Abstract
In the past decade, molecular-targeted drugs have been focused upon for the treatment of cancer. In 2002, gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor became available in Japan for the treatment of non-small cell lung cancer (NSCLC). Over 80% of selected patients, such as EGFR mutation-positive patients, respond to gefitinib treatment; however, most patients develop acquired resistance to gefitinib within a few years. Recently, many studies have been performed to determine precisely how to select patients who will respond to gefitinib, the best timing for its administration, and how to avoid the development of acquired resistance as well as adverse drug effects. This article reviews the use of gefitinib for the treatment of NSCLC from a pharmaceutical viewpoint.
Keywords: gefitinib, EGFR, KRAS, NSCLC, lung cancer
Introduction
Lung cancer, which is responsible for 1.38 million annual deaths worldwide, is the most common cause of cancer mortality in men and the second most common cause in women.1 In the treatment of lung cancer, surgery, chemotherapy, radiotherapy, or their combination are selected depending on the histological diagnosis, stage of cancer, and age of the patient. Despite the significant progress that has been made in treatment and substantial research efforts undertaken, the prognosis for lung cancer remains poor, and the development of more effective treatments is one of the most important topics in the field of oncology.
Lung cancers are classified according to their histological type. Because each variant has different biological and clinical properties, including response to treatment, a precise classification is essential to provide appropriate therapy for individual patients. Lung cancer consists of two broad categories—non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC)—and NSCLC itself is subdivided into adenocarcinoma (AC), squamous cell carcinoma (SCC), and large cell carcinoma (LCC). Approximately 40%–50% of lung cancers are AC, which is the most common form.2 SCC accounts for ~30% of lung cancers and typically occurs close to large airways. Approximately 10% of lung cancers are LCC, which takes its name from the presence of many large cells with a large nucleus and conspicuous nucleolus. SCLC is an aggressive malignant disease, with the majority of patients presenting with distant metastasis at presentation.3 Generally, SCLC grows quickly and spreads early in the course of the disease. Although the majority of patients with SCLC are diagnosed with advanced cancer with distant metastasis, SCLC also shows high sensitivity to chemotherapy. The response rate (RR) for SCLC is reportedly 60%–80%, even in the advanced stage, although complete remission is observed in only 15%–20% of patients.4 Among the histological types of NSCLC, AC is considered to have a higher sensitivity to chemotherapy than SCC or LC; however, the 20%–40% RR to chemotherapy for patients with NSCLC is still insufficient.
In the past decade, molecular-targeted drugs have been focused upon for the treatment of cancer and have improved its treatment by promoting research, especially of cancer-related genes and proteins. In 2002, gefitinib (ZD1839; AstraZeneca) (Fig. 1), the first epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, became available in Japan as an innovative molecular-targeted drug for the treatment of unresectable NSCLC. Initially, many NSCLC patients were expected to respond to gefitinib because many solid tumors, including NSCLC, are known to overexpress EGFR, which has a role in tumor proliferation and is used as a biomarker to predict poor prognosis. Indeed, gefitinib was shown to have a dramatic effect on a limited number of patients; however, it was found to be ineffective in 70%–80% of patients with NSCLC.5,6 Furthermore, there have been some reports of death caused by interstitial pneumonia (IP), one of the critical adverse drug reactions (ADRs) associated with gefitinib use.7,8 Therefore, we are currently in need of tools that can predict the effects of gefitinib, and we are also in need of criteria for selecting patients who could be treated with gefitinib.
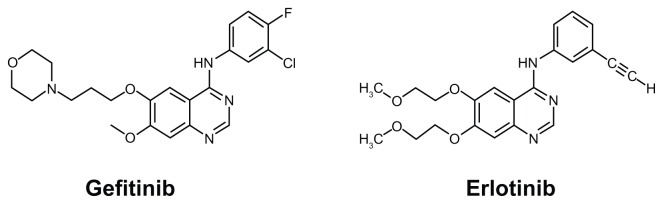
Figure 1.
Chemical structure of gefitinib (left) and erlotinib (right).
In 2004, Lynch et al9 and Paez et al10 each published, on the same day, sensational reports in the New England Journal of Medicine and Science, identifying somatic mutations in the tyrosine kinase domain of the EGFR gene in patients with gefitinib-sensitive lung cancer, as compared with none of the patients who had no response. Therefore, screening for EGFR mutations in lung cancer showed potential for identifying patients who would respond to gefitinib therapy. Many later studies clarified that patients with EGFR mutations in the area of the gene coding for the ATP-binding pocket of the tyrosine kinase domain responded to gefitinib.11–15 Consequently, the EGFR genotyping has been used to select patients who will respond to gefitinib. Other genetic mutations have also been reported as indicators of the response or resistance to gefitinib; for example, mutations of the KRAS gene are associated with primary resistance to gefitinib.16,17 Thus, at the present time, screening of EGFR and KRAS is used to predict the effects of gefitinib and to select patients who will respond to gefitinib in the clinical setting. Conversely, it was also reported that ~20% of patients with an EGFR mutation do not respond to gefitinib, while 10% of patients without an EGFR mutation respond to gefitinib.18,19 Furthermore, the majority of patients develop resistance to gefitinib within a few years. To define more precise criteria for the selection of patients who can be treated with gefitinib and to develop a method to improve the outcome of gefitinib therapy, more detailed and elaborate studies are currently in progress.
This article reviews the use of gefitinib for the treatment of NSCLC from a pharmaceutical viewpoint.
Mechanism of Action, Metabolism, and Pharmacokinetic Profile
Mechanism of action
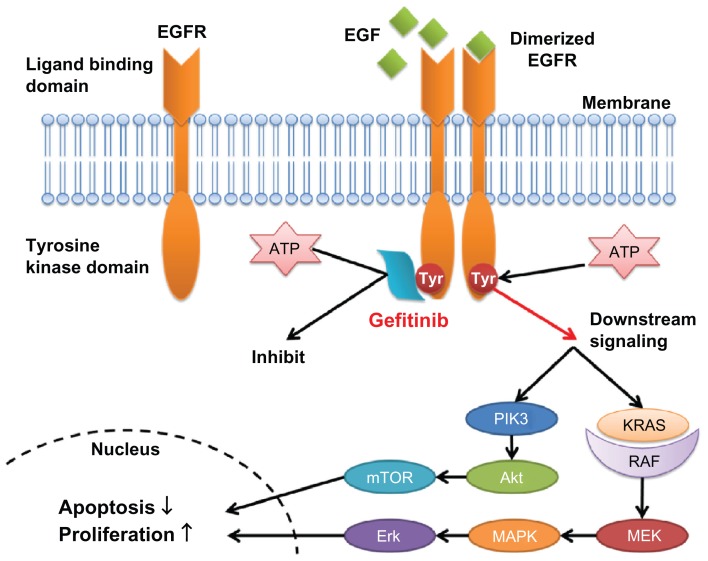
Gefitinib, an anilinoquinazoline (4-quinazolinamine, N-(3-chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazolin-4-amine) with a molecular weight of 446.90, is an oral tyrosine kinase competitive inhibitor. Its antineoplastic effect is exerted by inhibiting the activation of ErbB-1 (EGFR), one of the subtypes of the ErbB family. The ErbB family consist of four transmembrane receptor subtypes: EGFR (ErbB-1), HER2 (ErbB-2), ErbB-3, and ErbB-4.22 The ErbB family receptors fundamentally consist of an extracellular ligand binding domain, hydrophobic transmembrane domain, and intracellular tyrosine kinase domain that can bind with adosine triphosphate (ATP), with the exception of ErbB-3. Inactive ErbB transmembrane receptors are present as a monomer, which is difficult to phosphorylate, and when proliferative factors such as EGF bind to the extracellular ligand binding site, the conformation of the ErbB protein changes to form a heterodimer or homodimer. In the dimeric conformation, the tyrosine kinase domain of ErbB can easily be autophosphorylated in the presence of ATP and can activate the downstream RAS/MAPK/Erk and PI3K/Akt pathways to induce cell proliferation and suppress apoptosis.20–22 In cancer cells, ErbB receptors can be activated mainly by an increase of the copy number of the ErbB gene, overexpression of ligands or receptors, genetic variations, or the production of autocrine ligands.23,24 The overexpression or accommodative insufficiency of EGFR or its ligands is known to be pathognomonic for many kinds of solid tumors, including lung cancer, and 40%–90% of NSCLCs reportedly overexpress EGFR.25,26 The role of EGFR as a prognostic factor for NSCLC has been discussed in many studies, and most have shown that the overexpression, accommodative insufficiency, or genetic variation of EGFR correlates significantly with disease progression, poor prognosis, and decreased sensitivity to chemotherapy.27,28,29 Orally administered gefitinib is taken up by cancer cells, and it reversibly and competitively inhibits the binding of ATP to the phosphate-binding loop of the ATP binding site in the intracellular domain of EGFR. By inhibiting the binding of ATP to EGFR, gefitinib inhibits autophosphorylation and the activation of downstream signaling pathways, leading to the inhibition of cell proliferation and the induction of apoptosis in cancer cells (Fig. 2).30
Figure 2.
Action mechanism of epidermal growth factor receptor-tyrosine kinase inhibitors.
Metabolism and pharmacokinetic profile
Orally administered gefitinib is absorbed relatively slowly and reaches its peak level at 3–5 hours after administration. The elimination half-life of gefitinib in blood is approximately 48 hours, and 7–10 days are required to achieve a steady state concentration in the blood.31,32 Its bioavailability was reported to be ~60% in a study using the repeated administration of 250 mg/day gefitinib, and the plasma protein-binding rate of gefitinib was reportedly ~90%.33 Gefitinib has a very large distribution volume of ~1400 L, suggesting that it accumulates in a wide range of tissues such as in the liver, kidney, and lungs, in addition to cancer tissues.34 Gefitinib is concentrated in tissues at more than 10 times the level observed in the plasma.35 Furthermore, it was also reported that gefitinib is transported via ABCG2 and ABCB1.36 Galetti et al37 showed that gefitinib was actively taken up by cells, and its intracellular concentration was ~200 times higher than its extracellular concentration. In terms of clinical implications, mutations of ABCG2 were reportedly responsible for the incidence of diarrhea caused by gefitinib,38 and the usefulness of genotyping ABCG2 as a predictive factor of ADRs has been discussed.
Absorbed gefitinib is mainly metabolized in the liver and ~85% is passed in the feces, with less than 5% excreted in the urine.33 Its metabolic pathway was determined in in vitro and in vivo studies to be mediated by CYP3A4 and 2D6, and partly by CYP3A5 and 1A1;39 however, to date, the effects of genetic variations in these enzymes on the clinical outcomes of gefitinib have not been studied comprehensively.
Several studies focused on the influence of diet on the pharmacokinetics (PK) and pharmacodynamics (PD) of gefitinib. Swaisland et al40 reported that, for a 250 mg/day dose of gefitinib, there was an approximately two-fold difference in intra-subject variability and a 10 to 15-fold difference in inter-subject variability.40 These large differences have been considered to be due to diet, and the influence of diet on the PK of gefitinib has been considered to depend on intestinal pH. Conversely, Bergman et al41 used healthy volunteers to show that although the lower solubility of gefitinib in the human intestinal fluid compared to human gastric fluid is related to the difference in pH, gastric emptying, precipitation, and redissolution of gefitinib in the proximal human jejunum have no pronounced effects on the plasma concentration profile of gefitinib between subjects with a high clearance rate of gefitinib and those with a low clearance rate.41 They concluded that other mechanisms are likely to be more important in explaining the inter-individual differences in plasma exposure to gefitinib, such as polymorphisms in various enzymes and transport proteins, or possibly a combination of factors including gastrointestinal factors—none of which has a dominant effect in isolation.
Thus, until now, the effects of gefitinib have been predicted only by genotyping factors that play a role in the PD of gefitinib, such as EGFR and KRAS mutations. However, Nakamura et al42 presented an interesting report on the relationship between the blood concentration of gefitinib and its clinical effects.42 In their study of 23 NSCLC patients with EGFR mutations, the ratio of the gefitinib concentration on day 8 to that on day 3 after the first administration of gefitinib (C8/C3) correlated with the progression-free survival (PFS) period. They described that patients with a higher C8/C3 ratio had a significantly longer PFS (P = 0.0158, 95% confidence interval [CI]: 0.237–0.862). This report suggests the importance of the PK of gefitinib on its clinical outcome. At the same time, Chmielecki et al43 reported that maintaining a high concentration of erlotinib, another EGFR tyrosine kinase inhibitor (EGFR-TKIs) (Fig. 1) with the same mechanism of action as gefitinib, could delay the establishment of drug-resistant tumor cells and decrease the proliferation rate of drug-resistant cells compared to treatment using a lower concentration of erlotinib.43 These data suggest that in the near future, the relationship between the PK of gefitinib and its clinical outcome will be clarified, and therapeutic drug monitoring of gefitinib will become an essential tool to obtain the maximal therapeutic response.
Pharmacogenetic profile
Initially, gefitinib was expected to induce a response in patients with tumors that overexpressed EGFR because it exerts its antineoplastic effects by competitively inhibiting the binding of ATP to the ATP-binding site of EGFR, as described above. However, a post-marketing study and other studies have shown data contradicting this hypothesis: (1) while approximately 40%–80% of NSCLC overexpress EGFR,44 only 10%–20% of NSCLC patients respond to gefitinib; 5,6 and (2) while EGFR overexpression is known to be more common in SCC than AC, gefitinib shows a higher antineoplastic effect on AC than on SCC.45 Furthermore, some reports have shown that there was no correlation between the expression levels of EGFR and clinical outcomes.46 Thus, while gefitinib has a dramatic anticancer effect in a limited number of patients with NSCLC, the factors underlying its action have not been clarified.
In 2004, somatic mutations were identified in the EGFR tyrosine kinase domain of patients with gefitinib-responsive lung cancer, as compared with no mutations in patients exhibiting no response, and the presence of an EGFR mutation was highly correlated with a good response to gefitinib.9,10 The conformational change of the EGFR ATP-binding site caused by genetic mutations constitutively activates the EGFR downstream signaling pathway and increases the malignancy of cancer. Conversely, the conformational change of the ATP-binding site can also increase its affinity for gefitinib; therefore, gefitinib can inhibit the downstream signaling pathway more easily, strongly induces apoptosis, and reduces the proliferation of cancer cells.32 Before the publication of these reports, gefitinib was considered to be effective for non-smokers, AC, females, and Asian populations because EGFR mutations were common in these groups. Currently, the presence of an EGFR mutation, but not being a non-smoker, AC, female, or of Asian descent, is considered to be an independent factor that largely affects the therapeutic effect of gefitinib.11,47
In contrast, Cappuzzo et al48 studied which biomarker was most effective for predicting the clinical outcome of gefitinib—a high EGFR gene copy number, high protein expression levels, or mutations. They concluded that only a high EGFR gene copy number was associated with prolonged survival on multivariable analysis.48 In a clinical trial comparing erlotinib as an EGFR-TKI and a placebo for the treatment of NSCLC, Tsao et al49 also demonstrated with multivariate analysis that patients with AC, never-smokers, and EGFR expression were associated with an objective response and survival after treatment with erlotinib was not influenced by EGFR expression, number of EGFR copies, or presence of an EGFR mutation. They also concluded that among NSCLC patients who received erlotinib, the presence of an EGFR mutation may increase their responsiveness to the agent, but this was not indicative of a survival benefit.12
Conversely, in a prospective clinical trial that assessed the efficacy of gefitinib for untreated NSCLC patients harboring EGFR mutations, gefitinib was reported to show cytoreductive effects in 70%–80% of this population. This finding was also supported by two key clinical trials in the Japanese population reported by Mitsudomi et al13 and Maemondo et al14 in 2010. Furthermore, in 2011, Fukuoka et al15 reported the results of biomarker analyses in the IRESSA Pan-Asia Study (IPASS), namely that EGFR mutations were the strongest predictive biomarkers for PFS and tumor response to first-line gefitinib therapy versus carboplatin/paclitaxel therapy, and that the predictive value of the EGFR gene copy number was driven by a coexisting EGFR mutation.15 These reports have made it increasingly clear that the presence of EGFR mutations, but not the EGFR gene copy number, is the strongest predictive biomarker for the therapeutic efficacy of gefitinib.
Mutations in exons 18–21 of EGFR are predictive factors for the clinical efficacy of gefitinib; deletions in exon 19 and missense mutations in exon 21 account for ~90% of these mutations.9,10,19,47,50 The G719 and L861 mutations have also been reported as minor mutations that affect the efficacy of gefitinib; however, other minor mutations such as E709 and S768 have reportedly no influence on its efficacy.51 Thus, at least at the present time, the detection of EGFR mutations in exons 19 and 21 is considered to be essential to predict the clinical efficacy of gefitinib. In 2005, Pao et al16 reported that mutations of the RAS or RAF genes, whose products are located in the downstream signaling pathway of EGFR, were potent factors for predicting resistance to gefitinib,16 and that these factors are considered to be important in the selection of patients who can be treated with gefitinib. In particular, RAS mutations were reported not to exist simultaneously with EGFR mutations in one tissue sample, and the detection of KRAS mutations has also been used by some clinical facilities to select patients who can be treated with gefitinib.19,52 However, it has been infrequently reported that some patients have simultaneous EGFR and KRAS mutations, and it is controversial whether patients can be selected for treatment with gefitinib only on the basis of detecting an EGFR or KRAS mutation.53
Acquired resistance
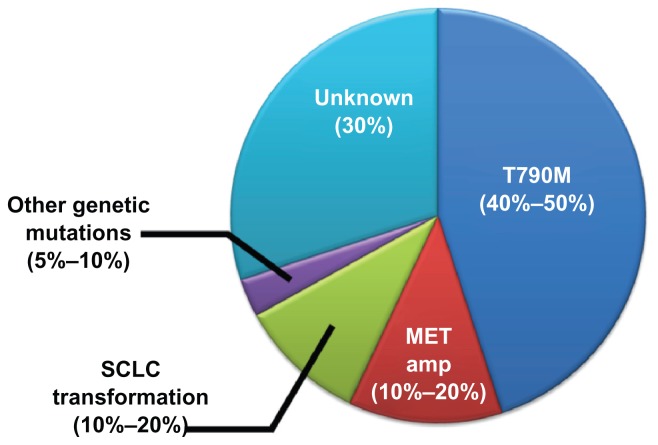
Unfortunately, all responders eventually develop resistance to gefitinib, most commonly because of genetic variations or pathological changes. Recently, therapeutic strategies to treat and to avoid the establishment of gefitinib-resistant cancer cells have been focused upon and discussed. In 2005, an EGFR mutation in exon 20, which substitutes methionine for threonine at amino acid position 790 (T790M), was reported to be one of the main causes of acquired resistance to gefitinib.54,55 The EGFR T790M variant changes the structural conformation of the ATP-binding site, thereby increasing the affinity of ATP to EGFR, while the affinity of gefitinib to ATP is unchanged. Thus, the T790M variant reduces the relative efficacy of gefitinib and causes the development of acquired resistance. Balak et al56 reported that the T790M variant was found in ~50% of NSCLC patients with acquired resistance to gefitinib. However, the T790M variant was also found in some patients who were not treated with gefitinib, and the presence of the T790M mutation was reported to be significantly higher in advanced tumors than in early-stage tumors. Thus, Inukai et al57 proposed that, although the detection of T790M-positive tumor cells may be useful for predicting the clinical efficacy of gefitinib, gefitinib treatment might result in the selection of T790M mutant cells and that even a small fraction of T790M-positive tumor cells at the beginning of treatment could lead to clinical resistance as a result of the selective proliferation of T790M mutant cells.
In addition to the presence of the T790M variation, L747S, D761Y, and G796A variations were identified in NSCLC patients with acquired resistance to gefitinib; the G796A variant is especially known to cause strong resistance to EGFR-TKIs.51 In 2007, Engelman et al58 found that the amplification of MET caused gefitinib resistance by driving the ErbB3-dependent activation of PI3K, a pathway thought to be specific to the EGFR/ErbB family of receptors. In recent studies, amplification of the MET gene was found in approximately 10%–20% of all cases with drug resistance.59,60 In 2011, Sequist et al61 reported a very interesting account of the mechanisms underlying the establishment of drug resistance; in 37 patients with gefitinib-resistant NSCLC carrying EGFR mutations, five resistant tumors transformed from NSCLC into SCLC and were sensitive to standard SCLC treatments. They also reported that serial biopsies in three patients revealed that genetic mechanisms of resistance were lost in the absence of the continued selective pressure of EGFR inhibitor treatment, and such cancers were sensitive to a second round of treatment with EGFR inhibitors. In many studies, including the reports mentioned above, approximately 70% of the causes of acquired resistance have been clarified (Fig. 3). Now, for the more effective use of gefitinib, the establishment of a method to avoid or to prevent the development of acquired resistance is required and is being widely studied.
Figure 3.
Cause of acquired resistance to gefitinib.
Screening methods for EGFR and KRAS mutations
The detection of EGFR and KRAS mutations has been usually achieved by sequencing DNA amplified from tumor tissues; however, sequencing techniques are too complex, time-consuming, and expensive for routine screening in the clinical setting. Adding to the complexity is the problem that clinical samples often contain a subpopulation of mutant cells within an excess of normal tissue, sometimes causing the mutations to be missed by sequencing because of the limitations of the technology. Recently, several methods have been reported for the detection of EGFR and KRAS mutations, including polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), single-strand conformation polymorphism (PCR SSCP), mutant-allele-specific amplification, mutant-enriched assays, PCR-invader method, Cycleave-PCR, Scorpion-amplified refractory mutation system assay, TaqMan PCR, denaturing high-performance liquid chromatography, high resolution melting assay, high-resolution chipCE, immunohistochemistry, and the peptide nucleic acid-locked nucleic acid PCR clamp method. Some of these techniques have quite a high level of sensitivity,52,62–66 but most are still not ideal for routine clinical use in general hospitals or outpatient clinics due to their long turnaround times, high cost, and complexity. Subsequently, we reported a rapid, simple, and highly sensitive method, named SmartAmp2, for the detection of EGFR and KRAS mutations.67–69 We demonstrated that SmartAmp2 is useful for the detection of mutations from tissue samples with marked fibrosis or formalin-fixed, paraffin-embedded samples with damaged DNA.
In previous reports, KRAS mutations were estimated to occur in 3.0%–15.0% of lung ACs in Asian individuals.16,19,70 However, we demonstrated that the variation in the prevalence of these mutations is thought to be partly an artifact resulting from the varying sensitivities and accuracies of the different detection methods.67 The selection of an appropriate method to detect EGFR and KRAS mutations is essential to make an exact prediction of the efficacy of gefitinib in individual patients.
Clinical Studies
Phase II trials
Two randomized double-blind Phase II trials for gefitinib have been reported: the IRESSA Dose Evaluation in Advanced Non-small Cell Lung Cancer (IDEAL)-1 trial, which was carried out mainly in Japan, Europe, Australia, and South Africa, and IDEAL-2, which was performed mainly in the United States.5,6 These studies examined patients with locally advanced or metastatic NSCLC, which are less sensitive to chemotherapy (including therapies with a platinum compound), and they assessed the antitumor effect of orally administered gefitinib. In IDEAL-1, the RRs were 18.4% in the group using 250 mg/day gefitinib and 19% in the group using 500 mg/day; in IDEAL-2, the RRs were 11.8% and 8.8%, respectively. In the 250 mg/day group, treatment was discontinued in 14.5%–15.5% of patients. In these studies, although less than 1% of patients in the 250 mg/day group required a reduction in their daily dose of gefitinib because of adverse events, the dose of gefitinib was reduced in 22.8%–28.3% of patients, and in the 500 mg/day group, 8.8%–10.4% of patients could not continue gefitinib therapy. In the IDEAL studies, the factors predicting the response to gefitinib were reported to be Japanese, female, non-smokers, and AC.
In these two studies, the frequency of grade 3/4 adverse events was 8.7% and 6.9% in the 250 mg/day groups, respectively. Conversely, in the 500 mg/day groups, they occurred in 30.2% and 17.5% of patients, respectively. Although the clinical efficacy of gefitinib was almost equal in both groups, the adverse events were clearly fewer in the 250 mg/day groups; thus, the recommended dose was concluded to be 250 mg/day.
Phase III trials
To assess the additional benefit of gefitinib as a first-line treatment (in combination with gemcitabine plus cisplatin or paclitaxel plus carboplatin), two large-scale randomized clinical trials, IRESSA NSCLC Trial Assessing Combination Treatment (INTACT)-1 and -2,71,72 were carried out on 2,130 patients with advanced NSCLC (Table 1). Unfortunately, as a result of the INTACT studies, no additional effect of gefitinib in combination with standard chemotherapy was reported at the European Society for Medical Oncology (ESMO) congress in 2002. The following factors were suggested as causes of these negative results:73 (1) gefitinib was not administered completely as scheduled because of the incidence of adverse reactions, and in the 500 mg/day gefitinib group particularly, dose reduction occurred in ~50% of patients, and therapy was discontinued in ~25%; (2) they did not assess the status of EGFR overexpression or genetic mutations because studies indicating that EGFR mutations could be used as biomarkers to predict the clinical outcome of gefitinib were reported after the INTACT studies had finished. The population of patients used in these studies might make it difficult to perform a precise analysis of the effects of gefitinib; and (3) gefitinib exerts an antiproliferative effect and may not have an additive effect when all proliferating cells have already been killed by other forms of chemotherapy.
Table 1.
Large-scale clinical studies of gefitinib.
| Name | Stage | Screening of patients | 1st-line or treated | Comparison | Result |
|---|---|---|---|---|---|
| IDEAL-1 | Phase II | Q | Treated | Positive | |
| IDEAL-2 | Phase II | – | Treated | Positive | |
| INTACT-1 | Phase III | – | 1st | Gefitinib, GEM, CDDP vs. GEM, CDDP | Negative |
| INTACT-2 | Phase III | – | 1st | Gefitinib, PTX, CBDCA vs. PTX, CBDCA | Negative |
| ISEL | Phase III | – | Treated | Gefitinib vs. Best supportive care | Negative |
| V-15-32 | Phase III | Japanese | Treated | Gefitinib vs. DTX | Negative |
| INTEREST | Phase III | – | Treated | Gefitinib vs. DTX | Positive |
| IPASS | Phase III | Non-or slight smoker, adenocarcinoma, Asian | 1st | Gefitinib vs. CBDCA, PTX | Positive |
| WJTOG3405 | Phase III | EGFR mutant, Japanese | 1st | Gefitinib vs. PTX, CBDCA | Positive |
| NEJ002 | Phase III | EGFR mutant, Japanese | 1st | Gefitinib vs. DTX, CDDP | Positive |
Abbreviations: GEM, gemcitabine; CDDP, cisplatin; PTX, paclitaxel; CBDCA, carboplatin; DTX, docetaxel.
In 2004, the IRESSA Survival Evaluation in Lung Cancer (ISEL) Phase III placebo-controlled study was carried out on 1,692 patients with advanced NSCLC.74 The population was set as refractory to or intolerant of their latest chemotherapy. As a result, although gefitinib did not significantly prolong the median overall survival (OS) in all patients or patients with AC, as was the case in INTACT, gefitinib did significantly prolong the median OS compared with placebo in a subset analysis of 342 Asian patients (9.5 versus 5.5 months, respectively; hazard ratio [HR] 0.66, 95% CI 0.48–0.91, P = 0.01) and 374 nonsmokers (8.9 versus 6.1 months, respectively, HR 0.67, 95% CI 0.49–0.92, P = 0.012).
In 2008, two large clinical trials with conflicting results were reported. The V-15-32 study of 489 Japanese patients with treated advanced NSCLC did not confirm the non-inferiority of gefitinib in OS compared with docetaxel therapy (HR 1.12, 95.24% CI 0.89–1.40) according to the predefined criterion (upper CI limit for HR ≤ 1.25).75 Conversely, the IRESSA NSCLC Trial Evaluating Response and Survival versus Taxotere (INTEREST) with 1,466 treated patients showed the comparable effects of gefitinib to docetaxel (Taxotere) for the first time; the median OS was 7.6 months in the gefitinib group and 8.9 months in the docetaxel group, and the 1-year survival was 32% and 34% (HR 1.020, 96% CI 0.905–1.150), respectively.76 Differences in subsequent therapies were suggested as a reason for the different results of these two studies. In the V-15-32 study, while 36% of patients in the gefitinib group received docetaxel after the end of gefitinib therapy, at least 53% of patients in the docetaxel group received gefitinib after the end of docetaxel therapy. Thus, the OS of the docetaxel group might be prolonged by the use of gefitinib compared to the gefitinib group. Conversely, in INTEREST, 31% of the gefitinib group received docetaxel after gefitinib therapy, and 37% of the docetaxel group received EGFR-TKIs, including gefitinib, after docetaxel therapy.
From 2006 to 2007, one of the most important clinical trials, IPASS, was conducted with a selected population that could be expected to respond to gefitinib.15,77 In IPASS, 1,217 nonsmokers or slight smokers (≥ 15 years of not smoking), non-treated, advanced, AC, and Asian patients were distributed randomly to a gefitinib group or to a combination chemotherapy with carboplatin and paclitaxel group. The primary endpoint was set as PFS, while the secondary end-points were OS, RR, quality of life (QOL), and safety. In the analysis of the primary endpoint (announced at ESMO 2008)78 the superiority of gefitinib compared to carboplatin/paclitaxel combination chemotherapy was demonstrated in all eligible patients (HR 0.74, 95% CI 0.65–0.85, P < 0.001). Furthermore, additional analysis showed that the superiority of gefitinib was found to be more significant in the population of EGFR mutation-positive patients compared to all patients enrolled in IPASS, and this analysis clarified that gefitinib was quite effective in patients with EGFR mutations compared to carboplatin/paclitaxel combination chemotherapy. In the patients with EGFR mutations, gefitinib reduced the risk of disease progression by 52% compared with carboplatin/paclitaxel combination chemotherapy (HR 0.48, 95% CI 0.36–0.64, P < 0.001), and it improved the median PFS from 6.3 months to 9.5 months. Similar results were also shown in two key Phase III trials conducted in Japan for patients with EGFR mutations (WJTOG3405 and NEJ002).13,14 Thus, IPASS, WJTOG3405, and NEJ002 are considered to be key clinical trials for the formation of selection criteria for patients who will respond to gefitinib. However, the use of gefitinib as a first-line therapy did not improve OS because most patients randomized into the carboplatin/paclitaxel as first-line therapy group had used gefitinib at some stage of their therapy after first-line therapy failed.15
Safety
Treatments using molecular-targeted drugs including gefitinib are regarded as well tolerated compared with other forms of chemotherapy. The frequently observed adverse effects of gefitinib are rash (37%), diarrhea (27%), nausea (17%), and vomiting (14%).74 Conversely, the INTEREST study showed that myelosuppression, neurotoxicity, and renal toxicity, widely known as common ADRs of chemotherapy, were not observed in the patients using gefitinib.76 On the other hand, some serious ADRs were observed in such patients, and these prevented the continuation of gefitinib treatment.
Although EGFR overexpression is found in many tumors, non-tumor cells also express EGFR on their membranes, and the inhibition of EGFR on non-tumor cells is regarded as the cause of the majority of ADRs from gefitinib. The reduction of activated EGFR in cells composing skin tissue induces hyperkeratosis, folliculitis, and decreased hydration, and these conditions can lead to a skin rash.78 Furthermore, different skin manifestations are observed depending on the kind of cells in which EGFR is inhibited by gefitinib and the stage of therapy. Acneiform dermatitis, including edema and redness on the face or upper trunk, occurs in the early stage of gefitinib therapy and is caused by the inhibition of EGFR in basal keratinocytes. In the middle stage of gefitinib therapy, dry skin is caused by abnormal keratinocytes whose differentiation into the stratum corneum is interrupted. In the latter stage of gefitinib therapy, paronychia occurs frequently, and is caused by a brittle and slower growing nail plate in the nail matrix. Many of these symptoms can be managed by the use of moisturizers or steroids. In addition, the use of tetracycline antibiotics (doxycycline and minocycline) may be effective to treat rash.79 The effects of steroids on the care of skin disorders are attributed to their anti-inflammatory activity through the inhibition of neutrophil migration and suppression of active oxygen and free radical synthesis (Table 2).
Table 2.
Summary of patient demographics, characteristics, and ADR in the ISEL and INTEREST studies.
| Study | ISEL | INTEREST | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Gefitinib | Placebo | Gefitinib | Placebo | |||||
|
|
|
|
|
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Sex | ||||||||
| Total | 1129 | (100) | 563 | (100) | 733 | (100) | 733 | (100) |
| Male | 761 | (67.4) | 378 | (67.1) | 466 | (63.6) | 488 | (66.6) |
| Female | 368 | (32.6) | 185 | (32.9) | 267 | (36.4) | 245 | (33.4) |
| Race | ||||||||
| White | 843 | (74.7) | 431 | (76.6) | 550 | (75.0) | 540 | (73.7) |
| Asian | 235 | (20.8) | 107 | (19.0) | 154 | (21.0) | 169 | (23.1) |
| Black | 9 | (0.80) | 5 | (0.89) | 10 | (1.4) | 12 | (1.6) |
| Other | 42 | (3.7) | 20 | (3.5) | 19 | (2.6) | 12 | (1.6) |
| Adverse events | ||||||||
| Rash | 413 | (36.7) | 56 | (10.0) | 360 | (49.1) | 73 | (10.0) |
| Diarrhea | 309 | (27.4) | 52 | (9.3) | 255 | (34.8) | 177 | (24.1) |
| Nausea | 190 | (16.9) | 90 | (16.0) | 148 | (20.2) | 187 | (25.6) |
| Vomiting | 152 | (13.5) | 56 | (10.0) | 109 | (14.9) | 123 | (16.8) |
| Dry skin | 128 | (11.4) | 20 | (3.6) | 111 | (15.1) | 10 | (1.4) |
| Constipation | 108 | (9.6) | 71 | (12.6) | 79 | (10.8) | 121 | (16.5) |
| Pyrexia | 79 | (7.0) | 27 | (4.8) | 69 | (9.4) | 118 | (16.1) |
| Stomatitis | 68 | (6.0) | 22 | (3.9) | 67 | (9.1) | 93 | (12.7) |
| Anemia | ND | ND | 34 | (4.6) | 84 | (11.5) | ||
| Alopecia | ND | ND | 23 | (3.1) | 254 | (34.7) | ||
| Neutropenia | ND | ND | 35 | (4.8) | 514 | (70.1) | ||
| Febrile neutropenia | ND | ND | 9 | (1.2) | 72 | (9.8) | ||
| Neurotoxicity | ND | ND | 49 | (6.7) | 171 | (23.3) | ||
Abbreviation: ND, not detected.
Interstitial lung disease (ILD) is reported as one of the serious and critical ADRs of gefitinib, and the risk of ILD is higher in the early stage of therapy, especially within 4 weeks after administration. Post-marketing surveillance in Japan demonstrated that the incidence of ILD from gefitinib in the Japanese population was 5.8%, consistent with the rate of ILD observed in the Japanese V-15-32 study (5.7 versus 2.9% for docetaxel).80 However, the incidence of ILD in the Japanese population was reported to be higher than in other populations. According to the results of the INTEREST study, the incidence of ILD with gefitinib was 1.4%, which is the same as for docetaxel in the Caucasian population.76 Studies performed in other Asian countries also reported a lower rate of ILD than observed in Japan.80,81 The ISTANA study, performed in Korea, reported an incidence of 3.7% for ILD in patients receiving gefitinib and 3.9% in the docetaxel group;82 a study in Taiwan also showed a lower incidence of ILD when gefitinib was used as a first-line therapy.83 A nested case-control study investigating relative risk and risk factors for the incidence of ILD with gefitinib therapy was performed in the Japanese population and it was found that the use of gefitinib was a stronger risk factor for ILD than other forms of chemotherapy (OR 3.23). In addition to gefitinib, smoking history, poor performance status (PS), preexisting ILD, and a low occupancy rate of the normal lung were demonstrated as risk factors.84 Although the rate of ILD remains controversial, patients should be monitored carefully, at least within 4 weeks of the start of gefitinib therapy.
As a mechanism for the increased incidence of ILD caused by gefitinib, Ishiguro et al85 discussed the contribution of transporters. They demonstrated that gefitinib reduces phosphatidylcholine biosynthesis by inhibiting cellular choline uptake in A549 and rat ATII cells, which is mainly mediated by CTL1, resulting in the abnormal secretion of lung surfactants, which could be one of mechanisms underlying the association of ILD with gefitinib. Although this hypothesis has not been demonstrated in any in vivo or clinical studies, this finding might provide an important insight into how to control the incidence of ILD caused by gefitinib.
Efficacy
Although the IDEAL study reported that the frequency and severity of the adverse effects associated with gefitinib depended on the dose, its efficacy was not significantly different between the 500 and 250 mg/day groups; therefore, 250 mg/day was decided as the optimal dose. This daily dose was reported to be sufficient to inhibit EGFR activity in skin biopsies and to ensure adequate exposure.32
In 2004, it was reported that EGFR mutations were associated with the RR to gefitinib,9,10 which is now known to reach 80%–90% in patients with an EGFR mutation.12,47,86,87 Furthermore, as described above, the IPASS study showed that gefitinib could significantly prolong PFS in patients with an EGFR mutation.78 Therefore, the efficacy of gefitinib is known to be more affected by EGFR gene status than clinical background factors. In addition, a recent study reported a favorable outcome for the use of gefitinib in EGFR mutation-positive patients who were contraindicated for other forms of chemotherapy (elderly or PS 3–4); the RR was 66% and the median OS was 17.8 months.88 This suggests that treatment with gefitinib might be effective for patients with an EGFR mutation and not suitable for other standard forms of chemotherapy because of some other reason such as advanced age or a poor PS.
Kim et al89 indicated that gefitinib might be more effective than erlotinib for patients with lung AC and asymptomatic synchronous brain metastasis. This might be due to the higher intracellular concentration of gefitinib compared to erlotinib. Although erlotinib is known to reach a higher concentration in plasma than gefitinib in the clinical dose setting, the ratio of the intratumor concentration of gefitinib to its plasma concentration was reportedly much higher than that of erlotinib.90 Although EGFR-TKIs are considered to have a lower permeability of the blood–brain barrier, gefitinib might be highly concentrated in cells regardless of its lower plasma concentration, and it may exert stronger effects upon brain metastasis.
Patient Preference
Because, as previously described, the effect of gefitinib depends on the presence of EGFR mutations and not on the copy number of the EGFR gene, a high copy number alone should not be used as a selection tool for first-line treatment of NSCLC with gefitinib; the presence of a mutation in EGFR exons 19 or 21 should be used instead.15 Although smoking history was also considered to be an important factor affecting the response to gefitinib, recent studies showed that EGFR mutations were frequently found in the tissues of nonsmoker NSCLC patients, and the presence of EGFR mutations was the strongest predictive biomarker for the therapeutic efficacy of gefitinib.91 Furthermore, because of the molecular mechanisms underlying the effect of EGFR mutations on the efficacy of gefitinib, the presence of EGFR mutations, but not smoking history, should be used to select patients.
Conversely, a high copy number of the EGFR gene has been considered to be the strongest factor in predicting the efficacy of erlotinib, and the presence of EGFR mutations was reported not to affect the response to erlotinib.92 Thus, at the present time, the presence of EGFR mutations should be one of the selection criteria for the use of the EGFR-TKIs gefitinib and erlotinib for NSCLC patients; gefitinib is suitable for patients with EGFR mutations, while erlotinib is suitable for patients without EGFR mutations. However, Farina et al93 presented the results of a Phase III study, the Tarceva Italian Lung Optimization Trial, which assessed the clinical benefits of erlotinib in an NSCLC population without EGFR mutations in comparison to docetaxel. They reported that docetaxel was significantly more effective for NSCLC patients without an EGFR mutation compared with erlotinib. Thus, the place of erlotinib therapy and how to choose which patients are suitable for gefitinib or erlotinib are still controversial.
To use EGFR-TKIs, the risk of ILD should also be considered because it is known as the most critical adverse effect of both of gefitinib and erlotinib, even though the incidence of ILD is controversial. Recently Ando et al reported that a history of IP could significantly increase the risk of ILD. Consequently, although patients with a smoking history were previously regarded as unsuitable for treatment with gefitinib, recent studies have indicated that exempting smoking patients from those patients who will respond to gefitinib is not appropriate because it is known that a smoking history does not reflect the history of IP. Furthermore, because IP consists of many clinicopathologic types, such as organizing pneumonia, usual IP, and lymphocytic IP, it is also not considered to be suitable to exclude patients with a history of IP alone.
Thus, at least at the present time, patients who will respond to gefitinib should be selected according to the presence of EGFR mutations, and gefitinib should be used while considering the risk of ILD, especially in patients with a history of smoking or IP.
Place in Therapy
Many studies reported that gefitinib could significantly improve the PFS and QOL of patients and suggested that it can be used at any stage of therapy for NSCLC with EGFR mutations;13 however, controversy remains over whether it should be used as a first-line therapy. Recently, the majority of studies assessing the usefulness of gefitinib in first-line therapy have shown that although it could significantly improve PFS, its use as a first-line drug had insignificant effects on OS because most of the patients randomized into the control group (which did not use gefitinib as a first-line therapy) had used gefitinib at a previous stage of therapy after their first-line therapy failed. Thus, no studies have been able to recommend the best timing for the use of gefitinib. However, some studies have reported the successful rechallenge of patients using gefitinib or therapy using standard SCLC treatments after acquiring gefitinib resistance. Moreover, although the possibility that gefitinib may not be suitable for patients with a poor PS in the later stage of therapy was reported,61 Inoue et al88 indicated that EGFR mutation-positive patients with an extremely poor PS benefit from first-line gefitinib therapy. Thus, there are some who think that gefitinib should be used in the early stage of therapy for patients with EGFR mutations and especially for patients with advanced age, poor PS, or advanced cancer to avoid missing the opportunity of using gefitinib. Furthermore, in 2011, Oxnard et al94 reported a significant improvement of OS by the continuation of EGFR-TKI therapy beyond that expected from disease progression. According to these reports and discussions, gefitinib may be used in the early stage of therapy, but not as a final tool for those patients expected to respond to gefitinib with a lower risk of ILD. Although the high efficacy of continuing gefitinib beyond disease progression has been reported, more studies are required to conclude the efficacy of gefitinib therapy beyond disease progression.
With regard to genetic analysis, we believe that at least the EGFR genotypes should be examined in all cases, and ideally, EGFR and KRAS genotypes should be examined from the clinical and basic research point of view. In Japan, EGFR genotyping for all cases and EML4-ALK genotyping for those patients with wild-type EGFR was recommended in the clinical situation. Patients with an EGFR mutation should receive EGFR-TKI therapy in the early stage of therapy. Although there is little evidence to support the idea that this strategy would benefit patients with mutations in both EGFR and KRAS given the extremely limited number of reported cases, we consider that these cases should be regarded as patients with an EGFR mutation and receive TKIs under careful observation, and these cases should be reported.
However, there are some problems associated with the genetic analysis of patient samples. For example, genetic analysis cannot be performed in facilities with limited resources because of its complexity, time consuming nature, and high cost. However, samples should be submitted to genetic analysis services, and a therapeutic strategy should be devised according to the genotypes. If genetic analysis is not available due to some reasons such as time or environmental constraints, a clinicopathological diagnosis or general status may help physicians to choose a target or non-target therapy.
Recently, some genetic analysis methods, which can be used at the bedside or in a general outpatient clinic, have been developed. We also reported a new rapid and highly sensitive method that can detect genetic variations within 1 hour. We believe that genetic analysis will be available in all facilities and at all bedsides in the near future.
Conclusions
The identification of the relationship between the presence of EGFR mutations and a higher response to gefitinib has dramatically improved the outcome of NSCLC therapy. Although there is a clinical benefit reported for patients with EGFR mutations receiving gefitinib as first-line therapy, genetic status does not appear to influence the survival of NSCLC patients receiving gefitinib as second- or third-line therapy when compared with other standard forms of chemotherapy. Exploratory biomarker analysis in the INTEREST study showed that there was no significant difference in the OS between gefitinib and docetaxel according to EGFR mutation status; however, there did appear to be improved PFS and higher RR for patients with EGFR mutations treated with gefitinib than in those treated with docetaxel.
Some studies are currently ongoing in Asian and other populations to further define the role of gefitinib in the treatment of advanced NSCLC. At the present time, we have many findings to identify the appropriate timing and appropriate patients for gefitinib; however, the impact of inter-individual differences for the PK of gefitinib on the inter-individual differences in clinical outcomes has not been clarified, and we cannot adjust the blood concentration of gefitinib for individual patients. We believe that, as a next stage, PK studies of gefitinib can improve its efficacy. Some genetic variations of CYPs, which metabolize gefitinib, and transporters, such as ABCG2, were reported, and the impact of those genetic variations on the PK of some drugs was also revealed. Furthermore, Nakamura et al42 indicated the effect of the blood concentration of gefitinib on its clinical effects. Consequently, although the IDEAL study assessed the optimal dose of gefitinib in all enrolled patients, analyzing the effects of genetic variations or the PK of gefitinib on the clinical outcome might be important in order to increase the effect of gefitinib in the future.
We believe that the identification of the cause of acquired drug resistance and incidence of ILD, as well as the establishment of a method to avoid both of them, will further improve the outcome of therapy using EGFR-TKIs.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: T Araki, HY, T Aomori, TH. Contributed to the writing of the manuscript: T Araki, KK, KS, KY. Agree with manuscript results and conclusions: KS, KK, KY. Jointly developed the structure and arguments for the paper: T Araki, HY, KS, T Aomori, TH, KK, TN, KY. Made critical revisions and approved final version: KS, KY. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25(5):561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 3.Demedts IK, Vermaelen KY, Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. 2010;35(1):202–15. doi: 10.1183/09031936.00105009. [DOI] [PubMed] [Google Scholar]
- 4.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep. 1973;34(2):31–42. [PubMed] [Google Scholar]
- 5.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21(12):2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 7.Sumpter K, Harper-Wynne C, O’Brien M, Congleton J. Severe acute interstitial pnuemonia and gefitinib. Lung Cancer. 2004;43(3):367–8. doi: 10.1016/j.lungcan.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361(9352):137–9. doi: 10.1016/S0140-6736(03)12190-3. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513–20. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 12.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24(21):3340–6. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. for West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, et al. for North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 16.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SW, Kim TY, Jeon YK, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res. 2006;12(8):2538–44. doi: 10.1158/1078-0432.CCR-05-2845. [DOI] [PubMed] [Google Scholar]
- 18.Bae NC, Chae MH, Lee MH, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet. 2007;173(2):107–13. doi: 10.1016/j.cancergencyto.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 20.Böni-Schnetzler M, Pilch PF. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci U S A. 1987;84(22):7832–6. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoeberl B, Eichler-Jonsson C, Gilles ED, Müller G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotechnol. 2002;20(4):370–5. doi: 10.1038/nbt0402-370. [DOI] [PubMed] [Google Scholar]
- 22.Wiley HS, Shvartsman SY, Lauffenburger DA. Computational modeling of the EGF-receptor system: a paradigm for systems biology. Trends Cell Biol. 2003;13(1):43–50. doi: 10.1016/s0962-8924(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 23.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira S, van Bergen en Henegouwen PM, Storm G, Schiffelers RM. Molecular biology of epidermal growth factor receptor inhibition for cancer therapy. Expert Opin Biol Ther. 2006;6(6):605–17. doi: 10.1517/14712598.6.6.605. [DOI] [PubMed] [Google Scholar]
- 25.Nakata A, Gotoh N. Recent understanding of the molecular mechanisms for the efficacy and resistance of EGF receptor-specific tyrosine kinase inhibitors in non-small cell lung cancer. Expert Opin Ther Targets. 2012;16(8):771–81. doi: 10.1517/14728222.2012.697155. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka K, Ohnishi H, Furuyashiki G, et al. Clinico-pathological and biological significance of tyrosine kinase domain gene mutations and overexpression of epidermal growth factor receptor for lung adenocarcinoma. J Thorac Oncol. 2006;1(8):787–95. [PubMed] [Google Scholar]
- 27.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37( Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 28.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 29.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-smallcell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Greulich H, Jänne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65(19):8968–74. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20(21):4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 32.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20(9):2240–50. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 33.Frampton JE, Easthope SE. Gefitinib: a review of its use in the management of advanced non-small-cell lung cancer. Drugs. 2004;64(21):2475–92. doi: 10.2165/00003495-200464210-00008. [DOI] [PubMed] [Google Scholar]
- 34.McKillop D, Hutchison M, Partridge EA, et al. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica. 2004;34(10):917–34. doi: 10.1080/00498250400009171. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Kamenecka TM, Cameron MD. Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol. 2009;22(10):1736–42. doi: 10.1021/tx900256y. [DOI] [PubMed] [Google Scholar]
- 36.Ozvegy-Laczka C, Hegedus T, Várady G, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65(6):1485–95. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 37.Galetti M, Alfieri RR, Cavazzoni A, et al. Functional characterization of gefitinib uptake in non-small cell lung cancer cell lines. Biochem Pharmacol. 2010;80(2):179–87. doi: 10.1016/j.bcp.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Cusatis G, Sparreboom A. Pharmacogenomic importance of ABCG2. Pharmacogenomics. 2008;9(8):1005–9. doi: 10.2217/14622416.9.8.1005. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Zhao M, He P, Hdalgo M, Baker SD. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13(12):3731–7. doi: 10.1158/1078-0432.CCR-07-0088. [DOI] [PubMed] [Google Scholar]
- 40.Swaisland HC, Smith RP, Laight A, et al. Single-dose clinical pharmacokinetic studies of gefitinib. Clin Pharmacokinet. 2005;44(11):1165–77. doi: 10.2165/00003088-200544110-00004. [DOI] [PubMed] [Google Scholar]
- 41.Bergman E, Forsell P, Persson EM, et al. Pharmacokinetics of gefitinib in humans: the influence of gastrointestinal factors. Int J Pharm. 2007;341(1–2):134–42. doi: 10.1016/j.ijpharm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Sano K, Soda H, et al. Pharmacokinetics of gefitinib predicts antitumor activity for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1404–9. doi: 10.1097/JTO.0b013e3181e59a7b. [DOI] [PubMed] [Google Scholar]
- 43.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–30. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 45.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(6):1103–9. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 46.Cappuzzo F, Gregorc V, Rossi E, et al. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J Clin Oncol. 2003;21(14):2658–63. doi: 10.1200/JCO.2003.01.039. [DOI] [PubMed] [Google Scholar]
- 47.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97(9):643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 49.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 50.Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11(3):1167–73. [PubMed] [Google Scholar]
- 51.Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–21. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 52.Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23(4):857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 53.Miyamae Y, Shimizu K, Hirato J, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep. 2011;25(4):921–8. doi: 10.3892/or.2011.1182. [DOI] [PubMed] [Google Scholar]
- 54.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 56.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12(21):6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 57.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66(16):7854–8. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 58.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 59.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11. doi: 10.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 61.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Endo K, Konishi A, Sasaki H, et al. Epidermal growth factor receptor gene mutation in non-small cell lung cancer using highly sensitive and fast TaqMan PCR assay. Lung Cancer. 2005;50(3):375–84. doi: 10.1016/j.lungcan.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Minarik M, Gassman M, Belsanova B, et al. A novel high-resolution chipCE assay for rapid detection of EGFR gene mutations and amplifications in lung cancer therapy by a combination of fragment analysis, denaturing CE and MLPA. Electrophoresis. 2010;31(21):3518–24. doi: 10.1002/elps.201000156. [DOI] [PubMed] [Google Scholar]
- 64.Nagai Y, Miyazawa H, Huqun, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid locked nucleic acid PCR clamp. Cancer Res. 2005;65(16):7276–82. doi: 10.1158/0008-5472.CAN-05-0331. [DOI] [PubMed] [Google Scholar]
- 65.Nomoto K, Tsuta K, Takano T, et al. Detection of EGFR mutations in archived cytologic specimens of non-small cell lung cancer using high-resolution melting analysis. Am J Clin Pathol. 2006;126(4):608–15. doi: 10.1309/N5PQNGW2QKMX09X7. [DOI] [PubMed] [Google Scholar]
- 66.Thomas RK, Nickerson E, Simons JF, et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med. 2006;12(7):852–5. doi: 10.1038/nm1437. [DOI] [PubMed] [Google Scholar]
- 67.Mitani Y, Lezhava A, Kawai Y, et al. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat Methods. 2007;4(3):257–62. doi: 10.1038/nmeth1007. [DOI] [PubMed] [Google Scholar]
- 68.Araki T, Shimizu K, Nakamura K, et al. Usefulness of peptide nucleic acid (PNA)-clamp smart amplification process version 2 (SmartAmp2) for clinical diagnosis of KRAS codon 12 mutations in lung adenocarcinoma: comparison of PNA-clamp SmartAmp2 and PCR-related methods. J Mol Diagn. 2010;12(1):118–24. doi: 10.2353/jmoldx.2010.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araki T, Shimizu K, Nakamura T, et al. Clinical screening assay for EGFR exon 19 mutations using PNA-clamp smart amplification process version 2 in lung adenocarcinoma. Oncol Rep. 2011;26(5):1213–9. doi: 10.3892/or.2011.1391. [DOI] [PubMed] [Google Scholar]
- 70.Toyooka S, Tsukuda K, Ouchida M, et al. Detection of codon 61 point mutations of the K-ras gene in lung and colorectal cancers by enriched PCR. Oncol Rep. 2003;10(5):1455–9. doi: 10.3892/or.10.5.1455. [DOI] [PubMed] [Google Scholar]
- 71.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22(5):777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol. 2004;22(5):785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson E. Surprise phase III failure for ZD1839. Lancet Oncol. 2002;3(10):583. doi: 10.1016/s1470-2045(02)00883-5. [DOI] [PubMed] [Google Scholar]
- 74.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 75.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small- cell lung cancer. J Clin Oncol. 2008;26(26):4244–52. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 76.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 77.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 78.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–12. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 79.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54(2):258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Jiang H. Overview of gefitinib in non-small cell lung cancer: an Asian perspective. Jpn J Clin Oncol. 2009;39(3):137–50. doi: 10.1093/jjco/hyn139. [DOI] [PubMed] [Google Scholar]
- 81.Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol. 2008;26(16):2745–53. doi: 10.1200/JCO.2007.15.6695. [DOI] [PubMed] [Google Scholar]
- 82.Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 83.Lie CH, Chang HC, Chao TY, et al. First- or second-line gefitinib therapy in unknown epidermal growth factor receptor mutants of non-small-cell lung cancer patients treated in Taiwan. Clin Lung Cancer. 2011;12(2):116–24. doi: 10.1016/j.cllc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Kudoh S, Kato H, Nishiwaki Y, et al. for Japan Thoracic Radiology Group. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177(12):1348–57. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 85.Ishiguro N, Oyabu M, Sato T, alameda T, Minami H, Tamai I. Decreased biosynthesis of lung surfactant constituent phosphatidylcholine due to inhibition of choline transporter by gefitinib in lung alveolar cells. Pharm Res. 2008;25(2):417–27. doi: 10.1007/s11095-007-9362-9. [DOI] [PubMed] [Google Scholar]
- 86.Tamura K, Okamoto I, Kashii T, et al. for West Japan Thoracic Oncology Group. Multicentre prospective phase UU trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJOTG0403) Br J Cancer. 2008;98(5):907–14. doi: 10.1038/sj.bjc.6604249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26(15):2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 88.Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27(9):1394–400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 89.Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351–4. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 90.McKillop D, Partridge EA, Kemp JV, et al. Tumor penetration of gefitinib (Iressa), an epidermal growth factor receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2005;4(4):641–9. doi: 10.1158/1535-7163.MCT-04-0329. [DOI] [PubMed] [Google Scholar]
- 91.Toyooka S, Takano T, Kosaka T, et al. Epidermal growth factor receptor mutation, but not sex and smoking, is independently associated with favorable prognosis of gefitinib-treated patients with lung adenocarcinoma. Cancer Sci. 2008;99(2):303–8. doi: 10.1111/j.1349-7006.2007.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu CQ, da Cunha Santos G, Ding K, et al. for National Cancer Institute of Canada Clinical Trials Group Study BR.21. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26(26):4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 93.Farina G, Longo F, Martelli o, et al. Rationale for treatment and study design of tailor:a randomized phase 3 trial of second-line erlotinib versus docetaxel in the treatment of patients affected by advanced non-small-cell lung cancer with the absence of epidermal grouth factor receptor mutations. Clin Lung Cancer. 2011;12(2):138–41. doi: 10.1016/j.cllc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]