Abstract
Background and Purpose
Cytochrome P450 epoxygenase metabolites of arachidonic acid (EETs) have multiple cardiovascular effects, including reduction of blood pressure, protection against myocardial ischemia-reperfusion injury, and attenuation of endothelial inflammation and apoptosis. The present study was aimed to determine potential neuroprotective roles for EETs in cerebral ischemia.
Methods
Transgenic mice with endothelial overexpression of CYP2J2 (Tie2-CYP2J2-Tr) were subjected to global cerebral ischemia induced by bilateral common carotid artery occlusion (BCCAO) for 10 minutes, Cerebral EET production, infarct size, and apoptosis were examined after 24 hours of reperfusion. The action mechanisms of EETs on cerebral ischemia was also studied in cultures of astrocytes and Neuro-2a cells exposed to oxygen-glucose deprivation (OGD).
Results
In Tie2-CYP2J2-Tr mice, CYP2J2 expression and 14, 15-EET production in both brain tissue and plasma significantly increased while brain infarct size and apoptosis after ischemia decreased, accompanied increased activation of the PI3K/AKT and ERK1/2 pathways, decreased activation of JNK, and higher ratios of Bcl-2/Bax and Bcl-xl/Bax in ischemic brain compared to wild type mice. In cells, addition of exogenous EETs or CYP2J2 transfection attenuated OGD-induced apoptosis by activation of ERK1/2 and PI3K/AKT pathways, inhibition of JNK, which were reduced by pretreatments with inhibitors of the PI3K (LY294002), the MAPK (PD98059) and EETs (EEZE), respectively.
Conclusions
We conclude that CYP2J2 overexpression exerts marked neuroprotective effects against ischemic injury by a mechanism linked to increased level of circulating EETs and reduction of apoptosis. These data suggests the possibility for clinical therapy of cerebral ischemia by enhancing EET levels.
Keywords: arachidonic acid, cytochrome P450 epoxygenase, global cerebral ischemia, neuroprotection, stroke
INTRODUCTION
Arachidonic acid (AA) is a polyunsaturated fatty acid normally found esterified to cell membrane glycerophospholipids. AA can be released by phospholipases in response to many stimuli such as ischemia 1. Free AA is then available for metabolism by cyclooxygenases, lipoxygenases and cytochrome P450 (CYP) monooxygenases to generate numerous metabolites, collectively termed eicosanoids 2, 3. CYP epoxygenases metabolize AA to four biologically active, regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12- and 14,15-EETs). EETs synthesized in cells are hydrolyzed to the corresponding and less biologically active dihydroxyeicosatrienoic acids (DHETs) by epoxide hydrolases. Previous work has demonstrated that soluble epoxide hydrolase (sEH or EPHX2) is the main enzyme involved in the in vivo hydrolysis of the EETs. Thus, changes in the expression and/or activity of specific CYP epoxygenase or epoxide hydroxylase enzymes can alter the delicate balance between EETs and DHETs 4.
EETs can induce multiple signal transduction pathways to produce a variety of effects in many different tissues 4. In the endothelium, EETs have anti-inflammatory and anti-apoptotic actions through activation of a PI3K/AKT, ERK1/2 and endothelial nitric oxide synthase (eNOS) 5, 6. Either exogenous EET application or cardiomyocyte-specific CYP2J2 overexpression enhance cardiac functional recovery and decrease infarct size after ischemia and reoxygenation 7.
Cerebral ischemia or stroke is a major cause of death and disability of adults in worldwide, especially in China 8, 9. The factors and mechanisms of cerebral tissue damage after ischemia are very complex. Mounting evidence supports the fact that apoptosis of cells in brain may be a major contributor to the injury which occurs following cerebral ischemic injury and PI3K/AKT plus MAPK/Erk1/2 signaling pathways play a critical role in the protection of cultured cerebral cortical astrocytes against ischemic injury 10.
In the brain, EETs are synthesized by astrocytes through a mechanism that is linked to mGluR and adenosine A (2B) receptors 11. EETs also reduce brain ischemia and infarct size in stroke 2, 12. In the brain, EETs play an important role in cerebral blood flow (CBF) regulation and neurovascular coupling 11, 13. Furthermore, ischemic preconditioning increases the expression of P450 epoxygenases in brain, and protects against ischemic stroke induced in rat by middle cerebral artery occlusion (MCAO). More recently, it had been demonstrated that EETs protect neurons 14 and astrocytes 15 against ischemic cell death induced in vitro by oxygen-glucose deprivation (OGD). Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia in the absence of changes in CBF suggesting that EETs exert a cytoprotective effect independent of blood vessel dilation 2, 16, 17.
In humans, a major enzyme involved in the production of EETs is the CYP2J2 epoxygenase which preferentially metabolizes AA to 11,12- and 14,15-EETs. Transgenic mice with cardiomyocyte-specific overexpression of CYP2J2 demonstrated improvement in heart functional recovery and decreased infarct size after ischemia/reperfusion injury 7, 18, 19. This data suggests a potentially promising role for CYP2J2 in cerebral ischemia/reperfusion. We hypothesized that mice with endothelial overexpression of CYP2J2 would have increased cerebral vascular EET biosynthesis and this would lead to reduced apoptosis and less infarction after global brain ischemia. In the current study, we subjected mice with endothelial overexpression of CYP2J2 (Tie2-CYP2J2-Tr) and wild type (WT) control mice to sham operations or bilateral common carotid artery occlusion (BCCAO). We compared CYP2J2 protein expression, DHET levels, infarct size, and various signaling pathways in WT and Tie2-CYP2J2-Tr mice. Furthermore, in the cultures of primary cortical astrocytes and Neyro-2a cells subjected to oxygen-glucose deprivation (OGD) with exogenous addition of EETs or CYP2J2 overexpression, we confirmed protective effects of EETs and identified related signaling pathways. Our findings suggest that endothelial CYP2J2 expression is protective against ischemic brain injury. This protection is linked to the increased generation of EETs and activation of pro-survival signaling pathways, including ERK1/2 and PI3K/AKT.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s nutrient mixture F-12 (F-12) medium, DMEM medium and fetal bovine serum were purchased from Gibco BRL (Grand Island, NY, USA). PI3K, Phosphor-p42/p44 ERK, phosphor-JNK, and phosphor-(Thr308)-Akt antibodies were from Cell Signaling (Beverly, MA). Akt, JNK, Bcl-2, Bcl-xl, Bax, c-Jun and phosphor-c-Jun were from Santa Cruz (Santa Cruz, CA). Antibody against CYP2J2 was purchased from Abcam Inc (Cambridge, MA), Horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti- mouse IgG and goat anti-rabbit IgG) were purchased from KPL (Gaithersburg, MA). Polyvinylidene difluoride (PVDF) membranes, prestained protein markers, and SDS-PAGE gels were from Bio-Rad, Inc. (Hercules, CA). 8, 9-, 11, 12- and 14, 15-EET were purchased from Sigma Chemical Co. (St. Louis, MO). PI3K inhibitor-LY294002 and ERK inhibitor-PD98059 were from Cayman Chemical Co. (Ann Arbor, MI), EET inhibitor EEZE was gift from Dr. J.R. (Camille) Falck (University of Texas Southwestern Medical Center), Bicinchoninic acid (BCA) protein assay reagent was from Pierce (Rockford, IL). Enzymes and other chemicals were from Sigma (St. Louis, MO).
Animal preparation
Mice with Tie2 promoter-driven, endothelial-specific CYP2J2 transgene overexpression were generated at NIEHS/NIH on a pure C57BL/6 background as described 20. Transgenic mice were identified by two polymerase chain reactions using tail genomic DNAs 21, 22. All studies used heterozygous Tie2-CYP2J2-Tr mice and age/sex-matched WT littermate control mice. All studies were performed in accordance with principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Mice were housed in an isolator caging system in air-conditioned animal room at room temperature. All experimental procedures described were approved by the Experimental Animal Research Committee of Tongji Medical College, Huazhong University of Science and Technology.
Furthermore, we evaluated whether selective inhibitor of CYP2J2, compound 26 (C26), blocked EETs production and attenuated the protective effect of CYP2J2 overexpression on cerebral infarction in BCCAO. C26 dissolved in dimethyl sulfoxide was administered orally to CYP2J2-Tr mice (n=6) for 14 days at a dose of 0.25 mg/kg/day before BCCAO 23.
Bilateral common carotid artery occlusion model in mice
Transient global cerebral ischemia (GCI) was induced in adult male mice (4 to 8 weeks old, 20 to 25 g body weight) by bilateral common carotid artery occlusion (BCCAO) as previously described 14, 24–27. Briefly, mice were deeply anesthetized with 2% sodium pentobarbital (50 mg/kg body weight). A femoral artery was cannulated with a polyethylene tube (PE-10) to monitoring blood pressure. Body temperature was strictly regulated at 37°C for the duration of the procedure. A midline cervical incision was made and both common carotid arteries were exposed. Both common arteries were isolated using 4/0 silk thread, taking care not to damage the vagus nerve. After a 3-minute stabilization period, both arteries were occluded using microaneurysm clips applied bilaterally for 10 minutes. Both clips were then removed and restoration of blood flow was confirmed before the incision was sutured closed. After surgery, mice were placed in an incubator (28°C) for 1 hour before being returned to the standard animal housing unit. Exposure of bilateral common carotid arteries without BCCAO was used in sham-control animals. Equal numbers of WT and CYP2J2 mice were randomly operated on the same day.
Evaluation of cerebral infarction
After BCCAO, mice were observed and allowed to recover for 24 hours. Infarct size was measured in 2-mm thick coronal brain sections (5 total) using 2, 3, 5-triphenyltetrazolium chloride (TTC) staining and digital image analysis as previously described 2, 14. Briefly, after reperfusion, animals were reanesthetized by intraperitoneal injection of 2% sodium pentobarbital, and brains were quickly removed and frozen for 20 minutes at −20°C. Coronal slices (2 mm in thickness) were prepared from the frozen brain, incubated in 2% TTC in PBS for 30 minutes at 37°C, and then fixed in 4% formalin for 4–6 hours. The areas of infarcted (white) and uninfarcted (red) were quantified with MCID software (InterFocus) for each slice. The volumes of infarcted and noninfarcted brain were calculated by multiplying the area times the 2 mm slice thickness. Infarct size was expressed as the percentage of infarcted tissue relative to total brain tissue.
Protein extraction and western blotting
Protein extraction was performed as described previously with some modification 1, 28. 50–60mg samples were obtained from the ischemic brain tissue and incubated in lysis buffer (50 mM Tris-Cl, pH 7.6, 5 mM EDTA, 100 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 5% NP-40, 1mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin) for 30 minutes on ice. After the incubation, the brain tissue was homogenized and cleared by centrifugation at 12,000 × g at 4°C for 30 minutes. The protein concentration of the supernatant was determined using the Bradford method to ensure equal loading. Protein samples (30µg) were separated by SDS-polyacrylamide gel electrophoresis, transferred to PVDF membranes and blocked with 5% nonfat dry milk in TBS-T (10 mM Tris-Cl, pH 7.5, 100 mM NaCl, 0.1% Tween 20). Blots were incubated at 4°C overnight with the primary antibodies (1:2000 dilution), washed and incubated with peroxidase-conjugated secondary antibodies for 2–3 hours. The ECL system was used to visualize the separated proteins. Autoradiograms were scanned and band optical densities quantified with QuantityOne software (BioRad). Blots were stripped and reprobed with antibodies to β-actin or respective non-phosphorylated kinases as a loading control.
14, 15-DHET ELISA
14,15-DHET, the stable metabolite of 14,15-EET, was measured in plasma using a commercial ELISA kit (Detroit R&D, Inc, Detroit, Mich) as described previously 2, 14. Briefly, plasma was extracted 3 times with equal volume of ethyl acetate before acidification at room temperature for 18 hours with glacial acetic acid (pH 3 to 4). Samples were dried and extracted 3 times with ethyl acetate and resuspended in DMF. 14, 15-DHET concentrations were measured according to the manufacturer’s instructions. The ELISA was also used to measure levels of 14, 15-DHET in brain homogenates.
TUNEL staining for apoptosis evaluation
Apoptosis was determined in situ by terminal deoxynucleotidyl transferase (TdT)–mediated dUTP-biotin nick end-labeling (TUNEL) staining of fragmented DNA 29. Paraffin sections of ischemic brain were processed for histologic evaluation of neuronal injury. Deparaffinized and rehydrated sections were treated with 20 mg/ml proteinase K for 15–30 minutes at 37°C and then with 3% hydrogen peroxide in methanol for 10 minutes at room temperature. The sections were incubated in the TdT reaction solution for 1 hour at 37°C and immersed in streptavidin-HRP at 37°C for 30 minutes, followed by reaction with diaminobenzidine and hydrogen peroxide for 10 minutes. Sections were counterstained with hematoxylin for 30 minutes.
Cell culture
Primary astrocyte and Neuro-2a cells were maintained in DMEM/F12 and DMEM supplemented with 10% FBS, 2 mM glutamine, and penicillinstreptomycin, respectively. The mouse neuroblastoma cell line, Neuro-2a was obtained from American Type Culture Collections (ATCC) (Rockville, MD, USA), while Rat primary astrocytes were prepared as previously described with minor modifications 30, 31. Briefly, forebrains of newborn Sprague-Dawley rats were removed aseptically from the skulls, freed of the meninges, dissociated by trypsinization and mechanically disrupted in DMEM/F12 containing penicillin (100U/ml) and streptomycin(100 µg/ml). The cells were seeded into poly-L-lysine-coated culture flasks. After in vitro culture for 10 days, highly purified astrocytes were isolated by shaking of the culture flasks for 48h by rotary shaker (150 rpm) at 37 °C with the floating cells discarded and reseeded into 6-well plates for assays. The purity of astrocyte cultures was greater than 90%, as determined by glial fibrillary acidic protein (GFAP) immunofluorescence staining. The passage 2–4 cells were used in all experiments.
Hypoxia–reoxygenation model and treatments of cultured cells
Oxygen-glucose deprivation (OGD) is established and used according to the reported method 32. Briefly, plated cells were grown for 24 h in culture to reach 80–90% confluence in an environment of 5% CO2–95% air (balanced nitrogen and 85% humidity). For experiment groups, culture plates were given fresh DMEM medium without glucose and serum and placed in a gas-tight humidified chamber flushed with 1% O2 -5% CO2-95% N2 at 37°C. After 8 h of hypoxia, the media of all the plates were changed and the cells were recovered in normal conditions for the next 24 h. The cells were pretreated with various inhibitors, LY494002 (10 µM), PD98059 (50 µ M), and EEZE (100 nmol) for 60 min prior to the addition of EET (100 nmol), which were applied 60 min before OGD to the end of experiments. Normal control cells underwent the same procedures except for OGD. The cultures were used for Western Blot analysis and assay of caspase-3 activity
Cell survival via trypan blue staining
Primary astrocytes and Neuro-2a (3×105) were seeded in six well plates. Cells were trypsinized and then stained with 0.4% trypan blue after OGD. Vital cells (white) and dead cells (blue) were counted and a minimum of 100 cells per count were analyzed 33.
Recombinant Adeno-Associated Virus and Gene Transfection
The recombinant adeno-associated virus (rAAV) vector was used to pack into rAAV containing CYP2J2 cDNA (rAAV-2J2) as described previously 3. Neuro-2a was infected with rAAV-CYP2J2 or rAAV-GFP (~50 virions/cell) in six-well plates in triplicate and cultured for one week to obtain maximal expression, the percent of cells infected by rAAV-GFP was over 60% according to routine microscopic observation 5.
Apoptosis assay by flow cytometric assay
To further identify the effect of CYP2J2 overexpression on apoptosis of Neuro-2a, we analyzed cell apoptosis after treatment with EEZE and after infection with rAAV-2J2 3. After OGD and EEZE added as above, transfected cells were resuspended and stained with fluoresce in isothiocyanate-conjugated annexin V and fluorescent dye propidium iodide (PI) and analyzed by flow cytometry (FACS, Vantage, BD, USA). The relative number in apoptotic cells was calculated as a percentage in rAAV-2J2 or rAAV-GFP infected cells with or without EEZE.
Assay of Caspase-3 Activity
The activity of caspase-3 was determined using a colorimetric protease assay kit ((R&D System, UK) 34. Cell lysates were prepared, lysed and centrifugated at 10,000 g for 1 min. A proteolytic reaction was carried out in a reaction buffer containing 50 µg of cytosolic protein extract and 200 µM of N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA). The reaction mixture was incubated at 37°C for 2 h and the formation of p-nitroanilide (pNA) was measured at 405 nm using a microtiter plate reader. The level of caspase-3 activity, proportional to the colorreaction intensity was expressed as a percentage of control.
Statistical Analysis
All values are expressed as mean ± SEM. Differences in infarct size, DHET levels and blood pressure were analyzed with a t-test for two groups. Analysis of variance (ANOVA) followed by post hoc Newman-Keuls multiple range tests was used for multiple groups. Significance was defined as p≤0.05 in all statistical analyses.
Results
CYP2J2 overexpression in transgenic mouse brain
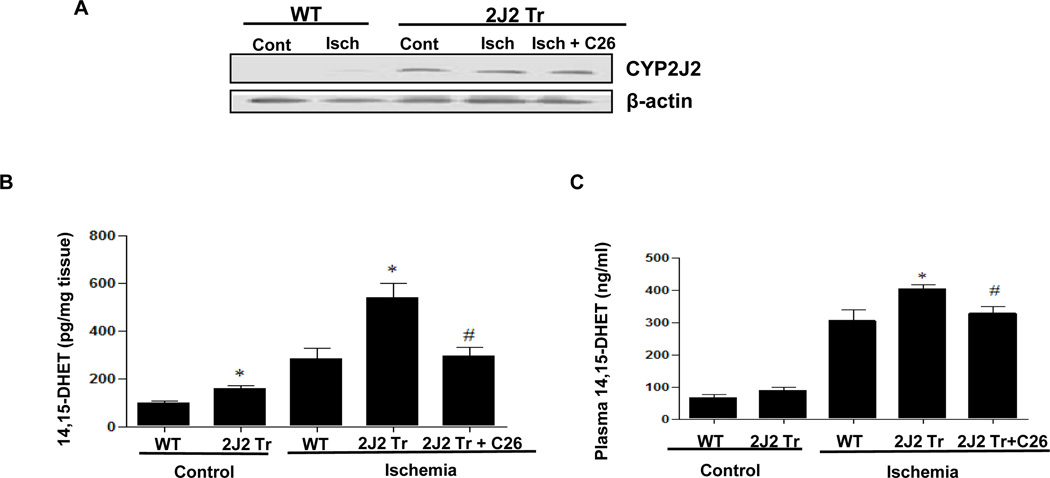
We previously reported the generation of Tie2-CYP2J2-Tr mice with endothelial overexpression human CYP2J2 20. Endothelial cells from these mice have increased EETs levels, and this leads to vasodilation and reduced blood pressure after angiotensin II treatment 20. To examine transgene expression in the brains of WT and Tie2-CYP2J2-Tr mice, we performed immunoblotting on brain homogenates using a selective antibody to human CYP2J2. A prominent band corresponding to human CYP2J2 was detected at approximately 55 kDa in the Tie2-CYP2J2-Tr mice but not in WT mice. These data confirm overexpression of the CYP2J2 transgene in Tie2-CYP2J2-Tr mouse brain. Brain expression of the CYP2J2 transgene was not altered after ischemia and administration of C26 did not affect protein expression of CYP2J2 (Fig. 1A and Supplementary Fig. 1A), which was consistent with previous report 23.
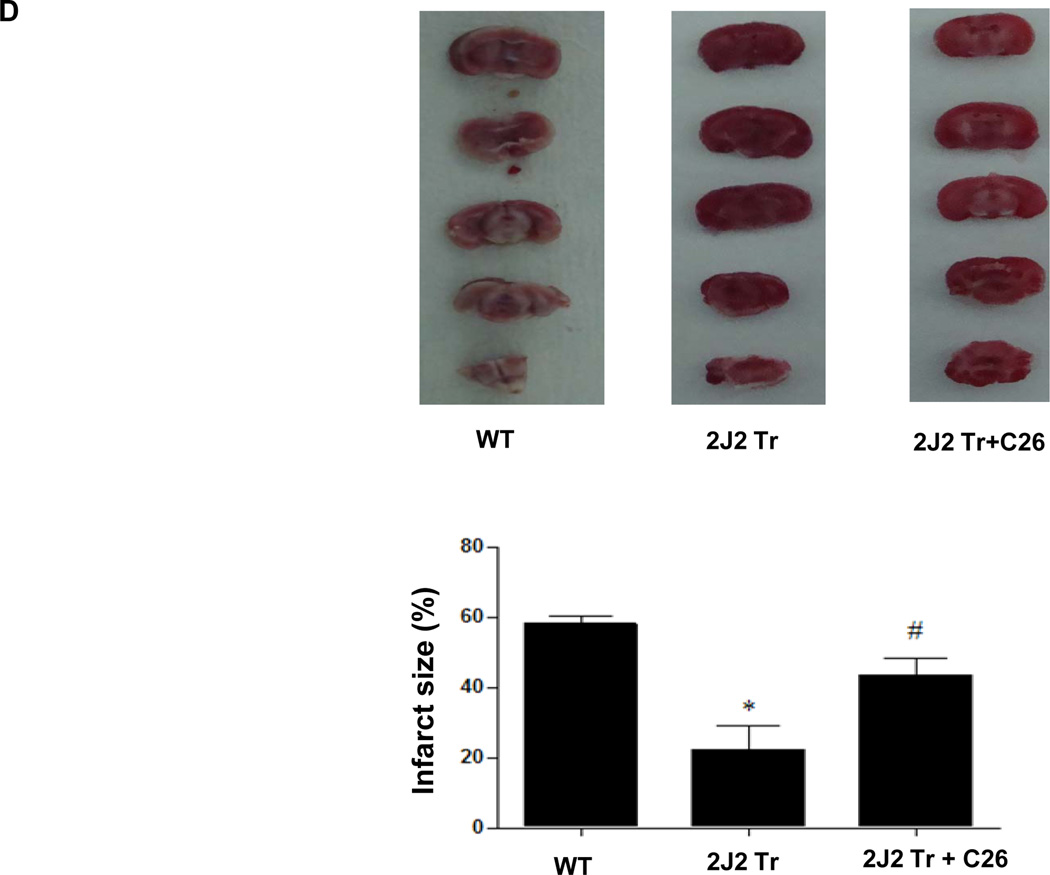
Figure 1.
Tie2-CYP2J2-Tr mice have overexpressed CYP2J2, increased 14, 15-DHET levels in brain and plasma and smaller brain infarcts than WT mice after BCCAO with or without C26. (A) Immunoblotting of brain lysates from WT and Tie2-CYP2J2-Tr (2J2-Tr) mice under control (sham) conditions and after BCCAO (ischemia) with or without C26 with the rabbit anti-human CYP2J2 antibody. WT brain lacks CYP2J2 immunoreactivity, while a prominent 55-kDa band is observed in brains of Tie2-CYP2J2-Tr mice and C26 cannot change the expression of Tie2-CYP2J2. (B, C) 14,15-DHET levels are significantly higher in brain homogenates (panel B) and Plasma (panel C) of Tie2-CYP2J2-Tr (2J2-Tr) mice compared to WT mice under both control and ischemic conditions while C26 decreased 14,15-DHET levels in Tie2-CYP2J2-Tr mice. (D) Representative TTC–stained brain slices from WT mice (left) and Tie2-CYP2J2-Tr (2J2 Tr) mice with (right) or without C26 (middle) after global ischemia and 24 hours reperfusion. White area indicates infarction and red area represents viable brain tissue. Infarct size (panel B) was significantly smaller in Tie2-CYP2J2 Tr mice than in WT mice. C26 increased the infarct size reduced in Tie2-CYP2J2 Tr mice. Results are representative of three independent experiments. N=6 mice per group, *p<0.05 vs. WT of same treatment group; #p<0.05 vs. Tie2-CYP2J2 under BCCAO group.
14, 15-DHET levels in brain and plasma
Ischemia resulted in increased levels of 14, 15-DHET in WT mouse brain and plasma compared to control (sham). Brain 14, 15-DHET levels were significantly higher in Tie2-CYP2J2-Tr mice than in WT mice under both control and post-ischemic conditions (176 ± 30 pg/mg in Tie2-CYP2J2-Tr mice vs. 79 ± 11 pg/mg in WT mice under control conditions, p<0.05; 537 ± 60 pg/mg in Tie2-CYP2J2-Tr mice vs. 283 ± 40 pg/mg in WT mice under ischemic conditions, p<0.05) (Fig. 1B and Supplementary Fig 1B). Plasma levels of 14, 15-DHET were also increased in Tie2-CYP2J2-Tr mice compared to WT mice after ischemia (404 ± 11 ng/ml vs. 305 ± 33 ng/ml, p<0.05) (Fig. 1C and Supplementary Fig 1C), and, as expected, C26 caused a significant decrease in the level of 14, 15-DHET either in brain or plasma under ischemic conditions (295 ± 36 pg/mg vs. 537 ± 60 pg/mg in brain, p<0.05; 326 ± 24 ng/ml vs. 404 ± 11 ng/ml, p<0.05 in plasma) (Fig. 1B and 1C), which indicated C26 reduce production of DHET by inhibiting CYP2J2. These data indicate that the Tie2-CYP2J2-Tr mice have increased brain AA epoxygenase activity after ischemia.
Evaluation of cerebral infarction after BCCAO
Transient global cerebral ischemia was induced in Tie2-CYP2J2-Tr and WT mice by BCCAO and the amount of viable and infarcted brain tissue was estimated using 2,3,5-triphenyltetrazolium chloride (TTC) staining. The amount of infarcted brain was less in Tie2-CYP2J2-Tr mice than in WT mice. Likewise, the percentage of infarcted brain tissue was significantly less in Tie2-CYP2J2-Tr mice compared to WT mice after BCCAO (23 ± 6% vs. 55 ± 5%, p<0.05) and this effect was attenuated by oral administration of C26 in Tie2-CYP2J2-Tr mice (23 ± 6% vs. 43 ± 3%, p<0.05) (Fig. 1D). These data indicate that Tie2-CYP2J2-Tr mouse brains are protected from infarction after global cerebral ischemia, which consistant with previous results (Supplementary Fig. 1D) and the inhibition in EETs production (Fig. 1B and 1C), suggesting the inhibition of CYP2J2 abolished the protective effect of CYP2J2 overexpression on infarction after cerebral ischemia.
Effect of CYP2J2 overexpression on PI3K/AKT and MAPK signaling pathways after BCCAO
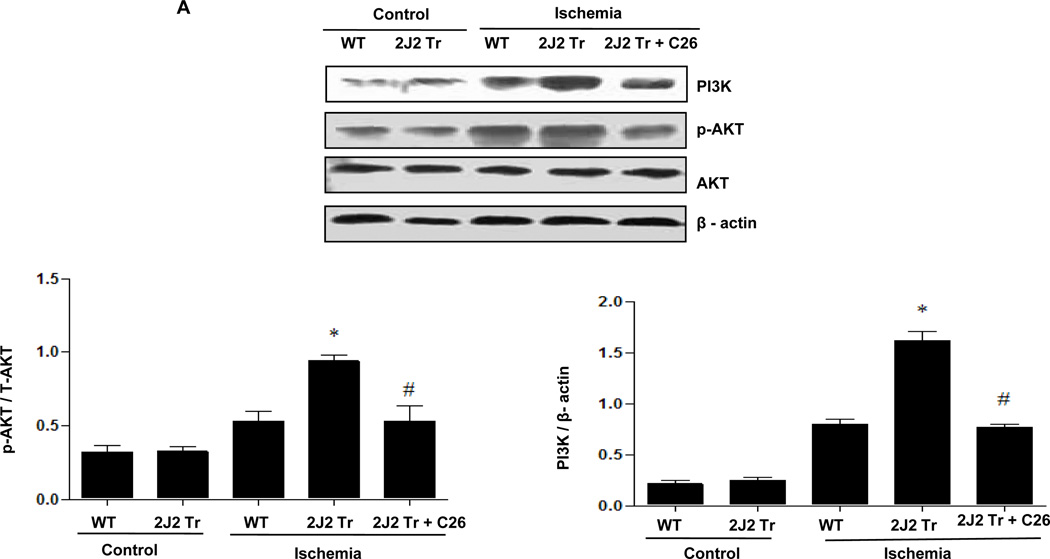
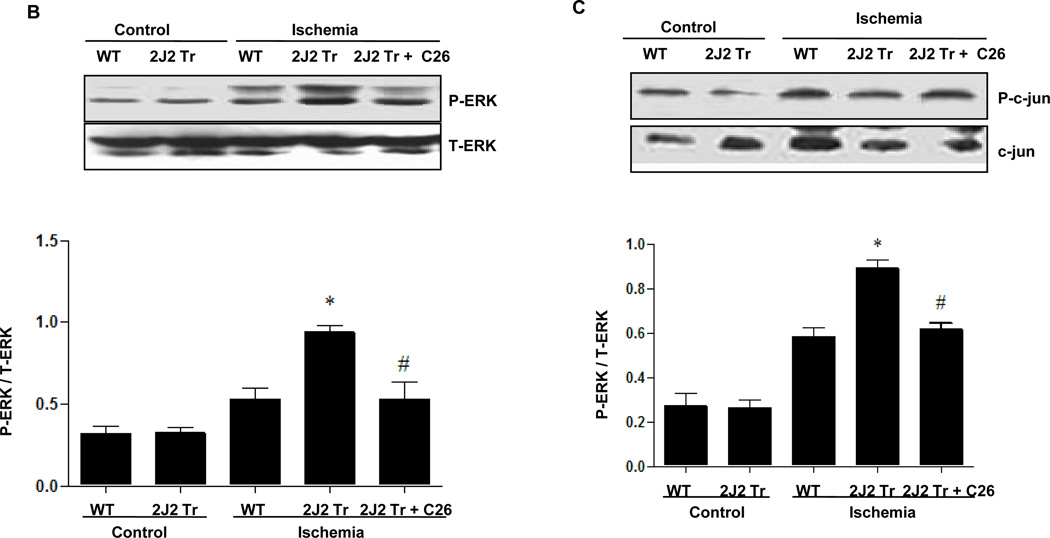
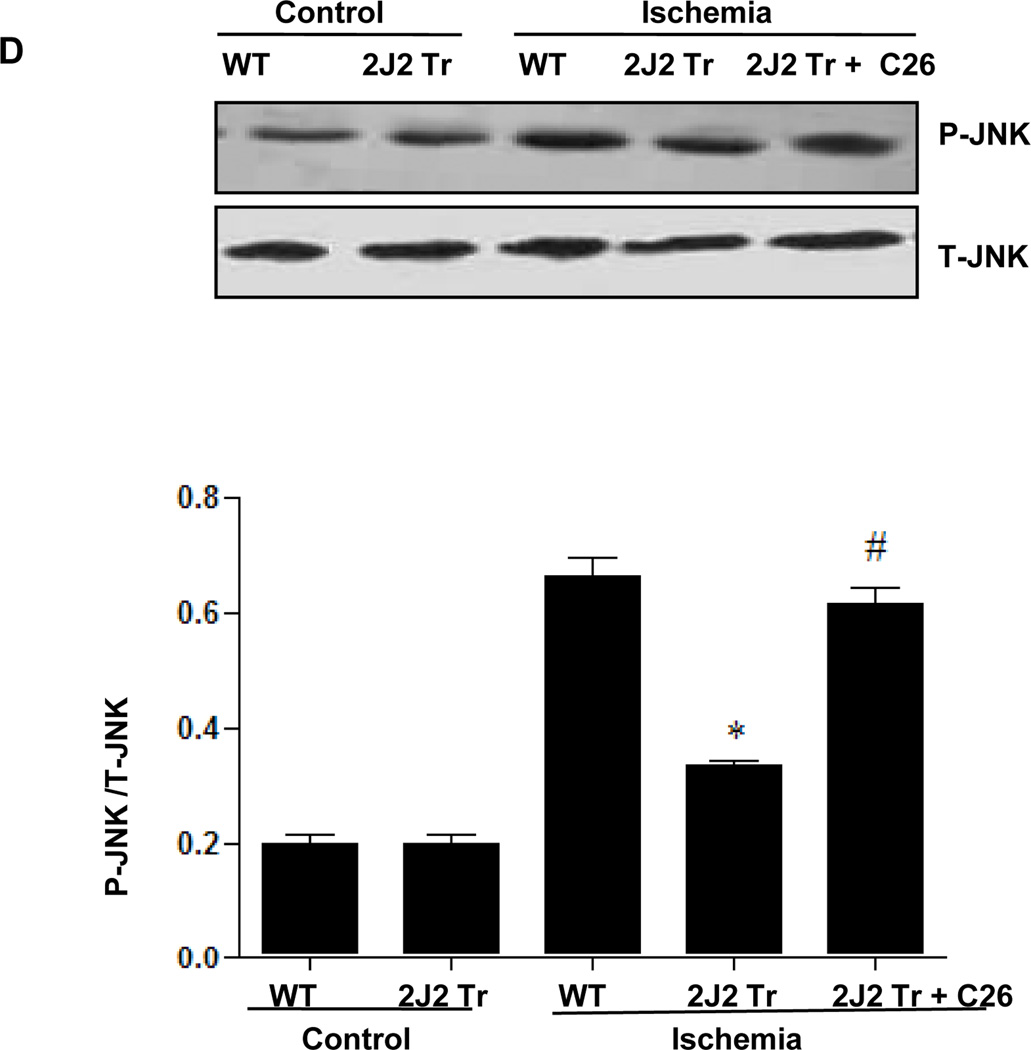
To investigate the mechanisms through which CYP2J2 overexpression protects against cerebral infarction, we examined activation of MAPK and PI3K/AKT signaling pathways after BCCAO. Protein extracts from hippocampus were used for immunoblotting analysis. BCCAO increased phosphorylation of AKT and PI3K expression compared to control in WT mouse brains (Fig. 2A and Supplementary Fig. 2A). Interestingly, CYP2J2 overexpression enhanced AKT activation and PI3K expression after ischemia (p<0.05). ERK1/2 phosphorylation also increased after ischemia in WT mouse brains, an effect that was potentiated by CYP2J2 overexpression (Fig. 2B and Supplementary Fig. 2B). In contrast, while c-Jun (Fig. 2C and Supplementary Fig. 2C and JNK phosphorylation (Fig. 2D and Supplementary Fig. 2D) increased after ischemia in WT mice, phosphorylation of these proteins was reduced in mice with CYP2J2 overexpression (p<0.05). However, pretreated with C26 reduced these effects of CYP2J2 (Fig. 2). These data indicate that ischemia leads to activation of PI3K/AKT, ERK1/2 and c-Jun/JNK signaling pathways, and that overexpression of CYP2J2 is associated with enhanced PI3K/AKT and ERK1/2 activation, and reduced c-Jun/JNK activation.
Figure 2.
Endothelial CYP2J2 overexpression increases PI3K/ AKT activation and altered ERK1/2 and c-Jun/JNK activation after BCCAO after BCCAO. (A) Immunoblotting (upper) of p-AKT ,total AKT and PI3K and densitometric analysis (bottom) using nuclear extract isolated from cortex 24 hours after BCCAO. p-AKT and PI3K protein upregulation after ischemia was augmented in Tie2-CYP2J2 Tr (2J2 Tr) mice, C26 inhibited the level of p-AKT and PI3K up regulated in 2J2 Tr mice. (B, C, D) Phospho-ERK1/2, ERK1/2, phosphor-c-Jun, c-Jun, phosphor-JNK and total-JNK expression in brain homogenates was assessed by immunblotting and densitometric analysis. Phospho-ERK1/2 (panels B) was significantly increased after BCCAO in Tie-CYP2J2-Tr (2J2 Tr) mice compared to WT. Phosphorylation of c-Jun (panels C) was increased after ischemia in WT but not in Tie2-CYP2J2 Tr brains. Phosphorylation of JNK (panels D) was increased after ischemia in WT but not in Tie2-CYP2J2-Tr brains. However, applied C26 reduced these effect. Blots are representative of three independent experiments. *p<0.05 vs. WT ischemia; #p<0.05 vs. Tie2-CYP2J2 ischemia.
Effect of CYP2J2 overexpression on the levels of Bcl-2, Bcl-xl, Bax, and caspase-3 after BCCAO
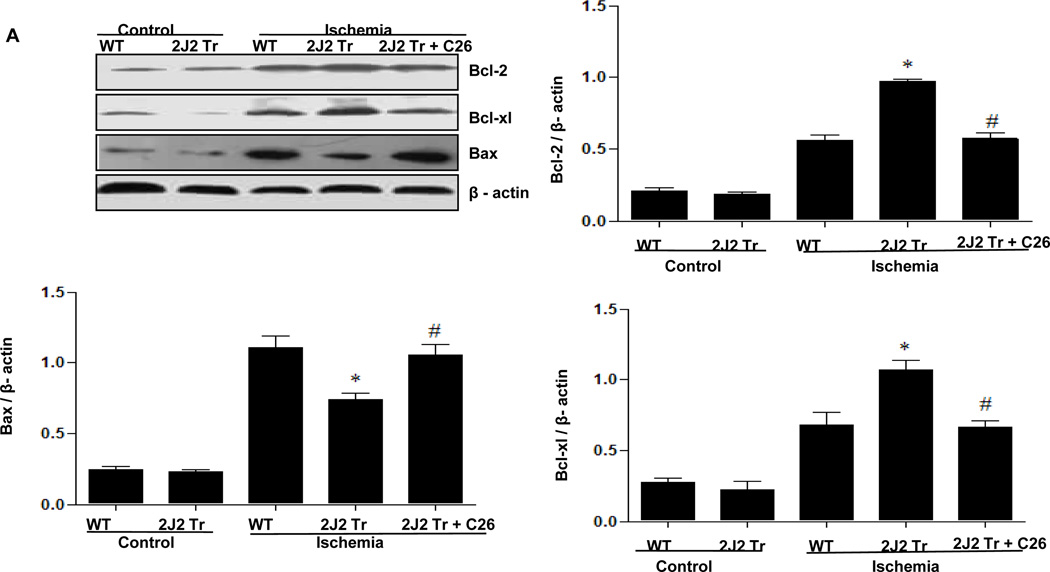
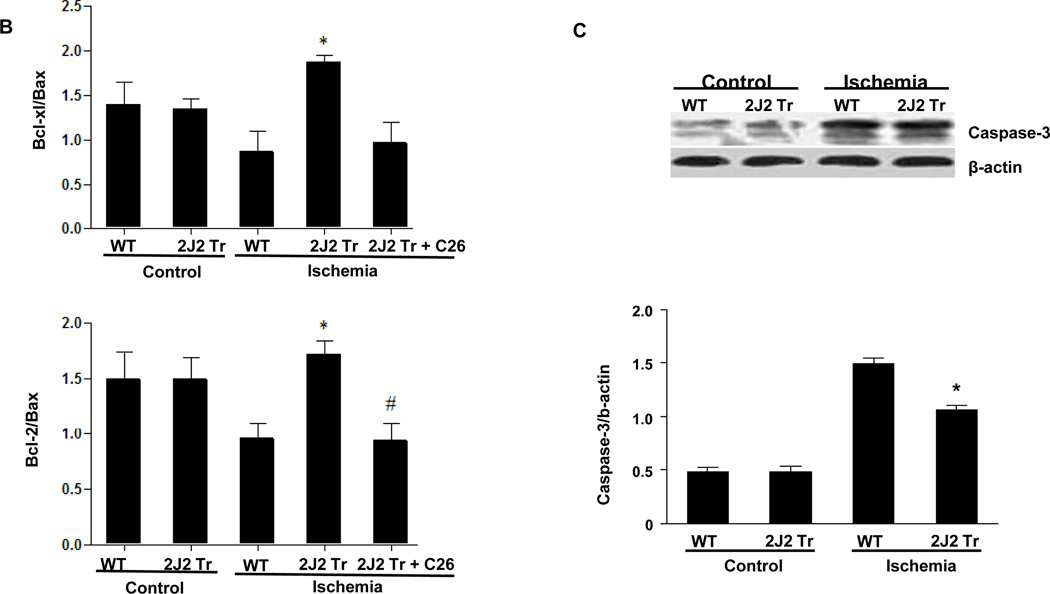
To investigate the effects of CYP2J2 overexpression on apoptosis in this model, we examined the apoptosis-related proteins Bcl-2, Bcl-xl, Bax and caspase-3 in brain. Ischemia increased brain expression of both anti-apoptotic (Bcl-2 and Bcl-xl) and pro-apoptotic (Bax and caspase-3) proteins. Tie2-CYP2J2-Tr brains showed augmented levels of the anti-apoptotic Bcl-2 and Bcl-xl and decreased levels of the pro-apoptotic Bax after ischemia compared to WT brains (Fig. 3A). The ratios of Bcl-2/Bax and Bcl-xl/Bax were significantly higher in Tie2-CYP2J2-Tr brains than in WT brains after ischemia (Fig. 3B). Conversely, Tie2-CYP2J2-Tr mice exhibited an attenuated rise in caspase-3 after ischemia compared to WT mice (Fig. 3C). However, pretreated with C26 attenuated these effect of CYP2J2. Together, these data indicate that cerebral ischemia activates apoptotic signaling pathways, and that overexpression of CYP2J2 has anti-apoptotic effects.
Figure 3.
Tie2-CYP2J2-Tr mice have altered levels of Bcl-2, Bcl-xl, Bax and caspase-3 after BCCAO. Immunoblots of brain homogenates show that CYP2J2 overexpression increases levels of Bcl-2 and Bcl-xl (panels A) compared to WT after ischemia. Ischemia results in increased expression of Bax (panels A) and caspase-3 (panels C), an effect that is attenuated in Tie2-CYP2J2-Tr (2J2-Tr) mice. Ratios of Bcl-2/Bax and Bcl-xl/Bax (panel B) are reduced in WT mice after ischemia, but significantly higher in Tie2-CYP2J2-Tr mice. The above effects of CYP2J2 were inhibited by C26, the increased level of Bcl-2 and Bcl-xl (panels A) were decreased with the level of Bax up regulated in Tie2-CYP2J2 Tr mice applied C26 compared to Tie2-CYP2J2-Tr mice alone. Ratios of Bcl-2/Bax and Bcl-xl/Bax (panel B) are reversed when Tie2-CYP2J2-Tr mice administrated C26. Blots were scanned and relative protein levels normalized to β-actin were determined from three independent experiments. *p<0.05 vs. WT ischemic; #p<0.05 vs. Tie2-CYP2J2 ischemia.
TUNEL staining
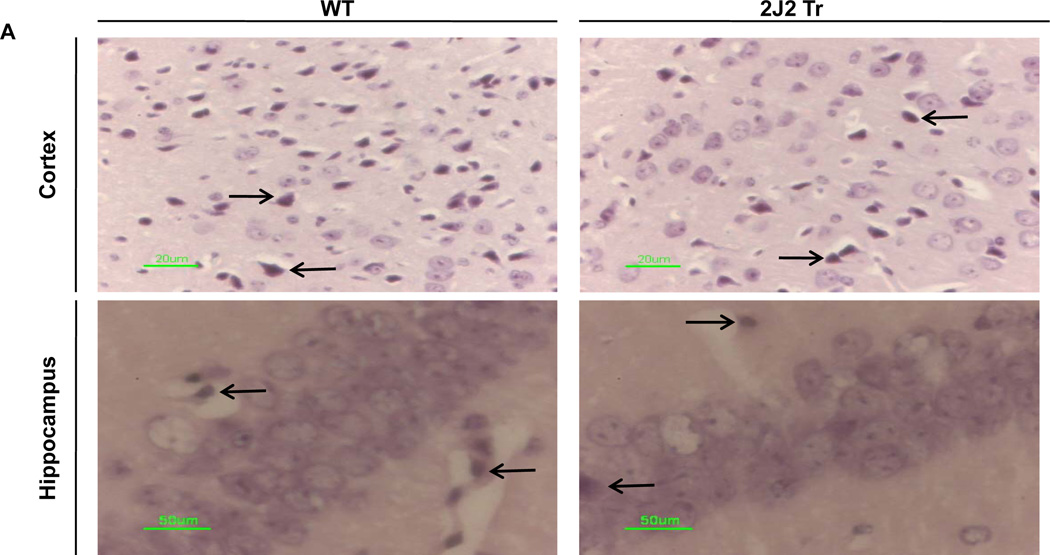
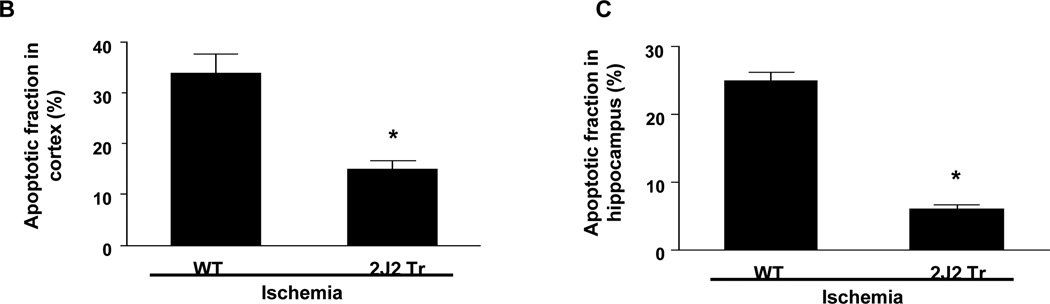
We also examined neuronal apoptosis by TUNEL staining. Many TUNEL-positive cells were observed in the cortex and hippocampus of WT mice. In contrast, TUNEL-positive cells were significantly less abundant in the cortex and hippocampus of Tie2-CYP2J2-Tr mice (Fig. 4A). Thus, the percentage of apoptotic cells was significantly lower in Tie2-CYP2J2-Tr mice than in WT mice in both the cortex (10.2 ± 3.3% vs. 34.7 ± 9.8%, p<0.05) (Fig. 4B) and hippocampus (8.4 ± 7.0% vs. 48.9 ± 15.3%, p<0.05) (Fig. 4C).
Figure 4.
TUNEL staining of coronal brain sections after BCCAO. Brains from WT and Tie2-CYP2J2-Tr (2J2-Tr) mice were stained with TUNEL (panels A). There were abundant TUNEL-positive apoptotic cells in the cortex and hippocampus of WT mice. TUNEL-positive cells were much less frequent in the cortex and hippocampus of the Tie2-CYP2J2-Tr mice. The fraction of apoptotic cells in the cortex (panel B) and hippocampus (panel C) was significantly lower in Tie2-CYP2J2-Tr mice compared to WT. More than 5 fields of view at 40X magnification from WT and Tie2-CYP2J2-Tr mice (n=4 for each) were examined for TUNEL-positive cells. Data are expressed as percentage of apoptotic cells in total number of cells. *p<0.05 vs. WT ischemic.
EETs or CYP2J2 overexpression decreases OGD-induced cell death or apoptosis
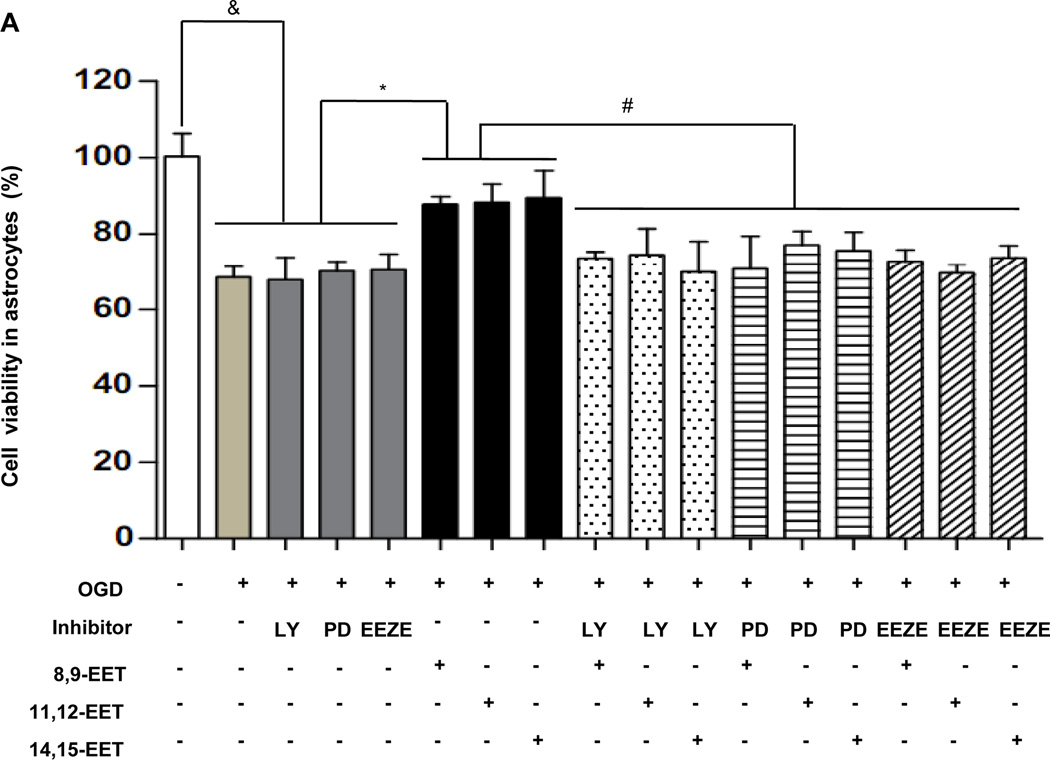
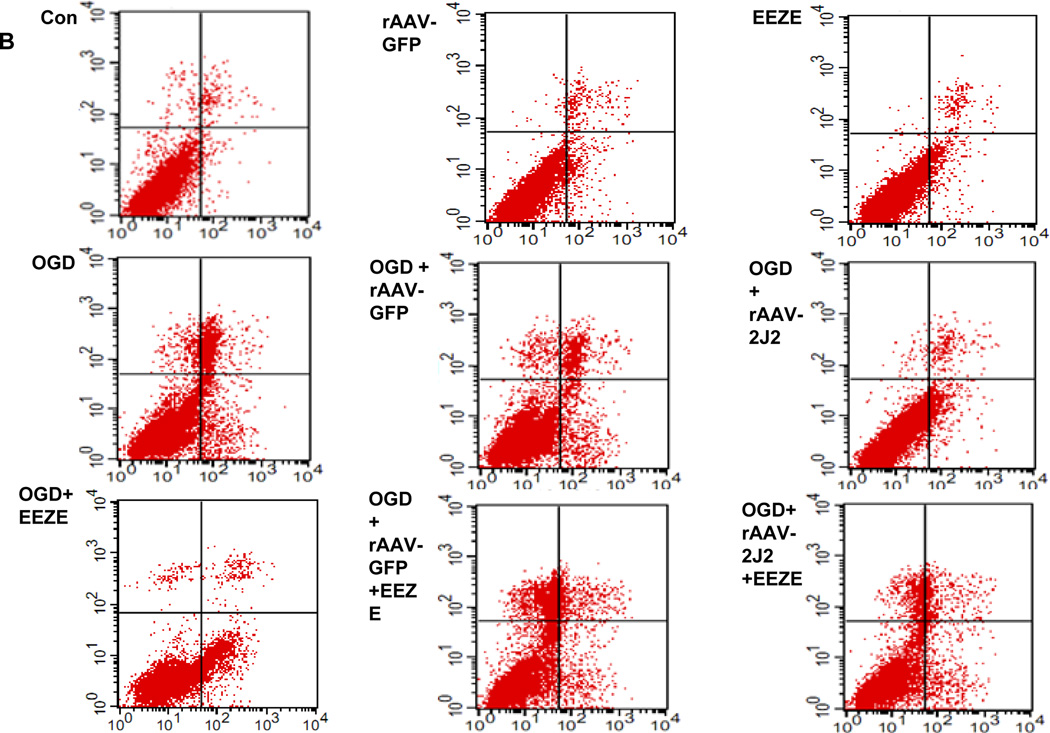
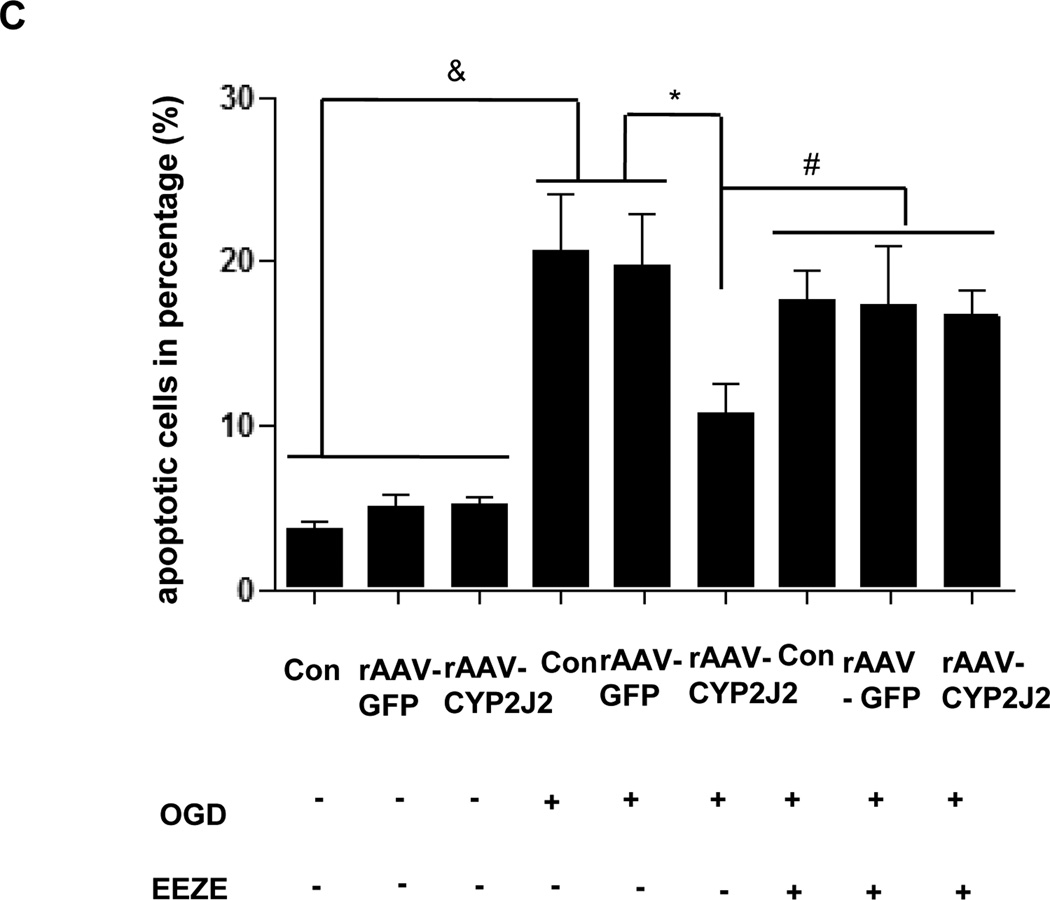
Trypan blue staining was performed for astrocytes and Neuro-2a after OGD. Compared with EETs treatment, OGD resulted in a significant reduction of vital cells in astrocytes (68.55 ± 2.78% vs. 89.57 ± 7.12%, p < 0.05) and in Neuro-2a group (68.38 ± 1.3% vs. 87.78 ± 1.67 %, p < 0.05), respectively. Additional application of EETs inhibitor EEZE attenuated the effects of EETs and led to a marked reduction of cell viability (73.77 ± 3.20% vs. 89.57 ± 7.12%, p<0.05 in astrocytes and 70.45 ± 1.8% vs. 87.78 ± 1.67 %, p<0.05 in Neuro-2a, respectively). Similarly, inhibitors of PI3K LY294002 and MAPK PD98059 also inhibited effects of EETs (Fig. 5A and Supplementary Fig. 4). Furthermore, we overexpressed CYP2J2 in Neuro-2a cells via transfected with rAAV-CYP2J2 and also observed effects of EETs blocker EEZE (Fig. 5B). Results showed that CYP2J2 overexpression significantly reduced apoptosis induced by OGD (10.75 ± 1.794 vs. 20.66 ± 3.4%, p<0.05), and in contrast, EEZE markedly attenuated the antiapoptic effects of CYP2J2 (17.66 ± 1.54% vs. 10.75 ± 1.794, p<0.05) (Fig. 5C). These data suggest that EETs have important protective role in cerebral ischemia and CYP2J2 functions via increased EETs level.
Figure 5.
The protective effect of EETs in astrocytes and Neuro-2a exposed to OGD. (A) The viability of primary astrocyte determined by Trypan blue staining with PI3K inhibitor LY294002, MAPK inhibitor PD98059 and EETs inhibitor EEZE. Treatment with EETs reversed the reduction of vital cells induced by OGD, However, pretreatment cells with PI3K inhibitor LY294002, MAPK inhibitor PD98059 and EETs inhibitor EEZE attenuated this protective effect of EETs. Bar graph: mean ± SEM, n =3. *p < 0.05 versus OGD, #p < 0.05 versus EETs combined with inhibitors, &p < 0.05 versus control cells. (B) Representative results of flow cytometry plots by annexin-V-FITC/propidium iodide (PI) staining to show the apoptosis of Neuro-2a exposed to OGD. Cells with negative staining of both PI and annexin V are living; PI-negative and annexin V-positive staining are early apoptotic cells; PI-positive and annexin V-positive staining are cells in a late stage of apoptosis. (C) Average percentage of apoptotic cells from three independent flow cytometry experiments and the values shown are mean ± SEM ; &p < 0.05 vs. normoxia; *p<0.05 vs. OGD; #p<0.05 vs. OGD + rAAV- 2J2.
Involvement of PI3K/AKT and MAPK activation in EETs against cell death
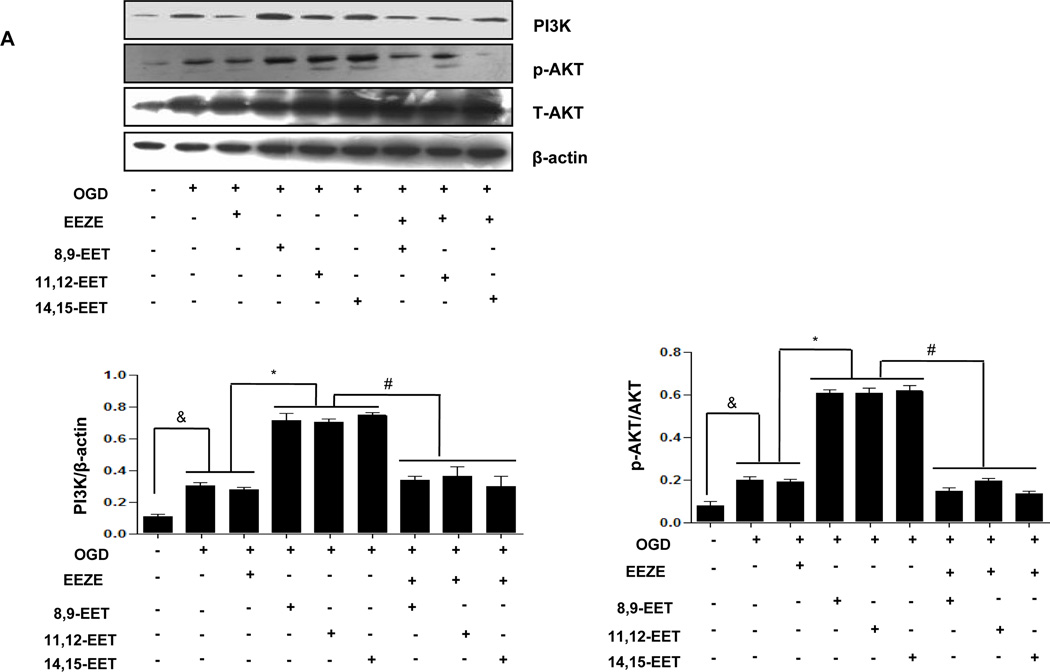
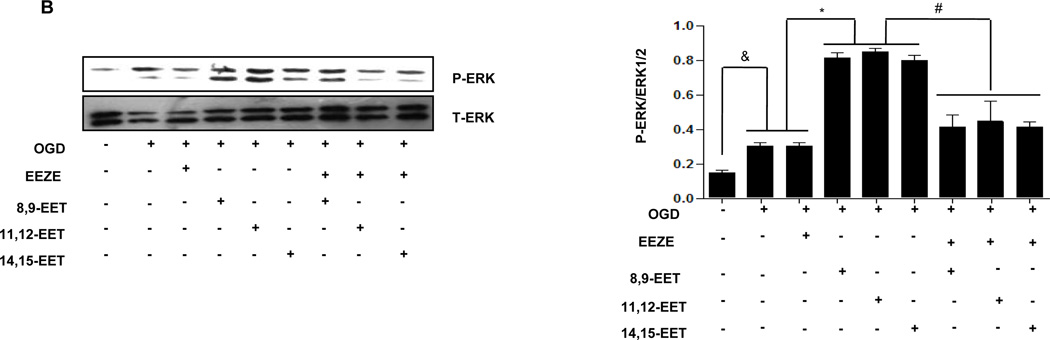
To evaluate the possible involvement of PI3K/AKT signaling pathway in CYP2J2 induced protection against cerebral ischemia, we pretreated primary cortical astrocytes and Neuro-2a with the PI3K inhibitor LY294002 (10µM), the MAPK kinase inhibitor PD98059 (50µM) or the EETs inhibitor EEZE (100nM) respectively and then evaluated related signaling molecules including apoptosis related protein levels by immunoblotting. Under OGD conditions, p-Akt, PI3K and MAPK1/2 were slightly increased in comparison with normoxia in astrocytes. Interestingly, exogenous EETs caused a significant activation of p-Akt, PI3K (Fig. 6A,) and MAPK1/2 further (Fig. 6B,), which was in consistence with finding in animals. EETs-dependent PI3K/Akt and MAPK activation was significantly depressed by pretreatment with PI3K inhibitor LY294003 and ERK1/2 inhibitor PD98059 (Supplementary Fig. 5), respectively. Furthermore, addition of EETs inhibitor EEZE completely reversed EETs-induced activation of these signaling pathways (Fig. 6 A–B). These effects were also observed in Neur0-2a (Supplementary Fig. 7). These results suggest that PI3K/AKT and MAPK signaling pathways involved in anti-ischemia effect of EETs.
Figure 6.
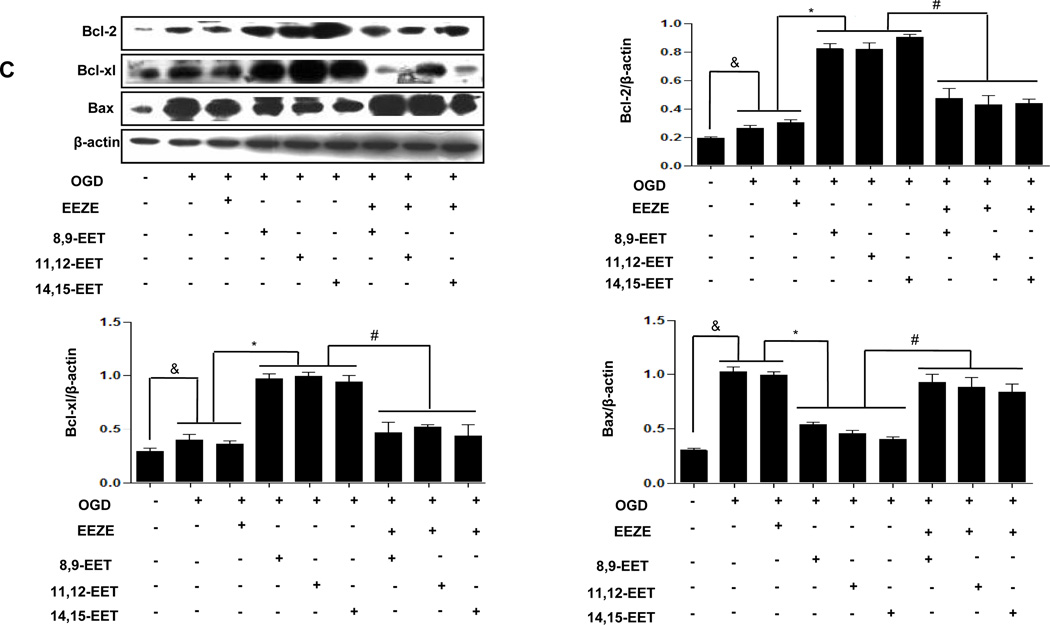
Representative Western blots showing protective effects of EETs and CYP2J2 overexpression in astrocytes. (A–C) Cell lysates were analyzed by immunoblotting using antibodies against PI3K, p-AKT (A), p-Erk1/2 (B) and antibody recognizing Bcl-2, Bcl-xl and Bax (C) and densitometric analysis of band intensity of these signaling molecules to their internal controls were calculated respectively. The data from three independent experiments are given as mean ± SEM; &p < 0.05 vs. normoxia; *p<0.05 vs. OGD; #p<0.05 vs. OGD + EETs. (D) Effect of EETs on caspase-3 activity in cultured cortical astrocytes exposed to OGD and inhibitors. The results are shown as percentages of the control value in the normoxia conditions. Each value is mean ± SEM from five independent experiments; &p < 0.05 vs. normoxia; *p < 0:05 vs. OGD; #p < 0.05 vs. OGD + EETs.
Role of Bcl-2, Bcl-xl, Bax expression in EETs against cell death
As is known, the importance of PI3K/AKT pathway in cell growth and survival has been widely documented 35, one important downstream target of the PI3K/Akt cell survival pathway is the Bcl-2 family 36. We determined the expression of Bcl-2, Bcl-xl and Bax protein in astrocytes exposed to OGD and results showed that OGD suppressed expression of Bcl-xl and Bcl-2, but promoted the expression of Bax in cultured astrocytes, which were all attenuated by EETs treatments. Such changes were reversed by LY294002 (Supplementary Fig. 6A) and PD98059 (Supplementary Fig. 6B) as well as EEZE (Fig. 6C). The same effects appeared in Neuro-2a (Supplementary Fig. 8A–C). These results suggest that in cultured neurons one of the intracellular targets mediating the protective effect of EET is Bcl-2 family, further confirm that activation of PI3K/AKT and ERK function upstream of EET-induced apoptosis.
CYP2J2 transfection inhibited OGD-mediated Neuro-2a apoptosis
Western blot analysis revealed that the effect of rAAV-CYP2J2 transfection was similar with EETs, that is, CYP2J2 significantly increased the level of Bcl-2 and Bcl-xl, decreased the level of Bax compared with OGD alone or rAAV-GFP transfeced group exposed to OGD, but EEZE treatment effectively attenuated the effect of CYP2J2, not rAAV-GFP group (Supplementary Fig. 8D), which indicated CYP2J2 mediated the protective effect against cerebral ischemia.
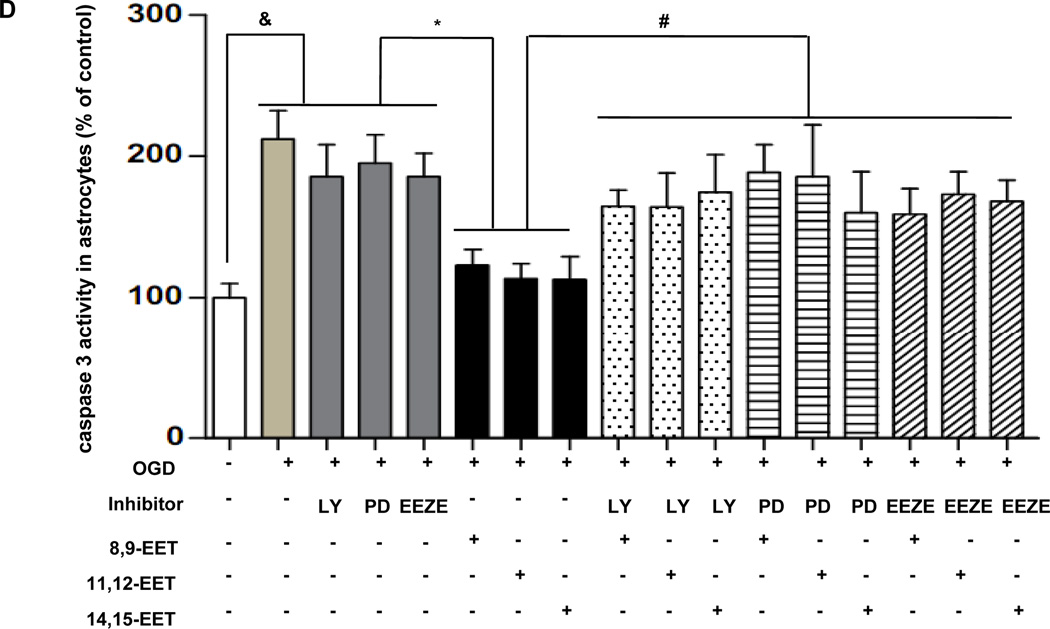
Influence of EET on Caspase-3 Activity
We examined role of caspase-3 activation in OGD-induced cell death. Exogenous EETs caused reduction in increased caspase-3 activity in astrocytes as well as in Neuro-2a cells exposed to OGD, its effect was inhibited by PD98059, LY294002 and EEZE (Fig. 6D, Neuro-2a cell data not shown). These data further suggested that EETs decreased injury and apoptosis in cells exposed to hypoxia, and PI3K/AKT plus ERK1/2 intracellular signaling pathways involved in this effect.
Discussion
In the present study, we tested the hypothesis that endothelial-specific overexpression of human CYP2J2 can protect the brain from global ischemic damage in mice. Our results show that Tie2-CYP2J2-Tr mice have increased AA epoxygenase activity in brain and plasma following ischemia. After ischemia/reperfusion, infarct size was significantly reduced in the Tie2-CYP2J2-Tr mice compared to WT mice. Immunoblotting demonstrated that CYP2J2 overexpression enhanced activation of ERK1/2 and PI3K/AKT in the ischemic brain. In contrast, activation of the pro-inflammatory c-Jun/JNK pathway was reduced in Tie2-CYP2J2-Tr mice compared to WT in the ischemic brain. In addition, CYP2J2 overexpression increased levels of the anti-apoptotic proteins Bcl-2 and Bcl-xl, and attenuated the rise in pro-apoptotic proteins Bax and caspase-3. These results parallel histopathological analyses showing that neurons in Tie2-CYP2J2-Tr mouse brains were well-preserved after ischemia. To confirm the specific role of the PI3K/AKT and MAPK/Erk1/2 kinase signaling pathway in the mechanism of EETs action, the effect of the PI3K inhibitor LY294002 (10 µM), Erk1/2 inhibitor PD98059 (50 µM) and EETs inhibitor EEZE (100nM) were examined. The addition of PD98059 to the culture medium of cells exposed to OGD and EETs resulted in a significant decrease in EETs induced up-regulation of Erk1/2 expression. LY294002 and EEZE resulted in strong attenuation of PI3K/AKT and ERK1/2. Moreover, EETs effectively protected astrocytes and Neuro-2a cells against OGD-induced apoptosis through increased Bcl-xl, Bcl-2 expression plus decreased Bax expression with attenuation of caspase-3 activity, these effects were blocked by three inhibitors, indirectly indicating the involvement of PI3K/AKT and Erk1/2 in EETs protective role. Together, these results indicate that CYP2J2 exerts significant neuroprotective effects against ischemic injury and suggest that CYP2J2 and its metabolites have therapeutic potential in management of ischemic brain injury.
The infarction produced by global ischemia includes not only neuronal damage but also damage to astrocytes, oligodendrocytes, and endothelial cells. Furthermore, circulatory disturbances may be critical to expansion of cerebral infarction after global ischemia 37, 38. The release of arachidonic acid and the protective effect of sEH gene disruption on transient global cerebral ischemia have been previously reported 2. EETs protect neurons and astrocytes against ischemic cell death induced in vitro by oxygen-glucose deprivation, suggesting that EETs may exert a cytoprotective effect independent of their effects on cerebral blood flow. However, there have been no reports showing that overexpression of CYP2J2 was protective against selective neuronal vulnerability after global ischemia in vivo. CYP2J2 overexpression may protect against cerebral infarction in several ways, with activation of pro-survival kinases and suppression of apoptotic signaling molecules as primary effectors. Activation of PI3K/AKT and ERK1/2 signaling pathways protect endothelial cells from apoptosis 5. AKT is known to play a critical role in controlling the balance between survival and apoptosis. The upregulation of Bcl-2 and Bcl-xl in cultured neurons has been shown to be protective against various noxious stimuli which induce apoptosis 37. In addition, enhanced neuronal survival in Tie-CYP2J2-Tr neurons was associated with increased epoxygenase activity, as measured by levels of the stable EET metabolite, 14, 15-DHET. There is substantial evidence supporting the involvement of apoptosis in infarction following cerebral ischemia 39–42. Suppression of apoptosis by CYP2J2 overexpression may be a key to neuronal protection after transient global ischemia. The observed decreased number of TUNEL positive cells in the Tie2-CYP2J2-Tr mice is consistent with the importance of apoptosis in neuronal injury after ischemia.
In addition to anti-apoptotic actions, some signal molecules, such as Bcl-2, have been shown to act as antioxidants 43. Since reperfusion after transient cerebral ischemia produces oxygen free radicals 44, 45, Bcl-2 upregulation may play a second important role. Neuronal death can be significantly reduced through treatment with superoxide dismutase or other antioxidants 46. Thus, the antioxidant actions of Bcl-2 may contribute, at least in part, to the neuroprotection observed in our study.
EETs are known to have anti-inflammatory effects, which may also play a role in protection against ischemic neural damage. Indeed, EETs have been show to inhibit NF-κB activation and upregulation of endothelial adhesion molecules 47. Our results show that CYP2J2 overexpression also reduces activation of the JNK pathway which is involved in the production of pro-inflammatory cytokines 48. Thus, EETs may limit secondary inflammation and thus reduce infarction after ischemia.
This study demonstrates that CYP2J2 overexpression is associated with altered signaling of multiple pathways after ischemia/reperfusion. However, the precise molecular mechanisms through which CYP2J2 or EETs activate these pathways remain unclear. EETs are thought to bind a G-protein-coupled-receptor, although no such receptor has been identified 4. There are also additional questions regarding the precise mechanisms of neuroprotective downstream of EETs. For instance, increased levels of Bcl-2 and Bcl-xl are protective, but the mechanisms are not clear 49. Our results imply that PI3K/AKT and ERK1/2 signaling pathways are activated after transient ischemia. Further studies are needed to clarify the whether CYP2J2 overexpression also influences other events including superoxide radical production, output of excitatory amino acids, calcium overload, activation of nitric oxide synthase, appearance of inflammation, as well as activation of signaling pathways other than PI3K/AKT, ERK1/1 and c-Jun/JNK after ischemia.
In conclusion, our results suggest a possible therapeutic potential for CYP2J2 overexpression after ischemia in the brain. The post-ischemic neuroprotective effects of CYP2J2 and its products reported in this paper have important implications with respect to development of novel therapeutic approaches for the management of stroke patients. Future studies should further explore the mechanisms underlying CYP2J2 neuroprotection.
Supplementary Material
Acknowledgements
This work was supported by grants from National Nature Science Foundation Committee of China (No. 30770882, 30800458, 30930039), “973” projects (No. 2007CB512004) and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025043).
Footnotes
Disclosures: None
References
- 1.Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–7930. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 4.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, et al. Cytochrome p-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via mapk and pi3k/akt signaling pathways. Am J Physiol Heart Circ Physiol. 2007;293:H142–H151. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, et al. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase c signaling pathways. J Pharmacol Exp Ther. 2003;307:753–764. doi: 10.1124/jpet.103.052787. [DOI] [PubMed] [Google Scholar]
- 7.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferri CP, Schoenborn C, Kalra L, Acosta D, Guerra M, Huang Y, et al. Prevalence of stroke and related burden among older people living in latin america, india and china. J Neurol Neurosurg Psychiatry. 2011 doi: 10.1136/jnnp.2010.234153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Pu S, Zhang W, Yang N, Shen G, Yin J, et al. Sex differences in risk factors, etiology, and short-term outcome of cerebral infarction in young patients. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Di Iorio P, Ballerini P, Traversa U, Nicoletti F, D'Alimonte I, Kleywegt S, et al. The antiapoptotic effect of guanosine is mediated by the activation of the pi 3-kinase/akt/pkb pathway in cultured rat astrocytes. Glia. 2004;46:356–368. doi: 10.1002/glia.20002. [DOI] [PubMed] [Google Scholar]
- 11.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, et al. Soluble epoxide hydrolase: A novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77:1601–1610. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 14.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, et al. Polymorphisms in the human soluble epoxide hydrolase gene ephx2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27:4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z, Zhang Y, Chen XQ, Lam PY, Yang H, Xu Q, et al. Apoptosis and activation of erkl/2 and akt in astrocytes postischemia. Neurochem Res. 2003;28:831–837. doi: 10.1023/a:1023206906164. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, et al. P-450 epoxygenase and no synthase inhibitors reduce cerebral blood flow response to n-methyl-d-aspartate. Am J Physiol Heart Circ Physiol. 2000;279:H1616–H1624. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- 17.Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain p-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996;271:H1541–H1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar A. Beating ischemia: A new feat of eets? Circ Res. 2004;95:443–445. doi: 10.1161/01.RES.0000142314.55287.a1. [DOI] [PubMed] [Google Scholar]
- 19.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome p450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, et al. Endothelial expression of human cytochrome p450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke Q, Xiao YF, Bradbury JA, Graves JP, Degraff LM, Seubert JM, et al. Electrophysiological properties of cardiomyocytes isolated from cyp2j2 transgenic mice. Mol Pharmacol. 2007;72:1063–1073. doi: 10.1124/mol.107.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in cyp2j2 transgenic hearts involves mitochondrial atp-sensitive k+ channels and p42/p44 mapk pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Li G, Liao W, Wu J, Liu L, Ma D, et al. Selective inhibitors of cyp2j2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J Pharmacol Exp Ther. 2009;329:908–918. doi: 10.1124/jpet.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K. Neuroprotection after transient global cerebral ischemia in wld(s) mutant mice. J Cereb Blood Flow Metab. 2004;24:62–66. doi: 10.1097/01.WCB.0000095798.98378.34. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Zhan RZ, Qi S, Fujihara H, Taga K, Shimoji K. A forebrain ischemic preconditioning model established in c57black/crj6 mice. J Neurosci Methods. 2001;107:101–106. doi: 10.1016/s0165-0270(01)00356-9. [DOI] [PubMed] [Google Scholar]
- 26.Ni B, Wu X, Su Y, Stephenson D, Smalstig EB, Clemens J, et al. Transient global forebrain ischemia induces a prolonged expression of the caspase-3 mrna in rat hippocampal ca1 pyramidal neurons. J Cereb Blood Flow Metab. 1998;18:248–256. doi: 10.1097/00004647-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Terashima T, Namura S, Hoshimaru M, Uemura Y, Kikuchi H, Hashimoto N. Consistent injury in the striatum of c57bl/6 mice after transient bilateral common carotid artery occlusion. Neurosurgery. 1998;43:900–907. doi: 10.1097/00006123-199810000-00102. discussion 907–908. [DOI] [PubMed] [Google Scholar]
- 28.Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic mice protects against neuronal cell death after transient focal ischemia by blocking activation of the bad cell death signaling pathway. J Neurosci. 2003;23:1710–1718. doi: 10.1523/JNEUROSCI.23-05-01710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing cuzn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshiro S, Kawamura K, Zhang C, Sone T, Morioka MS, Kobayashi S, et al. Microglia and astroglia prevent oxidative stress-induced neuronal cell death: Implications for aceruloplasminemia. Biochim Biophys Acta. 2008;1782:109–117. doi: 10.1016/j.bbadis.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Maeda S, Nakajima K, Tohyama Y, Kohsaka S. Characteristic response of astrocytes to plasminogen/plasmin to upregulate transforming growth factor beta 3 (tgfbeta3) production/secretion through proteinase-activated receptor-1 (par-1) and the downstream phosphatidylinositol 3-kinase (pi3k)-akt/pkb signaling cascade. Brain Res. 2009;1305:1–13. doi: 10.1016/j.brainres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Huang XJ, Zhang WP, Li CT, Shi WZ, Fang SH, Lu YB, et al. Activation of cyslt receptors induces astrocyte proliferation and death after oxygen-glucose deprivation. Glia. 2008;56:27–37. doi: 10.1002/glia.20588. [DOI] [PubMed] [Google Scholar]
- 33.Drescher C, Diestel A, Wollersheim S, Berger F, Schmitt KR. How does hypothermia protect cardiomyocytes during cardioplegic ischemia? Eur J Cardiothorac Surg. 2011 doi: 10.1016/j.ejcts.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Gabryel B, Adamczyk J, Huzarska M, Pudelko A, Trzeciak HI. Aniracetam attenuates apoptosis of astrocytes subjected to simulated ischemia in vitro. Neurotoxicology. 2002;23:385–395. doi: 10.1016/s0161-813x(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 35.Keszthelyi A, Jeney A, Kerenyi Z, Mendes O, Waalwijk C, Hornok L. Tagging target genes of the mat1-2-1 transcription factor in fusarium verticillioides (gibberella fujikuroi mp-a) Antonie Van Leeuwenhoek. 2007;91:373–391. doi: 10.1007/s10482-006-9123-5. [DOI] [PubMed] [Google Scholar]
- 36.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 37.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 38.Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Chopp M, Jiang N, Yao F, Zaloga C. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1995;15:389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- 40.Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993;24:2002–2008. doi: 10.1161/01.str.24.12.2002. discussion 2008–2009. [DOI] [PubMed] [Google Scholar]
- 41.Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, et al. Delayed neuronal death in the ca1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci. 1995;15:1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto M, Matsumoto M, Ohtsuki T, Taguchi A, Mikoshiba K, Yanagihara T, et al. Internucleosomal DNA cleavage involved in ischemia-induced neuronal death. Biochem Biophys Res Commun. 1993;196:1356–1362. doi: 10.1006/bbrc.1993.2402. [DOI] [PubMed] [Google Scholar]
- 43.Allsopp TE, Wyatt S, Paterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993;73:295–307. doi: 10.1016/0092-8674(93)90230-n. [DOI] [PubMed] [Google Scholar]
- 44.Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 45.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitagawa K, Matsumoto M, Oda T, Niinobe M, Hata R, Handa N, et al. Free radical generation during brief period of cerebral ischemia may trigger delayed neuronal death. Neuroscience. 1990;35:551–558. doi: 10.1016/0306-4522(90)90328-2. [DOI] [PubMed] [Google Scholar]
- 47.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome p450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. Mapk signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec (Hoboken) 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- 49.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, et al. Gene transfer of hsp72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: Influence of bcl-2. Ann Neurol. 2002;52:160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.