytic subunit by GTP loaded p21ras. One other possibility is that the TCR induces PI-(3,4,5)-P3 accumulation by acute inhibition of the catalytic activity of PTEN via the production of reactive oxygen species. PTEN can be transiently inactivated as a result of oxidation of essential cysteine residues in its catalytic core [89] and TCR triggering is known to induce a transient increase in cellular levels of reactive oxygen species [90]. Moreover, as PTEN deletion is sufficient to induce the accumulation of PI-(3,4,5)-P3 in T cells transient inactivation of PTEN would have the same effect [91].
Phosphoinositide-Dependent Protein Kinase-1 (PDK1) - a mis-named kinase?
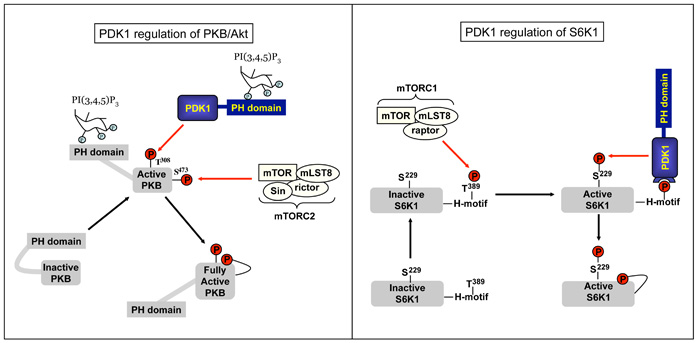
PI(3,4,5)P3, is a high-affinity docking site for pleckstrin homology (PH) domains in a large variety of signaling molecules and the increased levels of PI(3,4,5)P3 in the plasma membrane of activated T cells creates a signaling platform for the assembly of molecules such as TEC family tyrosine kinases, Rac/Rho guanine nucleotide exchange proteins and the serine/threonine kinases, Phosphoinositide-Dependent Protein Kinase-1 (PDK1) and Protein Kinase B (PKB)/Akt. PDK1 is an essential kinase in T cells because it activates multiple AGC kinases including PKB, p70 Ribosomal S6 kinases (S6Ks) and p90 Ribosomal S6 kinase (RSK) by phosphorylating a specific Thr or Ser residue located within the catalytic domain of the kinase [19, 92]. The activation of PKB is dependent on increases in cellular levels of PI-(3,4,5)-P3 and requires the phosphorylation of PKB on Threonine 308 within the PKB catalytic domain by PDK1. Phosphorylation of PKB on Serine 473 in the carboxy-terminus of the molecule is also required for optimal kinase activity and can be mediated by the mammalian target of rapamycin (mTOR) complex-2 [93] and the DNA dependent Protein kinase [94].
PI3K/PDK1-mediated activation of PKB is important in T cells particularly during thymus development. Hence, loss of PDK1 in T cell progenitors phenocopies loss of PI3Ks and PKBs and causes a block in T cell development at the pre-T cell stage [95-98]. T cell progenitors that lack PI3K p110γ and δ subunits [99] or PDK1 or PKB isoforms fail to develop primarily because they cannot up-regulate sufficient glucose, iron and amino acid transport mechanisms to meet the nutrient demands of thymus differentiation [96, 97]. Despite the importance of the PDK1/PKB link, the role of PDK1 in T cell progenitors extends beyond its ability to link PI-(3,4,5)-P3 production to PKB activation. Hence substitution of wild type PDK1 with a PDK1 L155E mutant that supports full PKB activation in T cells is not sufficient to restore normal thymocyte development [96]. This L155E mutation disrupts the integrity of an important PDK1 domain termed the PIF binding pocket. This domain is not required for PKB phosphorylation but it is essential for PDK1 to interact with phosphorylated carboxy-terminal hydrophobic motifs present in other PDK1-regulated AGC kinases, including S6Ks and RSKs [20, 100, 101]. This interaction then allows PDK1 to activate these AGC kinases by phosphorylating their catalytic domains. The role of PDK1 in T cell progenitors thus reflects that this kinase is essential for the phosphorylation and activation of multiple members of the AGC kinase family [96].
One confusing issue about PDK1 is whether it is a direct mediator of PI-(3,4,5)-P3 signal transduction. PDK1 does have a PH domain that binds PI-(3,4,5)-P3 with high affinity [102]. Moreover, PDK1 phosphorylation of PKB is PI-(3,4,5)-P3 dependent, which was originally interpreted to mean that PDK1 activity was PI3K dependent; hence the naming of this kinase as a 3-phosphoinositide-dependent protein kinase-1 (PDK1) [103]. However, subsequent work has since shown that PDK1 is a constitutively active kinase and that binding of PI-(3,4,5)-P3 to its PH domain is not required for its catalytic activity but instead targets PDK1 to the plasma membrane where it can co-localise with PKB [104]. The PI-(3,4,5)-P3 dependence of PKB activation reflects that binding of this lipid to the PKB PH domain causes a conformational change that allows PDK1 to phosphorylate threonine 308 within the PKB catalytic domain and activate the kinase [105]. Interestingly, mutations in the PDK1 PH domain that block PI-(3,4,5)-P3 binding do not completely compromise PDK1 function [104] (unless they also destabilize the protein, [20]) and indeed are much more benign than mutations within the PDK1 PIF domain. Hence, mice homozygous for PDK1 deleted alleles or homozygous for PDK1-L155E alleles do not survive embryogenesis beyond E9.5 or E13.5 respectively [101, 106], whereas mice homozygous for a knock in mutant of PDK1 that is incapable of binding PI-(3,4,5)-P3 (PDK1 K465E) are viable and fertile albeit significantly smaller than normal mice and prone to insulin resistance [104]. The loss of PI-(3,4,5)-P3 binding to the PDK1 PH domain strongly reduces PKB phosphorylation and activity but the viability of the mice indicates that low levels of active PKB are sufficient for many cellular functions.
Importantly, PI-(3,4,5)-P3 binding to the PDK1 PH domain has no impact on the ability of PDK1 to bind to and activate RSK, S6K1 or the PKCs [20, 104]. PDK1-mediated phosphorylation of PKCs appears constitutive in most cells in accordance with the known high basal activity of PDK1 [22]. In contrast, phosphorylation of RSK and S6K1 by PDK1 is regulated by extrinsic stimuli, which reflects that a rate-limiting step in the activation of these kinases is the regulated phosphorylation of these enzymes on hydrophobic motifs that create docking sites for PDK1. For S6K1 the hydrophobic motif kinase is the mTORC1 complex, which phosphorylates S6K1 on Thr389, allowing PDK1 to dock and directly activate S6K1 via direct phosphorylation of S6K1 at its T loop site (T229) (Fig.2). PDK1 can also indirectly controls S6K1 catalytic activity by controlling the activity of the mTORC1 complex via a PKB-dependent pathway mediated by the Tsc-1/Tsc2 complex and the Rheb GTPases [107]. Activation of RSK by PDK1 also requires co-ordinated inputs from multiple signals [68]. RSKs have two distinct kinase domains and the initiating step for their activation is Erk1/2-mediated phosphorylation of Ser369, Thr365 and Thr577 in the carboxy-terminal catalytic domain. The activated C-terminal catalytic domain of RSK then phosphorylates itself on Ser386 to create a docking site for PDK1, which then phosphorylates Ser227 in the N-terminal RSK kinase domain thereby activating the enzyme and enabling the phosphorylation of downstream substrates.
Figure 1.
Cartoon depicting Erkl/2 pathways which may regulate the survival and trafficking of T cells in response to antigen receptor triggering and activation of PLCγ-DAG-RAS-RAF-MEK signalling cascades. See main text for further details.
PDK1 thus integrates multiple signalling pathways to control the activity of different AGC kinases and is at the core of PI-(3,4,5)-P3 signalling to PKB. However PDK1 is not dependent on phosphoinositides for many of its key cellular functions and hence could be considered to be mis-named.
PKB function in T cells
One evolutionarily conserved role for PKB is to regulate cell metabolism by controlling the expression of glucose transporters, amino acid transporters and transferrin receptors [108]. PKB also controls the activity of the mTORC1 complex, a critical regulator of ribosomal biogenesis and protein synthesis that controls cell growth [107]. T cell development in the thymus and the survival and immune function of peripheral T lymphocytes requires that T cells match energy metabolism to energy demands. In this context, PKB is an essential core regulator of metabolism in T cell progenitors in the thymus [96, 97, 109]. Accordingly, thymocytes that do not express PDK1 or are deleted of one or all three PKB isoforms fail to develop because they cannot up-regulate sufficient glucose, iron and amino acid transport mechanisms to meet the nutrient demands of thymus differentiation. It is generally assumed that PKB has an equally important role to control metabolism in peripheral T cells. Indeed, expression of constitutively active mutants of PKB can substitute for the cytokine IL-7 and stimulate glucose uptake and promote cell growth and survival of naïve T cells [108, 110]. PKB activation also controls peripheral T cell differentiation and can repress development of regulatory T cell populations. However, there are studies indicating that PKB may not work alone to regulate energy metabolism in peripheral T cells. For example, PKB-independent signaling pathways can control glucose uptake in peripheral T lymphocytes [111] but while glucose deprivation causes apoptosis in peripheral T cells, inhibition of PKB suppresses antigen receptor-induced proliferation but does not cause apoptosis [112]. It has also been described that inhibition of PKB following TCR triggering of naïve T cells promotes the differentiation of regulatory T cell populations that function to restrain autoimmunity [113, 114]. Premature termination of PKB activity in antigen receptor-triggered naïve T cells thus results in expression of the transcription factor Foxp3 and development of a transcriptional program normally associated with regulatory T cells rather than effector Th1/Th2/Th17 profiles [113, 114]. Hence PKB signalling pathways actively suppress Foxp3 expression. This type of cellular differentiation switch would not be possible if there was a complete failure of glucose uptake and energy production in peripheral T cells following PKB inhibition. At this point it would seem likely that PKB will play some role in controlling peripheral T cell metabolic responses but other signaling pathways must make critical contributions. What is clear is that the magnitude and kinetics of PKB activation will determine the fate of effector T lymphocytes.
Another conserved function for PKB is to phosphorylate members of the Forkhead box O (FOXO) family of transcription factors (Foxo1, 3A and Foxo4) [115]. Non-phosphorylated Foxo1, 3A and 4 are found in the nuclei of quiescent cells where they drive transcription of cell cycle inhibitors such as p27. Following activation of PKB, Foxos are phosphorylated on multiple residues, which leads to their cytosolic sequestration via binding to 14-3-3 proteins. In quiescent T cells, antigen receptor activation of PKB can thus promote cell cycle progression by terminating expression of p27 [116]. Other important Foxo gene targets in lymphocytes are pro-apoptopic members of the Bcl-2 family Bim and Noxa. For example, the cytokines IL-2 and IL-15 stimulate PKB, promoting the nuclear exclusion and transcriptional inactivation of Foxos and hence ensuring NK cell survival by keeping levels of Bim and Noxa low. Withdrawal of cytokine results in loss of PKB activity, thus inducing expression of high levels of Bim and Noxa that cause apoptosis [117]. One more recently identified Foxo gene target is the transcription factor KLF-2. This was first identified as a transcription factor that maintained lymphocyte quiescence and hence was down-regulated in immune activated cells [118]. More recently it has become apparent that the main role for KLF2 is to control transcription of genes encoding the adhesion molecules and chemokine receptors that control the migration of T lymphocytes [119-121].
Naïve T lymphocytes constantly circulate around the body via the blood, lymphatics and secondary lymphoid organs. Immune responses are initiated within secondary lymphoid organs, such as peripheral lymph nodes, when T cells encounter primed antigen presenting cells that express cognate antigen/major histocompatibility complexes together with appropriate co-stimulatory molecules. Entry of naïve T cells into lymph nodes from the blood occurs in specialized high endothelial venules (HEVs) and is dependent on chemokine receptors, such as CCR7, and molecules that mediate lymphocyte adhesion, such as CD62L (L-selectin) and integrins. The first step of transendothelial migration is CD62L mediated tethering and rolling of naïve lymphocytes on the endothelium of HEVs. T cell entry into lymph nodes is then guided by a chemotactic gradient mediated by the chemokine receptor CCR7 while T cells exit lymph nodes by responding to sphingosine-1 phosphate (S1P) gradients [79, 122, 123]. Accordingly, the balance of CD62L/CCR7 and S1P1 expression determines T cell entry into and retention within lymph nodes [124]. As a result of immune activation, striking changes are induced in T cell migratory patterns: effector T lymphocytes migrate to a greater extent to non-lymphoid tissues and sites of inflammation and have a reduced capacity to home to peripheral lymph nodes compared to naïve and memory T cells. Activated T cells thus down-regulate expression of CD62L/CCR7 and S1P1 and up-regulate expression of inflammatory chemokine receptors and adhesion molecules such as CCR3, CCR5 VLA-4 and CD44.
Changes in the expression of these trafficking molecules, and subsequent alterations in the migratory behaviour of T cells, are vitally important for immune responses and are controlled by a PI(3,4,5)P3/PKB/Foxo/KLF2 signalling pathway [74, 119, 121, 125]. In naïve T cells high levels of transcriptionally active Foxos maintain expression of KLF2, which then induces transcription of naïve T cell homing receptors (CD62L/CCR7 and S1P1) while repressing expression of inflammatory chemokine receptors. Activation of PKB results in phosphorylation and inactivation of Foxos and hence loss of KLF2 expression and reduced expression of naïve T cell homing receptors. Historically, loss of these receptors is used to distinguish naïve and antigen experienced T cells. In fact, the expression of molecules such as CD62L/CCR7 and S1P1 levels rather report the PKB/Foxo signaling status of a T cell. Hence PKB, a serine/threonine kinase more usually associated with the control of cell metabolism determines the expression of lymph node homing receptors and is a critical regulator of T cell migration.
Future perspectives
The last few years have seen quite large increases in our understanding of how the second messengers diacylglycerol and PI(3,4,5)P3 co-ordinate networks of serine/threonine kinases to control T lymphocyte function. Gene targeting strategies have identified key roles for serine/threonine kinases in T lymphocytes. However, we have only uncovered a fraction of the relevant information about how protein phosphorylation reversibly regulates key processes in T lymphocytes. The main problem is that we do not know the full repertoire of substrates for any of the key serine/threonine kinase. In this context there are emerging advances in proteomics technology including developments in phosphopeptide purification, mass spectrometry, and bioinformatics that are beginning to make analysis of the entire phosphoproteome of an activated T lymphocyte feasible. Advances in DNA sequencing and microarray analysis made transcriptional profiling of T cells routine. Comparable advances in proteomic technology will make it routine to similarly quantitate and define the pathways of protein phosphorylation that control T cell biology.
Fig. 2.
Cartoon dipicting the regulation of protein kinase B (PKB) and p70 ribosomal S6 kinases (S6Ks) by phosphoinositide-dependent protein kinase 1 (PDK1) and protein phosphorylation.
References
- [1].Spitaler M, Cantrell DA. Protein kinase C and beyond. Nature immunology. 2004 Aug;5(8):785–90. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- [2].Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Molecular biology of the cell. 2004 Jun;15(6):2932–42. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006 May;24(5):535–46. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- [4].Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochimica et biophysica acta. 2006 Aug;1761(8):827–37. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [5].Kupfer A, Kupfer H. Imaging immune cell interactions and functions: SMACs and the Immunological Synapse. Seminars in immunology. 2003 Dec;15(6):295–300. doi: 10.1016/j.smim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [6].Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Current opinion in cell biology. 2007 Oct;19(5):529–33. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. The Journal of experimental medicine. 2005 Oct 17;202(8):1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nature immunology. 2005 Dec;6(12):1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- [9].Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nature immunology. 2003 Sep;4(9):882–90. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- [10].Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nature immunology. 2006 Nov;7(11):1166–73. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- [11].Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nature immunology. 2006 Nov;7(11):1174–81. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- [12].Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, et al. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proceedings of the National Academy of Sciences of the United States of America. 2008 Aug 19;105(33):11909–14. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunological reviews. 2008 Aug;224:249–64. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Merino E, Avila-Flores A, Shirai Y, Moraga I, Saito N, Merida I. Lck-dependent tyrosine phosphorylation of diacylglycerol kinase alpha regulates its membrane association in T cells. J Immunol. 2008 May 1;180(9):5805–15. doi: 10.4049/jimmunol.180.9.5805. [DOI] [PubMed] [Google Scholar]
- [15].Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998 Oct 30;95(3):307–18. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- [16].Newton AC. Lipid activation of protein kinases. Journal of Lipid Res. 2008 doi: 10.1194/jlr.R800064-JLR200. epub ahead of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003 Mar 1;370(Pt 2):361–71. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Parker PJ, Murray-Rust J. PKC at a glance. Journal of cell science. 2004 Jan 15;117(Pt 2):131–2. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- [19].Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Seminars in cell & developmental biology. 2004 Apr;15(2):161–70. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- [20].McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, et al. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. The EMBO journal. 2004 May 19;23(10):2071–82. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wood CD, Kelly AP, Matthews SA, Cantrell DA. Phosphoinositide-dependent protein kinase-1 (PDK1)-independent activation of the protein kinase C substrate, protein kinase D. FEBS letters. 2007 Jul 24;581(18):3494–8. doi: 10.1016/j.febslet.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gruber T, Freeley M, Thuille N, Heit I, Shaw S, Long A, et al. Comment on “PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation”. Science (New York, NY. 2006 Apr 7;312(5770):55. doi: 10.1126/science.1115362. author reply. [DOI] [PubMed] [Google Scholar]
- [23].Liu Y, Graham C, Li A, Fisher RJ, Shaw S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem J. 2002 Jan 15;361(Pt 2):255–65. doi: 10.1042/bj3610255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee KY, D’Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science (New York, NY. 2005 Apr 1;308(5718):114–8. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- [25].Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS letters. 2000 Nov 10;484(3):217–23. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- [26].Collins BJ, Deak M, Murray-Tait V, Storey KG, Alessi DR. In vivo role of the phosphate groove of PDK1 defined by knockin mutation. Journal of cell science. 2005 Nov 1;118(Pt 21):5023–34. doi: 10.1242/jcs.02617. [DOI] [PubMed] [Google Scholar]
- [27].Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997 Jan 2;385(6611):83–6. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- [28].Sedwick CE, Altman A. Perspectives on PKCtheta in T cell activation. Mol Immunol. 2004 Jul;41(6-7):675–86. doi: 10.1016/j.molimm.2004.01.007. [DOI] [PubMed] [Google Scholar]
- [29].Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006 Jul;25(1):117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007 May 18;129(4):773–85. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- [31].Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase Ctheta. J Immunol. 2008 Oct 1;181(7):4852–63. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008 Oct;29(4):589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baier G. PKC isotype functions in T lymphocytes. Ernst Schering Foundation symposium proceedings. 2007;3:29–41. doi: 10.1007/2789_2007_061. [DOI] [PubMed] [Google Scholar]
- [34].Marsland BJ, Kopf M. T-cell fate and function: PKC-theta and beyond. Trends in Immunology. 2008 Apr;29(4):179–85. doi: 10.1016/j.it.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [35].Pfeifhofer C, Gruber T, Letschka T, Thuille N, Lutz-Nicoladoni C, Hermann-Kleiter N, et al. Defective IgG2a/2b class switching in PKC alpha−/− mice. J Immunol. 2006 May 15;176(10):6004–11. doi: 10.4049/jimmunol.176.10.6004. [DOI] [PubMed] [Google Scholar]
- [36].Ma JS, Haydar TF, Radoja S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J Immunol. 2008 Oct 1;181(7):4716–22. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- [37].Ma JS, Monu N, Shen DT, Mecklenbrauker I, Radoja N, Haydar TF, et al. Protein kinase Cdelta regulates antigen receptor-induced lytic granule polarization in mouse CD8+ CTL. J Immunol. 2007 Jun 15;178(12):7814–21. doi: 10.4049/jimmunol.178.12.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005 Dec;23(6):575–85. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [39].Hermann-Kleiter N, Gruber T, Lutz-Nicoladoni C, Thuille N, Fresser F, Labi V, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008 Aug;29(2):205–16. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Letschka T, Kollmann V, Pfeifhofer-Obermair C, Lutz-Nicoladoni C, Obermair GJ, Fresser F, et al. PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008 Dec 1;112(12):4617–27. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- [41].Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunological reviews. 2007 Aug;218:135–46. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- [42].Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. The EMBO journal. 2008 Jan 9;27(1):62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fagerholm S, Morrice N, Gahmberg CG, Cohen P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. The Journal of biological chemistry. 2002 Jan 18;277(3):1728–38. doi: 10.1074/jbc.M106856200. [DOI] [PubMed] [Google Scholar]
- [44].Fagerholm SC, Hilden TJ, Gahmberg CG. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends in biochemical sciences. 2004 Sep;29(9):504–12. doi: 10.1016/j.tibs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [45].Fagerholm SC, Hilden TJ, Nurmi SM, Gahmberg CG. Specific integrin alpha and beta chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. The Journal of cell biology. 2005 Nov 21;171(4):705–15. doi: 10.1083/jcb.200504016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. The Journal of biological chemistry. 2005 Apr 8;280(14):13205–8. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- [47].Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006 Jun;27(6):317–23. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [48].Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. The Journal of biological chemistry. 1998 Oct 16;273(42):27662–7. doi: 10.1074/jbc.273.42.27662. [DOI] [PubMed] [Google Scholar]
- [49].Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. The Journal of biological chemistry. 2001 Aug 31;276(35):32606–15. doi: 10.1074/jbc.M101648200. [DOI] [PubMed] [Google Scholar]
- [50].Dequiedt F, Van Lint J, Lecomte E, Van Duppen V, Seufferlein T, Vandenheede JR, et al. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. The Journal of experimental medicine. 2005 Mar 7;201(5):793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Molecular and cellular biology. 2004 Oct;24(19):8374–85. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Parra M, Kasler H, McKinsey TA, Olson EN, Verdin E. Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after T-cell receptor activation. The Journal of biological chemistry. 2005 Apr;8(14):13762–70. doi: 10.1074/jbc.M413396200. [DOI] [PubMed] [Google Scholar]
- [53].Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, et al. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Molecular and cellular biology. 2006 Feb;26(4):1569–77. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Martin M, Kettmann R, Dequiedt F. Class IIa histone deacetylases: regulating the regulators. Oncogene. 2007;26:5450–67. doi: 10.1038/sj.onc.1210613. [DOI] [PubMed] [Google Scholar]
- [55].Roose J, Weiss A. T cells: getting a GRP on Ras. Nature immunology. 2000 Oct;1(4):275–6. doi: 10.1038/79713. [DOI] [PubMed] [Google Scholar]
- [56].Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Current opinion in immunology. 2000 Jun;12(3):289–94. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- [57].Downward J, Graves JD, Warne PH, Rayter S, Cantrell DA. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–23. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- [58].Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature immunology. 2000 Oct;1(4):317–21. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- [59].Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000 May 15;95(10):3199–203. [PubMed] [Google Scholar]
- [60].Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003 Aug 15;102(4):1414–20. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- [61].Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Molecular and cellular biology. 2005 Jun;25(11):4426–41. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood. 2005 May 1;105(9):3648–54. doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- [63].Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Molecular and cellular biology. 2007 Apr;27(7):2732–45. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005 Oct;23(4):431–43. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- [65].D’Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008 Dec 1;181(11):7617–29. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Treisman R. Regulation of transcription by MAP kinase cascades. Current opinion in cell biology. 1996 Apr;8(2):205–15. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- [67].Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nature immunology. 2004 Mar;5(3):289–98. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- [68].Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008 Oct;9(10):747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- [69].Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008 Apr;28(4):499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [70].Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. The Journal of biological chemistry. 2004 Mar 5;279(10):8837–47. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- [71].Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. The EMBO journal. 2007 Jun 20;26(12):2856–67. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. Journal of cell science. 2005 Jun 1;118(Pt 11):2371–80. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- [73].Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, et al. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. Journal of cell science. 2008 Jan 15;121(Pt 2):149–54. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- [74].Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature immunology. 2008 May;9(5):513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Richards H, Longhi MP, Wright K, Gallimore A, Ager A. CD62L (L-selectin) down-regulation does not affect memory T cell distribution but failure to shed compromises anti-viral immunity. J Immunol. 2008 Jan 1;180(1):198–206. doi: 10.4049/jimmunol.180.1.198. [DOI] [PubMed] [Google Scholar]
- [76].Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006 Mar 23;440(7083):540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- [77].Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science (New York, NY. 2005 Sep 9;309(5741):1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- [78].Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science (New York, NY. 2007 Apr 13;316(5822):295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- [79].Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature immunology. 2007 Dec;8(12):1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- [80].Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003 Apr;3(4):317–30. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- [81].Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110delta isoform of PI3K. Trends in immunology. 2007 Feb;28(2):80–7. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Oak JS, Matheu MP, Parker I, Cahalan MD, Fruman DA. Lymphocyte cell motility: the twisting, turning tale of phosphoinositide 3-kinase. Biochemical Society transactions. 2007 Nov;35(Pt 5):1109–13. doi: 10.1042/BST0351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008 Sep 18;27(41):5398–415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- [84].Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994 May 26;369(6478):327–9. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- [85].Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nature immunology. 2002 Nov;3(11):1082–9. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- [86].Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nature immunology. 2002 Nov;3(11):1090–6. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- [87].Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nature immunology. 2003 Aug;4(8):749–55. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- [88].Garcon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008 Feb 1;111(3):1464–71. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- [89].Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008 Sep 18;27(41):5464–76. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- [90].Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. The Journal of experimental medicine. 2002 Jan 7;195(1):59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Buckler JL, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunological reviews. 2008 Aug;224:239–48. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000 Apr 20;10(8):439–48. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- [93].Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY. 2005 Feb 18;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- [94].Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Molecular cell. 2008 Apr 25;30(2):203–13. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- [95].Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nature immunology. 2004 May;5(5):539–45. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- [96].Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, et al. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. The EMBO journal. 2007 Jul 25;26(14):3441–50. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 17;104(29):12105–10. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, et al. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007 May 1;178(9):5443–53. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- [99].Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005 Sep 1;175(5):2783–7. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- [100].Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. The EMBO journal. 2001 Aug 15;20(16):4380–90. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. The EMBO journal. 2003 Aug 15;22(16):4202–11. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Komander D, Fairservice A, Deak M, Kular GS, Prescott AR, Peter Downes C, et al. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. The EMBO journal. 2004 Oct 13;23(20):3918–28. doi: 10.1038/sj.emboj.7600379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997 Apr 1;7(4):261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- [104].Bayascas JR, Wullschleger S, Sakamoto K, Garcia-Martinez JM, Clacher C, Komander D, et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Molecular and cellular biology. 2008 May;28(10):3258–72. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Calleja V, Alcor D, Laguerre M, Park J, Vojnovic B, Hemmings BA, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS biology. 2007 Apr;5(4):e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, et al. Essential role of PDK1 in regulating cell size and development in mice. The EMBO journal. 2002 Jul 15;21(14):3728–38. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. Journal of leukocyte biology. 2008 Oct;84(4):949–57. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nature immunology. 2005 Sep;6(9):881–8. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- [110].mWofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008 Feb 15;111(4):2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008 Apr 1;180(7):4476–86. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and puma-dependent cell death upon growth factor deprivation. The Journal of Bological Chemistry. 2008;283(3):6344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 3;105(22):7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of experimental medicine. 2008 Mar 17;205(3):565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008 Apr 7;27(16):2258–62. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- [116].Fabre S, Lang V, Bismuth G. PI3-kinase and the control of T cell growth and proliferation by FoxOs. Bulletin du cancer. 2006 May 1;93(5):E36–8. [PubMed] [Google Scholar]
- [117].Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nature immunology. 2007 Aug;8(8):856–63. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nature immunology. 2001 Aug;2(8):698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- [119].Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006 Jul 20;442(7100):299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- [120].Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007 Jun 15;178(12):7632–9. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- [121].Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nature immunology. 2008 Mar;9(3):292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- [122].Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature immunology. 2008 Sep;9(9):970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- [123].Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008 Aug 15;181(4):2265–70. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008 Jan;28(1):122–33. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008 Sep 1;181(5):2980–9. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]