Abstract
Background
Following our previous reports of an increased prevalence of insulin resistance and adiposity among acute lymphoblastic leukemia (ALL) survivors, particularly women treated with cranial radiotherapy (CRT), we aimed to (1) assess the relationships between adipokines (leptin and adiponectin), CRT, and measures of body fatness, and (2) determine correlates of insulin resistance, by gender.
Methods
We conducted cross-sectional evaluation of 116 ALL survivors (median age, 23.0 years; range, 18–37; average time from treatment: 17.5 years), including fasting laboratory testing (adiponectin, leptin, insulin, glucose), anthropometric measurements (weight, height, waist circumference), DXA (total body fat, truncal-to-lower-body-fat ratio), and abdominal CT (visceral fat). We estimated insulin resistance using the homeostasis model for assessment of insulin resistance (HOMA-IR). Analytic approaches included regression models and Wilcoxon rank sum testing.
Results
Mean leptin per kilogram fat mass was higher for females (0.7 ng/mL/kg) than males (0.4 ng/mL/kg, P<0.01), and among subjects who had received CRT compared to those who had not received CRT (females CRT =0.9 ng/mL/kg, no CRT=0.7 ng/mL/kg; P=0.1; males CRT=0.5 ng/mL/kg, no CRT=0.3 ng/mL/kg; P<0.01). Elevated HOMA-IR was nearly uniformly present, even among subjects with BMI<25 kg/m2, and was associated with higher leptin:adiponectin ratio (P<0.01).
Conclusions
Among survivors of childhood leukemia, higher leptin levels were associated with measures of body fat and insulin resistance. Anthropomorphic and metabolic changes many years after ALL treatment remain a major health problem facing survivors and may be related to central leptin resistance.
Keywords: ALL, adiponectin, insulin resistance, leptin, leukemia
INTRODUCTION
With improvements in the treatment of childhood cancers, survival has increased dramatically. Currently, more than 85% of children with acute lymphoblastic leukemia (ALL) are expected to become long-term survivors.[1–2] As the ALL survivor population ages, serious health conditions will increase in prevalence. When compared to their siblings, ALL survivors at a median age of 26 years were four times more likely to have a severe or life-threatening health condition.[3]
As we have previously reported, obesity and insulin resistance are highly prevalent among ALL survivors[4–7] and contribute to morbidity and premature mortality in this population.[4–9] Treatment factors, especially the use of cranial radiotherapy (CRT), have been partially implicated, but much of the metabolic derangement in ALL survivors remains unexplained.[7,10]
Leptin and adiponectin are peptide hormones secreted by adipocytes. Leptin is proportional to total body fat mass and communicates primarily with the hypothalamus regarding satiety and energy usage. Adiponectin is inversely proportional to fat mass and appears to communicate primarily with skeletal muscle and the liver with respect to energy storage. Both adipokines are part of a larger system of communication between the hypothalamus, the adipocyte, and the gut with respect to hunger, satiety, energy usage, and energy storage (Figure 1).[11–13]
Figure 1.
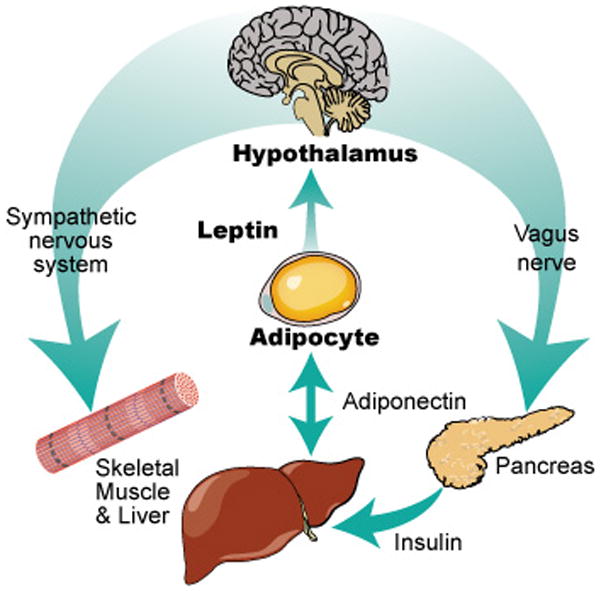
We conducted a cross-sectional analysis of 118 young adult survivors of childhood ALL. Each subject underwent extensive laboratory, anthropomorphic, and radiographic analysis. We assessed (1) the relationship between adipokines (leptin and adiponectin), CRT, and measures of body fatness and (2) correlates of insulin resistance, by gender. Our goal was to understand the hormonal and metabolic changes related to increased adiposity and risk for insulin resistance.
METHODS
Study population
From May 2004 to January 2007, 118 young adult survivors of childhood ALL participated in the Acute Leukemia Lifestyle Intervention for Everyday (ALLIFE) study. Detailed methods have been previously described.[7,14] Briefly, 118 young adult survivors of childhood ALL diagnosed between 1970 and 2000 and living in the Dallas area were enrolled. For the purposes of our analysis, since insulin modulators may impact adipokines, we excluded two subjects who were on diabetes medication for a total sample of 116 survivors. Remaining subjects were 55% female, had a median age of 23 years (range 18 – 37 years), and were an average of 17.5 years from treatment (Table I). All subjects provided written informed consent and the study was approved by the institutional review boards at The University of Texas Southwestern Medical Center and the Cooper Institute.
Table I.
Demographic characteristics of ALLIFE participants.
| Characteristic | Females | Males | P |
|---|---|---|---|
| N=64 % | N=52 % | ||
| Age at study | 0.47 | ||
|
| |||
| 18–24 y | 59 | 69 | |
| 25–34 y | 38 | 37 | |
| 35–37 y | 3 | 4 | |
|
| |||
| Race and ethnicity | 0.11 | ||
|
| |||
| White, Non-Hispanic | 69 | 81 | |
| Black, Non-Hispanic | 14 | 8 | |
| Hispanic or Latino | 17 | 8 | |
| Other | 0 | 4 | |
|
| |||
| Cancer therapy | |||
|
| |||
| Chemotherapy | |||
| Anthracycline | 66 | 80 | 0.09 |
| Cyclophosphamide | 39 | 46 | 0.45 |
| Dexamethasone | 14 | 4 | 0.37 |
| Cranial radiotherapy (CRT) | |||
| Any CRT | 38 | 29 | 0.42 |
| < 24 Gy | 9 | 10 | 0.28 |
| ≥ 24 Gy | 28 | 19 | 0.41 |
| Stem cell transplant | 5 | 8 | 0.70 |
| Total body irradiation | 5 | 8 | 0.70 |
Outcomes
Fasting laboratory testing
Venous blood samples were acquired from all participants after a 12-hour overnight fast. Serum was frozen at −80C until sent for batch analysis. Glucose was measured at the University of Texas Southwestern Medical Center GCRC Core Laboratory.[7] Leptin, adiponectin, and insulin were measured using commercial radioimmune assays (Linco Research, Inc., St. Charles, MO) as previously described.[14] Since plasma adiponectin and leptin levels are highly correlated with total body fat levels, we calculated adiponectin per kilogram fat mass and leptin per kilogram fat mass using total body fat mass from the dual-energy x-ray absorptiometry scan. Leptin:adiponectin ratio was calculated from the plasma leptin and adiponectin values. Insulin resistance was estimated using the homeostasis model for assessment of insulin resistance (HOMA-IR);[15] subjects were categorized as having abnormally high HOMA-IR when it was more than 2.86 (above the 75th percentile for HOMA-IR derived from the Third National Health and Nutrition Examination Survey).[7,16]
Anthropomorphic measures
At entry to the study, height, weight, and waist circumference (at the level of superior iliac crest to the nearest 0.1 cm) were measured using standard techniques. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). For our multivariate analysis, we coded BMI as a continuous variable. In order to further characterize differences in insulin resistance and adipokines among non-overweight, non-obese subjects compared to overweight and obese subjects, we dichotomized BMI as BMI<25 (non-overweight, non-obese) and BMI>=25 (overweight and obese). Of note, no subject had BMI<18.16. Three subjects with BMI 18.16 –18.49 were included in the non-overweight, non-obese category.
Radiographic measures
Abdominal CT
Computed tomography (CT) scans of the abdomen were performed on all subjects using an electron beam CT scanner (Imatron; General Electric, Milwaukee, Wisconsin) as previously described.[14] Standard procedures were used to obtain images, which were analyzed using specialized software (Tomovision, Montreal, Canada). A contiguous series of five to seven CT images between the L4–L5 and L3–L4 vertebral disc spaces were used to calculated visceral and subcutaneous abdominal fat mass.
Dual-energy x-ray absorptiometry scan (DXA)
A full-body DXA scan (Lunar DPX scanner, MEC, Minster, Ohio) was acquired on each subject and used to calculate total body fat mass, total body lean mass, percent body fat, and truncal-to-lower-body-fat ratio.
Analysis
We reported mean and standard deviation by gender and whether or not a subject had CRT in light of previous studies demonstrating differences in cardiovascular risk factors by these variables.[4–5,7–9,13] Wilcoxon rank sum test was used to test the covariate differences between females and males, between patients treated with CRT or not CRT according to gender, and between overweight and obese patients (BMI>=25) and non-obese, non-overweight patients (BMI<25), according to gender.
Multivariate regression models, controlling for history of CRT, race/ethnicity, age at diagnosis, and interval from cancer diagnosis, were used to examine the association between the natural logarithm transformation of the dependent variable (HOMA-IR) and the independent variables (leptin:adiponectin ratio, BMI, waist circumference, total body fat mass, total body lean mass, percent total body fat, truncal-to-lower-body-fat ratio and visceral adiposity) by gender. To compare the strengths of association between HOMA-IR and different covariates, we standardized the measurements by subtracting the mean and dividing by the standard deviation according to gender. Generalized estimation equations (GEE) were used to compare the regression coefficients associated with covariates using the methodology described by Pepe and colleagues. A Gaussian model with an identity link function and an independent working covariance matrix was used for this purpose. GEE models were built for females and males separately. R square was calculated by squaring the Pearson correlation coefficient from the simple regression models. To evaluate whether CRT modified the association between HOMA-IR and each of these independent variables, we included CRT and its interaction with each of the independent variables in separate models.
All statistical analyses were performed using STATA version 8 (College Station, Texas) and SAS version 9 (Cary, NC).
RESULTS
Insulin Resistance
Descriptive data are presented in Table II. Mean HOMA-IR was elevated in both males and females, indicating a high prevalence of insulin resistance, and did not differ by gender (P=0.56). Females exposed to CRT had higher mean HOMA-IR compared to females not exposed to CRT (5.2 vs. 3.7; P=0.01). Among males, those exposed to CRT had a higher mean HOMA-IR than males not exposed to CRT, but the difference was not statistically significant (CRT: HOMA-IR = 4.8, no CRT: HOMA-IR = 3.7; P=0.49).
Table II.
Hormones and fat measures in 116 survivors of childhood acute lymphoblastic leukemia, by gender and history of treatment with cranial radiotherapy (CRT).
| Entire Cohort | Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Females | Males | p | CRT | No CRT | p | CRT | No CRT | p | |
| N=64 | N=52 | N=24 | N=40 | N=15 | N=37 | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| HOMA-IR* | 4.3 (2.5) | 4.1 (2.5) | 0.56 | 5.2 (2.7) | 3.7 (2.1) | 0.01 | 4.8 (3.6) | 3.7 (2.0) | 0.49 |
| Leptin per kg fat (ng/mL/kg) | 0.7 (0.3) | 0.4 (0.2) | <0.01 | 0.9 (0.3) | 0.7 (0.3) | 0.1 | 0.5 (0.3) | 0.3 (0.2) | 0.01 |
| Adiponectin per kg fat (μg/mL/kg) | 0.6 (0.4) | 0.8 (0.7) | 0.75 | 0.4 (0.3) | 0.8 (0.5) | <0.01 | 0.6 (0.7) | 0.8 (0.6) | 0.02 |
| Leptin to adiponectin ratio | 2.2 (1.9) | 1.2 (1.9) | <0.01 | 3.0 (2.1) | 1.7 (1.7) | <0.01 | 2.2 (3.0) | 0.8 (1.1) | 0.01 |
| Truncal to lower body fat ratio | 1.2 (0.3) | 1.4 (0.4) | <0.01 | 1.4 (0.4) | 1.0 (0.3) | <0.01 | 1.6 (0.5) | 1.4 (0.3) | 0.04 |
| Total body fat (% mass) | 37.7 (7.5) | 23.6 (8.6) | <0.01 | 42.6 (4.9) | 34.6 (7.2) | 0.01 | 28.5 (6.3) | 21.7 (8.7) | 0.05 |
| Lean body mass (kg) | 43.7 (7.8) | 59.7 (9.1) | <0.01 | 42.3 (9.6) | 44.6 (6.5) | 0.22 | 56.8 (11.1) | 60.8 (8.1) | 0.21 |
| Waist: height ratio | 0.6 (0.1) | 0.5 (0.1) | <0.01 | 0.6 (0.1) | 0.5 (0.1) | <0.01 | 0.6 (0.1) | 0.5 (0.1) | 0.09 |
| Body mass index (kg/m2) | 28.7 (7.8) | 26.7 (5.6) | 0.21 | 31.7 (8.6) | 26.9 (6.8) | 0.01 | 27.8 (7.5) | 26.2 (4.5) | 0.54 |
| Waist circumference (cm) | 92.3 (16.6) | 90.8 (13.5) | 0.84 | 96.5 (16.7) | 89.9 (16.2) | 0.09 | 94.6 (16.3) | 89.2 (12.1) | 0.21 |
HOMA-IR: Homeostasis model of insulin resistance
Anthropomorphic measures and Adipokines
BMI, total body fat mass, and percent total body fat were higher, while total body lean mass was lower, among female subjects (P<0.01 for all comparisons; Table II). Leptin per kilogram fat mass and leptin: adiponectin ratio were also higher for females (P<0.01), although adiponectin per kilogram fat mass did not differ between males and females (P=0.75). Mean BMI was 31.7 among the female subjects who had received CRT, while it was 26.7 among those females who had not (P=0.01). CRT exposure was also associated with higher leptin per kilogram fat mass levels and higher measures of body fat, particularly among the female subjects (Table II). Male subjects with a history of exposure to CRT also had higher leptin, lower adiponectin, and worsened measures of body fat, but the magnitude of the differences was smaller and most comparisons were not significant (Table II).
Correlates of Insulin Resistance
As shown in Figure 2, the leptin:adiponectin ratio was significantly correlated with HOMA-IR level and the relationship differed by gender. In multivariate models adjusting for CRT, race/ethnicity, age at diagnosis, and interval from cancer diagnosis, the adipokines, BMI and measures of body fat including total body fat mass and visceral adiposity were highly correlated with HOMA-IR levels (Table III). The median HOMA-IR is 14% higher (100(e 0.13-1)%) per unit increase in leptin:adiponectin ratio. The relationship between leptin:adiponectin ratio and HOMA-IR did not differ by history of treatment with CRT; P values for the interaction terms were 0.22 and 0.13 for females and males, respectively. That is, although history of treatment with CRT was associated with higher leptin levels and a higher leptin:adiponectin ratio, and although CRT was associated with higher HOMA-IR levels, CRT did not modify the relationship between the adipokines and HOMA-IR.
Figure 2.
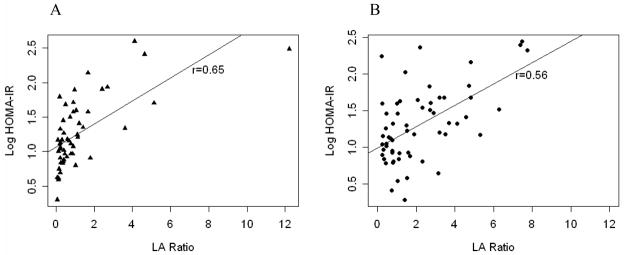
Insulin resistance as measured by log-transformed homeostasis model of insulin resistance (HOMA-IR) versus serum leptin:adiponectin ratio (LA Ratio) in 116 survivors of childhood leukemia, separated by gender: men (A) and women (B).
Table III.
Multivariate analyses of correlates of insulin resistance (estimated by the Homeostasis Model of Insulin Resistance) in 116 survivors of childhood acute lymphoblastic leukemia.
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| N=64 | N=52 | |||||||
|
| ||||||||
| Measurements | intercept | β | 95%CI | P† | intercept | β | 95%CI | P† |
| Leptin: adiponectin ratio | 0.93 | 0.13 | 0.06–0.19 | <0.01 | 0.55 | 0.17 | 0.10–0.23 | <0.01 |
| Body mass index (mg/kg2) | 0.44 | 0.04 | 0.02–0.05 | <0.01 | −0.14 | 0.05 | 0.04–0.07 | <0.01 |
| Waist circumference (cm) | 0.08 | 0.02 | 0.008–0.02 | <0.01 | −0.47 | 0.02 | 0.01–0.03 | <0.01 |
| Percent body fat (by DXA)* | −0.07 | 0.03 | 0.01–0.05 | <0.01 | 0.27 | 0.03 | 0.02–0.05 | <0.01 |
| Visceral adiposity (by CT)** | 1.02 | 1.98 | 1.23–2.73 | <0.01 | 1.15 | 1.46 | 0.82–2.01 | <0.01 |
| Lean body mass (kg) | 0.13 | 0.03 | 0.02–0.05 | <0.01 | 0.23 | 0.02 | 0.002–0.03 | 0.02 |
DXA: Dual-energy x-ray absorptiometry scan;
CT: Computed tomography;
P-value adjusting for Cranial radiation therapy, race/ethnicity, age at diagnosis, and interval from cancer diagnosis
Comparing Correlates of Insulin Resistance
Among women, we found no evidence of superiority of any correlate of insulin resistance. Among men, leptin:adiponectin ratio was more strongly associated with HOMA-IR than lean body mass (P = 0.04), but no other comparison was significant. After adjusting for history of treatment with CRT, ethnicity, age of diagnosis, and interval from cancer diagnosis, leptin:adiponectin ratio was more strongly associated with HOMA-IR than lean body mass, but the comparison lost significance (P = 0.08).
Overweight and Obesity
We examined levels of adipokines and HOMA-IR in overweight and obese subjects (BMI≥25) compared to non-obese, non-overweight subjects (BMI<25). HOMA-IR greater than 2.86 (above the 75th percentile for HOMA-IR derived from the Third National Health and Nutrition Examination Survey) was considered abnormal.[7,16] As expected, we found that leptin per kilogram fat mass was lower in non-obese, non-overweight subjects compared to obese and overweight subjects (Table IV). Non-obese, non-overweight male subjects had the lowest levels of leptin (mean 0.3, standard deviation 0.2). However, in this population of ALL survivors, even non-obese, non-overweight male subjects had mean HOMA-IR levels that met or exceeded normal levels.
Table IV.
Serum adipokines and insulin resistance by BMI* among 116 survivors of acute lymphoblastic leukemia, by overweight and obese (BMI 25 kg/m2) versus not (BMI <25 kg/m2).
| Females | Males | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| BMI<25 | BMI≥25 | p | BMI<25 | BMI≥25 | p | |
| N=21 | N=43 | N=20 | N=32 | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| HOMA-IR** | 2.8 (0.9) | 5.0 (2.7) | <0.01 | 3.0 (1.3) | 4.6 (2.9) | 0.01 |
| Leptin per kg fat mass (ng/mL/kg) | 0.6 (0.3) | 0.8 (0.3) | 0.04 | 0.3 (0.2) | 0.4 (0.2) | 0.14 |
| Adiponectin per kg fat mass (μg/mL/kg) | 1.1 (0.4) | 0.4 (0.2) | <0.01 | 1.3 (0.7) | 0.4 (0.3) | <0.01 |
| Leptin to adiponectin ratio | 0.7 (0.5) | 2.9 (2.0) | <0.01 | 0.4 (0.4) | 1.7 (2.3) | <0.01 |
BMI: body mass index;
HOMA-IR: Homeostasis model of insulin resistance
Insulin resistance
As the final stage in our examination of correlates of insulin resistance in this population, we compared subjects with HOMA-IR greater than or equal to 2.86 (“insulin resistant”) to subjects with HOMA-IR less than 2.86 (“not insulin resistant”) (Table V). We found differences in adipokines between the insulin resistant and not insulin resistant populations. When we divided subjects by gender, a pattern of higher mean leptin per kilogram fat mass and lower mean adiponectin per kilogram fat mass was seen in the insulin resistant subjects compared to the not insulin resistant subjects. This pattern was maintained in the overweight and obese subjects but lost significance in non-obese, non-overweight subjects (Table V). In other words, the derangement of the leptin-adiponectin system associated with insulin resistance in subjects with BMI≥25 is not seen in subjects with BMI<25. While this difference may be due to sample size considerations, these results suggest that another mechanism may be at work in this insulin resistant, non-obese, non-overweight population of ALL survivors.
Table V.
Serum adipokines in 116 survivors of acute lymphoblastic leukemia by gender, by overweight and obese (BMI* ≥ 25 kg/m2) versus not (BMI < 25 kg/m2), and by insulin resistant (HOMA-IR ≥ 2.86)** versus not (HOMA-IR < 2.86).
| Females | p | Males | p | BMI < 25 kg/m2 | p | BMI ≥ 25 kg/m2 | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOMA-IR
|
HOMA-IR
|
HOMA-IR
|
HOMA-IR
|
|||||||||
| < 2.86 | ≥ 2.86 | < 2.86 | ≥ 2.86 | < 2.86 | ≥ 2.86 | < 2.86 | ≥ 2.86 | |||||
| N=23 | N=41 | N=23 | N=41 | N=22 | N=18 | N=20 | N=54 | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Leptin per kg fat (ng/mL/kg) | 0.7 (0.3) | 0.8 (0.3) | 0.1 | 0.3 (0.2) | 0.4 (0.2) | 0.01 | 0.5 (0.3) | 0.5 (0.3) | 0.9 | 0.5 (0.3) | 0.7 (0.4) | 0.03 |
| Adiponectin per kg fat (μg/mL/kg) | 0.6 (0.5) | 0.5 (0.4) | <0.01 | 1.1 (0.8) | 0.5 (0.5) | <0.01 | 1.3 (0.6) | 1.0 (0.5) | 0.2 | 0.6 (0.2) | 0.3 (0.2) | <0.01 |
| Leptin to adiponectin ratio | 1.0 (0.7) | 2.8 (2.1) | <0.01 | 0.4 (0.4) | 1.7 (2.3) | <0.01 | 0.5 (0.6) | 0.6 (0.4) | 0.4 | 1.0 (0.7) | 2.9 (2.4) | <0.01 |
BMI: body mass index;
Homeostasis model of insulin resistance (HOMA-IR) ≥ 2.86 indicates above the 75th percentile for HOMA-IR derived from the Third National Health and Nutrition Examination Survey.(15)
DISCUSSION
In this cross-sectional study of 116 survivors of childhood ALL, leptin levels were higher among females and those who had received CRT. Adiponectin closely mirrored insulin resistance, while leptin reflected insulin resistance as well as anthropomorphic, DXA, and CT measures of body fat. Furthermore, insulin resistance was nearly uniformly present regardless of gender or treatment variables and was found in non-obese, non-overweight subjects.
In a series of recent papers, Moschovi and colleagues described leptin and adiponectin levels during diagnosis and treatment of childhood leukemia and lymphoma.[17–19] In their study of children with acute lymphoblastic leukemia, nine patients with ALL aged 2 to 7 years were found to have low adiponectin and high leptin levels at diagnosis; adipokines gradually moved towards non-cancer control levels during treatment.[18] When 121 subjects with Non-Hodgkin Lymphoma subjects were examined, higher adiponectin levels at baseline was associated with worse prognosis.[17] No prior study, however, has examined adipokines and their relationship to insulin resistance and measures of body fatness in survivors of acute leukemia.
Our findings contribute to a better understanding of how insulin resistance develops in long-term survivors of childhood ALL. Female gender and history of treatment with CRT were associated with higher leptin levels, lower adiponectin levels, and increased body fat as measured by BMI, waist circumference, DXA and abdominal CT, when compared to male gender and those without a history of treatment with CRT. Leptin, which communicates primarily with the brain regarding energy usage, was strongly correlated with insulin resistance in our overweight and obese subjects. We hypothesize that ALL or its therapy may result in a form of central leptin resistance so that, instead of processing a signal to burn energy, these subjects inappropriately store energy. Central leptin resistance in this population could be caused by damage to the hypothalamus itself, disruption of communication between the blood and the hypothalamus, or down-regulation of hypothalamic leptin receptors.
Our results support a mechanism for insulin resistance in this population that is in some part independent of BMI. Among subjects with BMI less than 25 (non-obese, non-overweight subjects), mean HOMA-IR was above normal levels, but leptin levels did not differ by HOMA-IR. While survivors of ALL are overweight and obese, on average, and are insulin resistant, on average, these two findings may not be completely interdependent. Further research among lean ALL survivors is needed.
Our study has some limitations. As noted, we conducted a cross-sectional analysis, so that causality cannot be inferred. Additionally, we do not have measures of body fatness and insulin resistance that pre-date the diagnosis of ALL in this population. Prior study of this population of ALL survivors has shown them to be more obese and more insulin resistant than non-cancer controls, however.[7] Furthermore, our population of long-term ALL survivors is one of the largest that has been studied, and no prior examination of adipokines and insulin resistance in ALL survivors has been reported.
In conclusion, in this study of 116 survivors of childhood ALL, we have shown derangements in adipokines which may help explain the body composition changes and insulin resistance commonly seen in this population. Furthermore, we describe insulin resistance and serum leptin changes even among non-overweight, non-obese subjects. Our findings suggest that anthropomorphic and metabolic changes many years after ALL treatment remain a major health problem facing survivors and may be related to central leptin resistance.
Acknowledgments
Support: This work was supported by research grants from the National Institutes of Health (R01-CA-100474), the Howard J. and Dorothy Adleta Foundation, and the Donald W. Reynolds Cardiovascular Research Center at Dallas, and the General Clinical Research Center (Grant M01-RR-00633 and CTSA UL1-RR-024982).
Footnotes
Presented in oral abstract form at the 11th International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer. June 11, 2010; Williamsburg, Virginia.
References
- 1.Tonorezos ES, Oeffinger KC. Survivorship after childhood, adolescent, and young adult cancer. Cancer J. 2008;14(6):388–395. doi: 10.1097/PPO.0b013e31818f5aba. [DOI] [PubMed] [Google Scholar]
- 2.LAG R, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Insitute; 1975–2003. [Accessed October 5, 2009]. < http://seer.cancer.gov/csr/1975_2003/>. [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Eng J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 5.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107(6):1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 6.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4635. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(22):3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kourti M, Tragiannidis A, Makedou A, et al. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol. 2005;27(9):499–501. doi: 10.1097/01.mph.0000181428.63552.e9. [DOI] [PubMed] [Google Scholar]
- 9.Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 10.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169(15):1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller D, Flier J. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 12.Lustig RH. Autonomic dysfunction of the beta-cell and the pathogenesis of obesity. Rev Endocr Metab Disord. 2003;4(1):23–32. doi: 10.1023/a:1021819318484. [DOI] [PubMed] [Google Scholar]
- 13.Askari H, Tykodi G, Liu J, et al. Fasting plasma leptin level is a surrogate measure of insulin sensitivity. J Clin Endocrinol Metab. 2010;95(8):3836–3843. doi: 10.1210/jc.2010-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janiszewski PM, Oeffinger KC, Church TA, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92(10):3816–3821. doi: 10.1210/jc.2006-2178. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2960–2965. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 17.Petridou ET, Sergentanis TN, Dessypris N, et al. Serum adiponectin as a predictor of childhood non-Hodgkin’s lymphoma: a nationwide case-control study. J Clin Oncol. 2009;27(30):5049–5055. doi: 10.1200/JCO.2008.19.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moschovi M, Trimis G, Vounatsou M, et al. Serial plasma concentrations of adiponectin, leptin, and resistin during therapy in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32(1):e8–13. doi: 10.1097/MPH.0b013e3181b8a50c. [DOI] [PubMed] [Google Scholar]
- 19.Petridou ET, Dessypris N, Panagopoulou P, et al. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr Blood Cancer. 2010;54(2):311–315. doi: 10.1002/pbc.22294. [DOI] [PubMed] [Google Scholar]


