Antenatal preventive administration of sulfadoxine-pyrimethamine did not potentiate pregnancy-associated malaria morbidity despite expansion and fixation of drug-resistant malaria parasites. Sulfadoxine-pyrimethamine may be a safe component of malaria prevention programs without causing adverse effects on birth outcomes.
Abstract
Background. Antenatal intermittent preventive therapy with 2 doses of sulfadoxine-pyrimethamine (IPTp-SP) is the mainstay of efforts in sub-Saharan Africa to prevent pregnancy-associated malaria (PAM). Recent studies report that drug resistance may cause IPTp-SP to exacerbate PAM morbidity, raising fears that current policies will cause harm as resistance spreads.
Methods. We conducted a serial, cross-sectional analysis of the relationships between IPTp-SP receipt, SP-resistant Plasmodium falciparum, and PAM morbidity in delivering women during a period of 9 years at a single site in Malawi. PAM morbidity was assessed by parasite densities, placental histology, and birth outcomes.
Results. The prevalence of parasites with highly SP-resistant haplotypes increased from 17% to 100% (P < .001), and the proportion of women receiving full IPTp (≥2 doses) increased from 25% to 82% (P < .001). Women who received full IPTp with SP had lower peripheral (P = .018) and placental (P < .001) parasite densities than women who received suboptimal IPTp (<2 doses). This effect was not significantly modified by the presence of highly SP-resistant haplotypes. After adjustment for covariates, the receipt of SP in the presence of SP-resistant P. falciparum did not exacerbate any parasitologic, histologic, or clinical measures of PAM morbidity.
Conclusions. In this longitudinal study of malaria at delivery, the receipt of SP as IPTp did not potentiate PAM morbidity despite the increasing prevalence and fixation of SP-resistant P. falciparum haplotypes. Even when there is substantial resistance, SP may be used in modified IPTp regimens as a component of comprehensive antenatal care.
Pregnancy-associated malaria (PAM) is an important, preventable cause of poor birth outcomes in malaria-endemic areas in sub-Saharan Africa [1]. Intermittent preventive therapy with antimalarials during pregnancy (IPTp) is a critical tool for reducing the incidence of PAM-attributable adverse birth outcomes [2]. Sulfadoxine-pyrimethamine (SP) is the most commonly administered drug for IPTp owing to its long half-life, favorable side-effect profile, and safety during pregnancy. However, Plasmodium falciparum strains harboring mutations associated with SP resistance are increasingly prevalent throughout sub-Saharan Africa.
The outcome of antimalarial therapy results from complex interactions between parasite pathogenicity, host immunity, drug characteristics, and parasite susceptibility to drug therapy. Recent in vitro studies have suggested that the removal of susceptible parasites from heterogeneous parasitemias by antimalarial therapy may facilitate the relative overgrowth of the remaining resistant parasites and therefore potentiate their pathogenicity [3]. A recent study in Tanzania documented increases in placental inflammation that were associated with SP receipt, suggesting that SP may potentiate placental pathology when applied to partially susceptible infections [4]. However, the effect of these placental findings upon birth outcomes was unclear. Given the prevalence of SP resistance and the current lack of appropriate, safe alternatives for use as IPTp, it is critical to explore the associations between IPTp-SP, SP resistance, and delivery outcomes in further contexts to inform malaria control policies.
Malawi adopted a policy of IPTp with SP in 1993, and despite the subsequent development of widespread SP resistance [5], a sustained decline in the prevalence of PAM was observed from 1997 to 2005 [6]. In view of the evidence from Tanzania [4], we hypothesized that the emergence of SP-resistant parasites would modify the effect of IPTp-SP and thereby potentiate placental inflammation and parasite densities and worsen birth outcomes. The Queen Elizabeth Central Hospital Epidemiology of Resistance in Pregnancy-Associated Malaria (QuEERPAM) study [7] was a serial cross-sectional analysis in which we explored the relationships between IPTp-SP, the presence of resistant parasites at delivery, and multiple measures of adverse delivery outcome, including parasite densities, placental histology, maternal hemoglobin concentration, and birth weight.
METHODS
Ethics Statement
Ethical approval for this study was granted by the review boards of the Malawi Health Sciences Research Committee, the University of Malawi College of Medicine, and the University of North Carolina at Chapel Hill.
Enrollment and Sample Collection
Patient enrollment and sample collection have been described elsewhere [6, 8]. In brief, pregnant women delivering between July 1997 and August 2006 at Queen Elizabeth Central Hospital in Blantyre, Malawi, were invited to participate. Those who consented to participate were queried regarding demographic and clinical information. The receipt of SP antenatally was obtained from antenatal clinic cards; from 1999 onward, the date of the last dose of SP was available.
Peripheral and placental blood was used to prepare thick blood smears, which were read by 2 microscopists for the presence and density of P. falciparum parasites. From 1998 onward, full-thickness placental biopsies were wax embedded, stained with modified Giemsa and/or hematoxylin and eosin, and assessed by 2 trained observers masked to other clinical data. Histologic indices included (1) density of parasitemia, expressed as percentage of all observed erythrocytes that were parasitized; (2) mononuclear cell infiltrate, expressed as percentage of all cells observed in the intervillous space; and (3) semiquantitative assessment of malaria pigment deposition in fibrin, as an indicator of chronic placental inflammation [9]. Maternal hemoglobin concentration was measured using HemoCue, and newborns were weighed within 1 day of birth.
Genotyping Procedures
A subset of 25% of available samples from women with positive peripheral blood thick smears for P. falciparum parasites was manually selected at random by personnel blinded to all clinical information. From these specimens, genomic DNA was extracted and P. falciparum parasites were genotyped for mutations in the dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) genes by direct sequencing [7]. To reduce contamination risk, separate work areas were maintained for molecular steps and filtered pipette tips were used for all procedures.
Definitions and Statistical Analyses
Mutations were investigated at codons 51, 59, 108, and 164 of dhfr and codons 437, 540, 581, and 613 of dhps. Haplotypes were assigned based upon codons 51, 59, and 108 in dhfr and 437 and 540 in dhps [10]. The “quintuple mutant” haplotype consisted of mixed or mutant alleles at all 5 loci in dhfr and dhps; “partial wild-type” haplotypes were those with a wild-type allele at any locus. Recent antenatal SP receipt was defined as within 60 days prior to delivery, owing to the prolonged half-life and activity of SP [11]. Maternal anemia was defined as a hemoglobin concentration <11 g/dL (any) and <9 g/dL (moderate), and low birth weight was defined as <2500 g. Antenatal SP use was categorized as “full IPTp” (≥2 doses) or “suboptimal IPTp” (<2 doses).
Antenatal indices and birth outcomes (parasite densities, placental histologic indices, maternal hemoglobin concentration, and birth weight) were compared between women who received suboptimal or full IPTp using the Kruskal-Wallis rank test, the Student t test, or the χ2 test. The prevalence of moderate anemia and low birth weight were compared between groups using the χ2 test. To assess for effect modification of the effect of IPTp upon birth outcomes, we stratified women into those harboring partial wild-type parasites and those with quintuple mutant parasites; after stratification, we repeated comparisons of birth outcomes between IPTp groups.
To account for covariates (including the effect of the year of delivery and the effect of resistant P. falciparum) in the analysis of the association between IPTp-SP and birth outcomes, we first used linear or logistic regression to compare maternal peripheral parasite density, placental parasite density, maternal hemoglobin concentration, birth weight, and the dichotomized proportions of moderate anemia and low birth weight between women who received suboptimal and full IPTp. Parasite densities were natural log-transformed prior to regression modeling. Subsequently, adjusted comparisons were then computed by repeating regression models after the inclusion of covariates representing delivery year and P. falciparum haplotype, as well as reported mediators of placental pathology: human immunodeficiency virus (HIV) infection [12], gravidity (primigravid versus multigravid) [4], and the recent receipt of SP.
Clinical data were initially entered into Epi Info or Microsoft Access; all statistical analyses were ultimately performed using Stata/IC, version 10.
RESULTS
Antenatal Indices
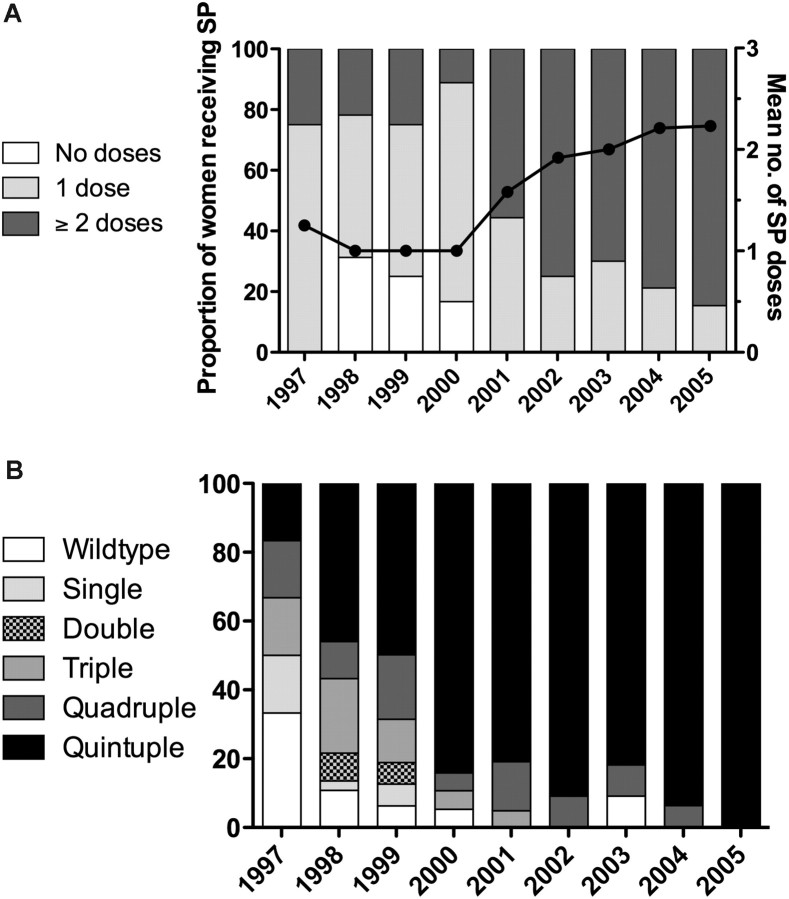
Full genotyping and antenatal data were available for 177 women with P. falciparum parasites on peripheral blood smear from 1997 to 2005. The patients in this subset were similar to the overall cohort from which they were drawn [6]. The mean (standard deviation [SD]) age was 21.7 years (4.8) and 52% were primigravidae (Table 1). Of 128 women tested for HIV, 45 (35.2%) were positive; 90.4% received SP antenatally, with a mean (SD) number of doses of 1.59 (0.9). Of 120 women who received SP for whom the date of receipt was recorded, 74 (61.7%) had received SP within 60 days prior to delivery. Between years there were significant differences in the mean number of SP doses (P < .001), the proportion of women receiving SP (P < .001) (Figure 1A), and the proportion of women who received SP within the preceding 60 days (P = .002) (data not shown).
Table 1.
Antenatal Indices by IPTp-SP Use and Plasmodium falciparum dhfr-dhps Haplotype
| Suboptimal IPTp |
Full IPTp |
|||||
|---|---|---|---|---|---|---|
| Overall | Partial Wild-type (n = 28) | Quintuple Mutant (n = 59) | Partial Wild-type (n = 12) | Quintuple Mutant (n = 78) | P Valuea | |
| Age, years, mean (SD) | 21.7 (4.8) | 21.2 (3.73) | 22.1 (4.8) | 19.9 (3.1) | 21.9 (5.4) | .730 |
| HIV positive,b % | 35.2 | 25 | 52.2 | 28.6 | 25.5 | .062 |
| Primigravid, % | 52.0 | 46.4 | 39.0 | 50.0 | 64.1 | .006 |
| SP doses, mean (SD) | 1.59 (0.9) | 0.64 (0.49) | 0.88 (0.33) | 2.33 (0.49) | 2.35 (0.66) | <.001 |
| % recent SPc | 61.7 | 25.0 | 30.8 | 85.7 | 81.8 | <.001 |
| Year of enrollment, median | 2001 | 1999 | 2000 | 2001 | 2004 | <.001 |
Haplotypes assigned per Kublin et al [10].
Abbreviations: HIV, human immunodeficiency virus; IPTp, intermittent preventive therapy with antimalarials during pregnancy; SD, standard deviation; SP, sulfadoxine-pyrimethamine.
a For comparison of suboptimal and full IPTp groups. Determined by Student t test, Kruskal-Wallis test, or χ2 test for continuous and categorical data.
b Data available only for 128 women.
c Last dose of SP received within 60 days prior to delivery. Data available only in years after 1999 for 120 women.
Figure 1.
Trends in sulfadoxine-pyrimethamine (SP) use and Plasmodium falciparum dhfr-dhps haplotypes over time. (A) Proportion of women receiving SP (gray bars) and the average number of doses received (black line); (B) the prevalence of dhfr-dhps haplotypes over time. Haplotypes assigned per Kublin et al [10].
Antenatal Associations With IPTp-SP and Drug Resistance
Overall, 67.9% of women harbored the quintuple mutant haplotype, the prevalence increasing from 16.7% in 1997 to 100% in 2005 (Figure 1B) [7]. Pure wild-type dhfr-dhps haplotypes declined from 33.3% to 0. Overall, differences in the prevalences of haplotypes were significant between years (P < .001). Using Poisson regression, the prevalence rate of the quintuple mutant haplotype increased by an average of 11% each year (1.11; 95% confidence interval, 1.08–1.16).
The mutation in codon 164 of dhfr was not detected in any year; for dhps, the mutation at codon 581 was detected in 1 sample in 2004, and the mutation in codon 613 was detected in 1 sample in 1998. No novel mutations were detected in the sequenced segments of dhfr and dhps.
Age and HIV infection were not associated with IPTp use (Table 1). Compared with women in the suboptimal IPTp group, women in the full IPTp group had a higher proportion of primigravidae (62.2% versus 41.4%; P = .006), greater mean number of doses of SP (0.8 versus 2.3; P < .001), and a higher proportion of women who had received SP within 60 days prior to delivery (82.2% versus 29.8%; P < .001).
Delivery Outcomes
Of the 177 women with full antenatal and genotyping data, missing data resulted in measurements of maternal hemoglobin, birth weight, peripheral parasite density, placental parasite density, and placental histology being available for 176, 152, 172, 108, and 133 women, respectively. Mean (SD) birth weight was 2834 g (417), and 13.8% of births were low birth weight (Table 2). Mean (SD) maternal hemoglobin concentration was 10.6 g/dL (1.8): 58% had any anemia and 15.9% were moderately anemic. Median (interquartile range [IQR]) parasite density in the maternal peripheral blood of parasitemic women was 526 parasites/μL (IQR, 60–3495) and in the placental blood was 1069 parasites/μL (IQR, 73–9185). On placental histologic analyses, median parasite density was 8.0% (IQR, 1.5%–22.1%), median mononuclear cell infiltrate was 4.8% (IQR, 1.8%–10.4%) of all cells in the intervillous space, and 18.7% of women had no malaria pigment deposition.
Table 2.
Bivariate and Multivariate Associations Between Receipt of IPTp-SP and Pregnancy Outcomes
| Overall | Suboptimal IPTp | Full IPTp | P Value (Unadjusted) | P Value (Adjusted)a | |
|---|---|---|---|---|---|
| Maternal parasite density, parasites/μL median (IQR) | 526 (60–3495) | 780 (105–8370) | 226 (49–2118) | .014 | .349 |
| Placental parasite density, parasites/μL median (IQR) | 1069 (73–9185) | 4898 (300–13 800) | 168 (52–1072) | <.001 | .659 |
P values determined by linear regression. Parasite densities were natural log-transformed prior to inclusion in regression models.
Abbreviations: IPTp, intermittent preventive therapy with antimalarials during pregnancy; IQR, interquartile range; SP, sulfadoxine-pyrimethamine.
a Adjusted for gravidity, human immunodeficiency virus infection, delivery year, and Plasmodium falciparum dhfr-dhps haplotype.
Parasite densities varied significantly between years: median maternal peripheral density decreased from 1997 to 2005 from 38 748 to 97 parasites/μL and median placental parasite density decreased from 10 470 (in 1998) to 68 parasites/μL (both P < .001; data not shown). Additionally, placental histology scores varied significantly between years: the proportion of placentae with no malaria deposition in fibrin increased from 7.4% to 63.6% (P < .001) and median placental parasitemia decreased from 23.5% to 8.1% (P < .001). Median mononuclear cell infiltrate in the intervillous space varied significantly between 2.2% and 7.9% (P = .039), but there was no overall trend. There were no differences between years in mean birth weight, maternal hemoglobin, or the prevalence of any anemia or low birth weight births.
Relationship Between IPTp-SP and Delivery Outcomes
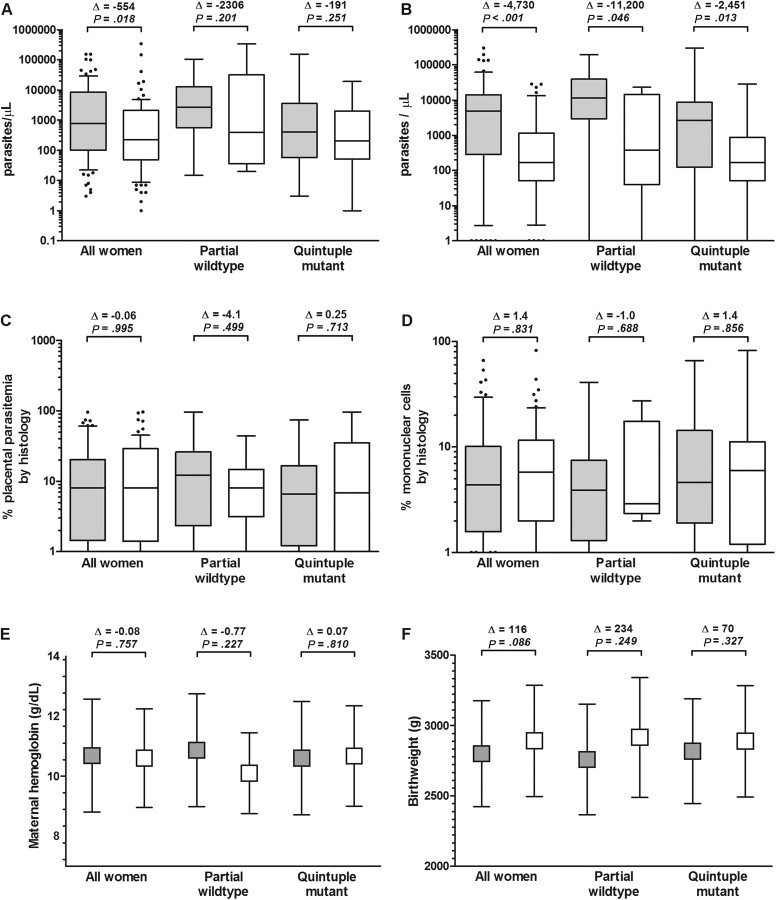
Compared with women who received suboptimal IPTp (n = 87), women who received full IPTp (n = 90) had lower median parasite densities in the peripheral blood (226 versus 780 parasites/μL; P = .018) and placenta (168 versus 4898 parasites/μL; P < .001; Figure 2A and 2B). There were no significant differences between women who received full and suboptimal IPTp in median placental parasitemia by histology (8.1% versus 8.0%; P = .995), placental inflammation (median mononuclear cell infiltrate, 4.4% versus 5.8%; P = .831), mean maternal hemoglobin level (10.6 versus 10.7 g/dL; P = .757), the prevalence of moderate anemia (13.5% versus 18.4%; P = .373), mean birth weight (2892 versus 2776 g; P = .086), or the prevalence of low birth weight (11.8% versus 15.8%; P = .481; Figure 2C–F).
Figure 2.
Parasitologic, histologic, and clinical delivery outcomes by intermittent preventive therapy with antimalarials during pregnancy (IPTp)–sulfadoxine-pyrimethamine use and stratified by Plasmodium falciparum dhfr-dhps haplotype. Gray boxes: suboptimal IPTp; white boxes: full IPTp. (A) Median (interquartile range [IQR]) maternal peripheral parasite densities; (B) median (IQR) maternal placental parasite densities; (C) median (IQR) percentage of placental parasitemia by histology; (D) median (IQR) percentage of mononuclear cells in the intervillous space; (E) mean (standard deviation) maternal hemoglobin (g/dL); (F) birth weight (g). Outcomes were compared between IPTp groups using the Student t test or the Kruskal-Wallis test. Δ indicates difference in median or mean of the full IPTp group relative to the suboptimal IPTp group.
To analyze whether the presence of resistant parasites modified the effect of IPTp, we repeated these analyses after stratifying by the presence of quintuple mutant parasites (Figure 2). Although women who received full IPTp had lower peripheral parasite densities in both the presence and the absence of quintuple mutant parasites, the differences within each parasite subgroup were not significant (Figure 2A). In contrast, the receipt of full IPTp was significantly associated with lower placental parasite densities in both the presence (168 versus 2620; P = .013) and the absence (374 versus 11 575; P = .046) of quintuple mutant haplotypes (Figure 2B). Thus, the presence of quintuple mutant parasites did not modify the association of full IPTp receipt with reduced parasite densities at delivery.
Multivariate Analyses of IPTp-SP and Delivery Outcomes
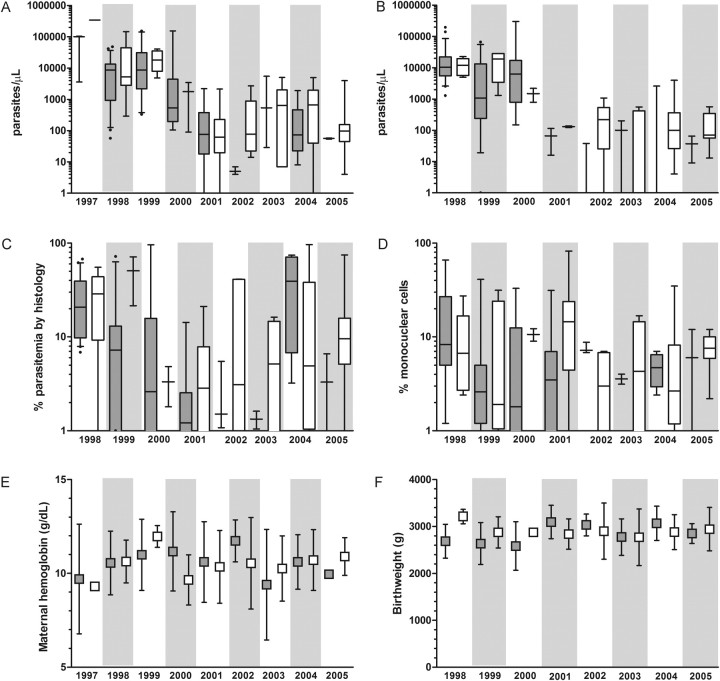
Between 1998 and 2005, there were significant decreases in parasite densities in women who received either suboptimal or full IPTp (Figure 3A and 3B; Supplementary Table), while histologic indices of inflammation and parasitemia and clinical outcomes of maternal hemoglobin and birth weight were unchanged (Figure 3B–D and 3F).
Figure 3.
Pregnancy outcomes over time stratified by receipt of intermittent preventive therapy with antimalarials during pregnancy (IPTp)–sulfadoxine-pyrimethamine. Gray boxes: suboptimal IPTp; white boxes: full IPTp. (A) Median (interquartile range [IQR]) maternal peripheral parasite density; (B) median (IQR) placental parasite density; (C) median (IQR) percentage of parasitemia by histology; (D) median (IQR) percentage of mononuclear cells in the intervillous space; (E) mean (standard deviation [SD]) maternal hemoglobin; (F) mean (SD) birth weight. Neither birth weight nor placental outcomes were available for 1997.
After adjusting for year of delivery, HIV infection, gravidity, and the presence of P. falciparum quintuple mutants, the receipt of full IPTp was not associated with parasite densities, placental histology, maternal hemoglobin concentration, or birth weight (Table 2). In these models, only the year of delivery was significantly associated with the birth outcomes. Thus, the difference in parasite densities between women who received suboptimal and full IPTp with SP was largely accounted for by differences in the year of delivery.
DISCUSSION
In this serial, cross-sectional observational study in which SP-resistant P. falciparum isolates emerged and fixed in parasite populations, the receipt of IPTp with SP was not associated with poor birth outcomes or with the exacerbation of placental pathology. Indeed, the receipt of ≥2 doses of antenatal SP was associated with lower parasite densities, although these differences were most closely associated with the overall sustained declines in morbidity over the 9 years of the study. These data, which indicate that IPTp with SP does not potentiate malaria infection or its impact during pregnancy in Malawi, suggest that the continuation or intensification of IPTp-SP programs will not exacerbate PAM morbidity.
Prior studies of the clinical consequences of SP-resistant parasites have been conflicting: a cross-sectional study in Tanzania suggested that late-term receipt of SP was associated with both higher parasite densities and a greater prevalence of resistant parasites [4]. However, in a randomized trial in Mozambique, the receipt of SP as IPTp was not associated with PAM morbidity [12]. Compared with these studies, our investigation benefits by capturing a greater spectrum of P. falciparum SP resistance and transmission. Some malarious areas are experiencing sustained declines in malaria transmission [13–15], and our data are the first to describe the association of drug resistance and PAM morbidity in this dynamic context: from 1997 to 2005, the prevalence of parasitemia at delivery at our study site declined from 23.5% to 5%. Our data suggest that despite the possibility that IPTp-SP may contribute to the selection of quintuple mutant resistant parasites, the use of IPTp-SP is not associated with higher parasite densities, greater placental inflammation, or worsened delivery outcomes.
Recent reports from African settings document a decline in IPTp-SP efficacy, highlighting the need for new PAM-preventive strategies [6, 16, 17]. Because we focused on a selected population of parasite-positive women (who largely failed preventive therapy), we could not fully assess drug efficacy. Nevertheless, we did not detect measurable benefit of full IPTp. This finding may be accounted for by the increasing uptake of insecticide-treated bed nets [6, 18] or increasing parasite drug resistance in the setting of relative underdosing of SP, because only 14% (24 of 177) of women received >2 doses of SP. Feasible options for the replacement of current IPTp-SP regimens include intermittent therapy with another drug [19], intermittent screening and treatment of antenatal parasitemia [20], or more frequent dosing of SP [21, 22]. More frequent SP dosing is attractive for several reasons: (1) SP's desirable antenatal safety profile, tolerability, and pharmacokinetics; (2) evidence of improved SP efficacy with more frequent dosing [21–24]; (3) the limited tolerability of proposed replacement drugs [19]; and (4) measurable success (though suboptimal) incorporating SP administration into existing antenatal care services throughout Africa [25], which may facilitate the adoption of a new policy. Though IPTp-SP will undoubtedly be eclipsed by other PAM preventive strategies, it is likely to remain an important antenatal intervention in many African contexts for some time, and our data from a setting where SP resistance spread early and quickly allay fears that SP may exacerbate PAM morbidity as resistance spreads.
Why was IPTp-SP administration in the presence of resistant parasites not associated with greater degrees of PAM morbidity as predicted by murine [3] and clinical [4] studies? Epidemiologically, the time period of our study covered an overall decline in parasite prevalence among pregnant women, potentially reducing the ability to detect differences. Pharmacologically, in the setting of partial host immunity, SP is capable of clearing infections comprising resistant parasites [26] and thus may have limited parasite growth and placental inflammation despite mutant parasites. Genetically, a key difference between our study and that in Tanzania [4] is the absence of the dhps581 mutation. Similar to a study in Mozambique [12], this mutation is not prevalent in Malawi, whereas its promotion in pregnant women was posited as a key consequence of SP exposure in Tanzania. Prior in vitro allelic exchange experiments [27] and clinical studies in children [28] suggest that dhps581 does not confer substantially greater resistance or virulence, whereas the quintuple mutant haplotype that we use as a proxy for SP resistance is consistently associated with clinical SP failure [10, 29]. Nevertheless, it should be noted that known drug resistance genotypes might not be the only determinant of parasite phenotype in placental malaria. Also, because drug resistance haplotypes in sub-Saharan Africa have regional origins [30], a mutation such as dhps581 may be linked to mediators of pathogenicity that are heretofore undescribed and that modify response to antimalarials.
This study is subject to several limitations. Comparison to some prior studies [4, 12, 31] is limited owing to our analysis of peripheral instead of placental parasitemias. Nevertheless, the prevalence of drug-resistant genotypes between compartments is tightly correlated [31, 32]. The number of women harboring parasites with partial wild-type haplotypes was very low in some years. Because of this, we chose to study a broad range of clinical, parasitologic, and histologic outcomes to maximize our ability to detect differential morbidity. Bed-net use was not analyzed because it is not postulated to mediate the effect of drug resistance and may not confer added benefit when IPTp-SP coverage is high, as in our study [21]. This successful provision of SP to most (>85%) women in our study precluded an analysis of outcomes in women who did not receive SP. However, accumulating evidence supports a dose-response relationship between SP receipt and clinical outcomes [21], suggesting that the comparison between suboptimal and full IPTp-SP is biologically and clinically relevant. Finally, despite our multivariate modeling, as an observational study that is subject to prevailing and dynamic clinical factors, we cannot rule out confounding of the relationships between IPTp-SP, parasite haplotype, and morbidity by unmeasured clinical or parasite variables.
In this longitudinal observational study that benefits from consistent patient enrollment designed to limit bias and the rigorous assessment of a broad range of clinical, parasitologic, and histologic delivery outcomes, the receipt of IPTp-SP and infection with SP-resistant parasites at delivery were not associated with the exacerbation of PAM morbidity. Our study provides reassurance that the spread of parasites harboring the “quintuple mutant” haplotype may not exacerbate PAM morbidity in the context of SP use as IPTp and demonstrates the value of longitudinal, integrated clinical and molecular surveillance to inform policies to prevent pregnancy-associated malaria and improve maternal and neonatal health.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Mike Mistarz for his work in the laboratory (University of North Carolina), Alfredo Mayor (Universitat de Barcelona), Michel Cot (Institut de Recherche pour le Développement, Paris), and Brian Greenwood (London School of Hygiene and Tropical Medicine) for their comments on an earlier version of this manuscript. We are indebted to the patients for their participation.
Financial support. This work was supported by the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine (to F. O. tK.). Sample collection was funded by a Career Development Fellowship and a Senior Overseas Biomedical Research Fellowship awarded by the Wellcome Trust (to S. J. R.) and by grants from the National Institutes of Health (NIH; AI 49084), NIH-Fogarty International Center (5 D43 TW00908), and the Center for AIDS Research at the University of North Carolina (to S. R. M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgements section.
References
- 1.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 2.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 3.Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc Natl Acad Sci U S A. 2007;104:19914–9. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci U S A. 2009;106:9027–32. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sridaran S, McClintock SK, Syphard LM, Herman KM, Barnwell JW, Udhayakumar V. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247. doi: 10.1186/1475-2875-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng G, Simpson JA, Chaluluka E, Molyneux ME, Rogerson SJ. Decreasing burden of malaria in pregnancy in Malawian women and its relationship to use of intermittent preventive therapy or bed nets. PLoS One. 2010;5:e12012. doi: 10.1371/journal.pone.0012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor SM, Antonia A, Feng G, et al. Adaptive evolution and fixation of drug-resistant Plasmodium falciparum genotypes in pregnancy-associated malaria: 9-year results from the QuEERPAM study. Infect Genet Evol. 2012;12:282–90. doi: 10.1016/j.meegid.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogerson SJ, Chaluluka E, Kanjala M, Mkundika P, Mhango C, Molyneux ME. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997-99. Trans R Soc Trop Med Hyg. 2000;94:549–53. doi: 10.1016/s0035-9203(00)90083-x. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–9. [PubMed] [Google Scholar]
- 10.Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–8. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 11.White NJ. Intermittent presumptive treatment for malaria. PLoS Med. 2005;2:e3. doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez C, Serra-Casas E, Scahill MD, et al. HIV and placental infection modulate the appearance of drug-resistant Plasmodium falciparum in pregnant women who receive intermittent preventive treatment. Clin Infect Dis. 2011;52:41–8. doi: 10.1093/cid/ciq049. [DOI] [PubMed] [Google Scholar]
- 13.O'Meara WP, Bejon P, Mwangi TW, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–62. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattarai A, Ali AS, Kachur SP, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceesay SJ, Casals-Pascual C, Erskine J, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372:1545–54. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis. 2011;53:224–30. doi: 10.1093/cid/cir376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am J Trop Med Hyg. 2010;83:1212–20. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menendez C, Bardaji A, Sigauque B, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briand V, Bottero J, Noel H, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis. 2009;200:991–1001. doi: 10.1086/605474. [DOI] [PubMed] [Google Scholar]
- 20.Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS One. 2010;5:e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiga OM, Kayentao K, Traore BT, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in Mali: a randomized controlled trial. Clin Infect Dis. 2011;53:215–23. doi: 10.1093/cid/cir374. [DOI] [PubMed] [Google Scholar]
- 22.Filler SJ, Kazembe P, Thigpen M, et al. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. J Infect Dis. 2006;194:286–93. doi: 10.1086/505080. [DOI] [PubMed] [Google Scholar]
- 23.Kapito-Tembo A, Meshnick SR, van Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–72. doi: 10.1093/infdis/jiq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill CJ, Macleod WB, Mwanakasale V, et al. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. J Infect Dis. 2007;196:1577–84. doi: 10.1086/522137. [DOI] [PubMed] [Google Scholar]
- 25.van Eijk AM, Hill J, Alegana VA, et al. Coverage of malaria protection in pregnant women in sub-Saharan Africa: a synthesis and analysis of national survey data. Lancet Infect Dis. 2011;11:190–207. doi: 10.1016/S1473-3099(10)70295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cravo P, Culleton R, Hunt P, Walliker D, Mackinnon MJ. Antimalarial drugs clear resistant parasites from partially immune hosts. Antimicrob Agents Chemother. 2001;45:2897–901. doi: 10.1128/AAC.45.10.2897-2901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triglia T, Wang P, Sims PF, Hyde JE, Cowman AF. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 1998;17:3807–15. doi: 10.1093/emboj/17.14.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gesase S, Gosling RD, Hashim R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce RJ, Pota H, Evehe MS, et al. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 2009;6:e1000055. doi: 10.1371/journal.pmed.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mockenhaupt FP, Bedu-Addo G, Eggelte TA, et al. Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis. 2008;198:1545–9. doi: 10.1086/592455. [DOI] [PubMed] [Google Scholar]
- 32.Mockenhaupt FP, Bedu-Addo G, Junge C, Hommerich L, Eggelte TA, Bienzle U. Markers of sulfadoxine-pyrimethamine-resistant Plasmodium falciparum in placenta and circulation of pregnant women. Antimicrob Agents Chemother. 2007;51:332–4. doi: 10.1128/AAC.00856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.