Abstract
Borneol, a monoterpenoid alcohol, is used widely, particularly in combined formulas for preventing and curing cardiovascular and cerebrovascular diseases in traditional Chinese medicine. In order to understand the blood and brain pharmacokinetics after intravenous, intranasal, or oral administration and to investigate the superiority and feasibility of intranasal administration, a simple gas chromatographic (GC) method with flame ionization detection (FID) was developed for the quantification of borneol. Blood samples and brain were collected from mice at 1, 3, 5, 10, 20, 30, 60, 90, and 120 min after intravenous, intranasal, or oral administration of borneol at a dosage of 30.0 mg/kg. Sample preparations were carried out by liquid-liquid extraction with an internal standard solution of octadecane. The pharmacokinetic parameters were calculated by the software of Kinetica. The calibration curves were linear in the range of 0.11–84.24 μg/ml and 0.16–63.18 μg/g for borneol in plasma and brain, respectively. The methodological and extraction recoveries were both in the range of 85%–115%. The intra-day and inter-day variabilities for plasma and brain samples were ≤5.00% relative standard deviation (RSD). The absolute bioavailabilities F of intranasal and oral administrations were 90.68% and 42.99%. The relative brain targeted coefficients Re of intranasal and oral administrations were 68.37% and 38.40%. The GC-FID method developed could be applied to determination and pharmacokinetic study. The borneol from injection was distributed and metabolized fast without absorption process. The borneol from oral administration was distributed more slowly and had the lowest absolute bioavailability. Nasal administration of borneol was quickly absorbed into the blood and brain, was easy to use and had a greater safety than infection, which makes it worthy of further development as an administration route for encephalopathy treatment.
Keywords: Borneol, Intravenous administration, Intranasal administration, Oral administration, Pharmacokinetics
1. Introduction
Borneol is a monoterpenoid component composed of many traditional Chinese medicine prescriptions. Because of the rare resources and high price of natural borneol, synthetic borneol is used in Chinese formulas as a substitute for natural product. However, synthetic borneol arouses wide safety concerns. In recent years, new sources of natural borneol have been discovered, mainly in certain species of Cinnamonum tree (consists of d-borneol) or Blumea tree (consists of l-borneol) as described in Chinese Pharmacopoeia (State Pharmacopoeia Committee of People’s Republic of China, 2010). Based on initial pilot experiments in our laboratory (Lu et al., 2011a; Ma et al., 2011; Song et al., 2011), we choose the borneol extracted from the branches and leaves of Blum bal-samifera (L.) D.C for further study. Borneol functions in inducing resuscitation, clearing heat, and alleviating pain, and has been mainly used to treat cardiovascular and cerebrovascular diseases. Borneol has sedative and anticonvulsant effects, so it has doubled the effect on the central nervous system (CNS). Many studies have shown that borneol can improve the bioavailability of drugs, accelerate the opening of the blood-brain-barrier (BBB), and enhance the distribution of drugs in brain tissue (Li et al., 2007; Cai et al., 2008; Dai et al., 2009; Lu et al., 2011b).
With the development of administration technology, intranasal administration has become more widely used to treat encephalopathy and neurological disorders (Hanson et al., 2009; Dhuria et al., 2010; Lochhead and Thorne, 2012). Intranasal delivery provides a practical and non-invasive method of bypassing the BBB to deliver therapeutic components to the brain (Hanson and Frey, 2008). However, borneol tissue distribution or pharmacokinetic studies of intranasal administrations is rarely reported (Huang et al., 2009). Obviously, the borneol in plasma and brain after different administrations possibly affects the functions of borneol. The purpose of this study was to research the pharmacokinetics of borneol after intravenous, intranasal, and oral administrations and investigate the differences between them, and further to investigate the superiority and feasibility of intranasal administration, which could lay the foundation for brain targeting preparation.
2. Materials and methods
2.1. Instruments
Instruments used in this study were centrifuge (Anke TGL-16G, Anting Scientific Instrument Company, Shanghai, China), vortex mixer (VDRTEX-5, Shanghai Medical University Instrument Company, China), and homogenizer (IKA T10BS25, Germany). Analytical balances (BT25S, BS110S, Sartorius Instrument Company, Beijing, China) were also used in this study. A gas chromatographic (GC) system with D7980 station (Tianmei Scientific Instrument Company, Shanghai, China) was used in the study.
2.2. Chemicals
Borneol reference standards were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China, batch number: 110881-200706). Octadecane was of analytical grade (Guangfu Fine Chemical Research Institute, Tianjin, China). Ethyl acetate was of high performance liquid chromatography (HPLC) grade (Fisher Scientific, American). Borneol was purchased from Guizhou Golden Pharmaceutical Co., Ltd. (batch number: 0910312).
2.3. Animals
Male ICR (Institute of Cancer Research) mice (weight (20±2) g, No. 0256504) were purchased from Weitong Biotechnology Inc. (Beijing, China). All animals were clinically healthy and biochemically normal throughout the experimental period. The animals were fasted for 12 h with free access to water prior to treatment.
2.4. Solution preparation
The compound solution, normal saline solution-ethanol-propylene glycol 40:30:30 (v/v/v), was prepared. A weight of 1.5 g of borneol fine powder was dissolved into 10 ml of the compound solution, and then dissolved completely in ultrasonic for 10 min. We named this solution as nasal drops. For injection, we diluted 1 ml of nasal drops with 1% Tween-80 solution into 50 ml and filtrated using a 0.22 μm filter. For the oral solution, we diluted 1 ml of nasal drops with 1% Tween-80 solution into 50 ml.
2.5. Pharmacokinetic study
One hundred and thirty-five male ICR mice were divided into three groups (A, B, and C) randomly. Group A was injected with a 30.0 mg/kg dose of borneol (0.2 ml borneol injection) via tail vein. Group B was treated with 4 μl of borneol nasal drops (a dosage of 30.0 mg/kg) by unilateral nostril under anesthesia using ether. Group C was administrated a 30.0 mg/kg dose of borneol (0.2 ml borneol oral solution) by gavage needle via the esophagus into the stomach. Blood samples were collected into heparinized tubes by picking eyeball and the corresponding brains were also collected at 1, 3, 5, 10, 20, 30, 60, 90, and 120 min after drug administration. Five mice at each time point were euthanized. After centrifugation, the plasma was obtained. The brains were blotted with filter paper and accurately weighed.
An aliquot of 100 μl plasma sample was placed into a centrifuge tube and 10 μl octadecane solution (dissolved in ethyl acetate, concentration was 46.6 μg/ml, as internal standard solution) was added (Lu et al., 2011a). After being vortexed for 1 min, 90 μl of ethyl acetate was added. The solution was vortexed for 1 min and the mixture solution was centrifuged at 10 000 r/min for 10 min. A total volume of 1 μl of supernatant fluid was injected into the GC system.
The brains were homogenized after mixing with normal saline solution 1.5 times to the weight. An aliquot of 100 μl brain homogenate was placed into a centrifuge tube and 10 μl octadecane solution (dissolved in ethyl acetate, concentration was 46.6 μg/ml, as internal standard solution) was added. After being vortexed for 1 min, 90 μl of ethyl acetate was added. The solution was vortexed for 1 min, and centrifuged at 10 000 r/min for 10 min. Then 1 μl of supernatant fluid was injected into GC system.
2.6. GC assay method
GC-flame ionization detection (FID) instrument and chromatographic conditions are as follows: chemical separation was carried out on a TM-1701 capillary column (30 m×0.32 mm inside diameter, 0.25 μm film, Tianmei, China). The oven temperature was programmed to rise from an initial temperature of 100 °C (remaining 5.5 min) to 200 °C at a rate of 30 °C/min and then held isothermally at 200 °C for 5 min. The carrier gas was nitrogen at a flow rate of 28 ml/min. Injection volume was 1 μl. The injector temperature and detection temperature were set at 250 °C and 300 °C, respectively.
2.7. Method validation
2.7.1. Method validation of plasma samples
Blank plasma was prepared from heparinized whole-blood samples collected from mice. Borneol (21.06 mg) was dissolved into 25 ml of ethyl acetate resulting in a final concentration of 842.40 μg/ml. The solution was diluted with ethyl acetate to give serial concentrations of 1.05, 8.42, 21.06, 84.24, 252.72, 336.96, and 421.20 μg/ml. A total volume of 10 μl of corresponding borneol working solution was added to 100 μl blank plasma for serial concentrations containing 0.11, 0.84, 2.11, 8.42, 25.27, 33.70, 42.12, and 84.24 μg/ml of borneol for GC analysis were prepared. The calibration curve was obtained with eight different concentrations, each in triplicate.
The extractive recoveries were calculated for three different borneol concentrations (0.21, 4.21, and 42.12 μg/ml) in plasma of three replicate samples by comparing with solutions without blank plasma.
For intra-day precision and accuracy of borneol concentrations, three replicate quality control samples at each concentration (0.21, 4.21, and 42.12 μg/ml) were assayed on the same day. The inter-day precision and accuracy were evaluated on three different days.
Samples containing 0.21, 4.21, and 42.12 μg/ml borneol with three duplicates were prepared. The samples were put in 20–25 °C environment for 0, 6, and 12 h and analyzed in order to evaluate the samples stability.
The limit of quantification was determined by establishing the minimum borneol concentration in plasma that could be quantified with acceptable precision and accuracy under the chromatographic conditions.
2.7.2. Method validation of brain samples
The blank brain homogenates were prepared. A total volume of 10 μl of borneol working solution mentioned above was added to 100 μl blank brain homogenate and a serial concentrations containing 0.16, 1.26, 3.16, 6.32, 12.64, 25.27, 31.59, and 63.18 μg/g of borneol for GC analysis were prepared. The calibration curve was obtained with eight different concentrations each in triplicate.
The brain homogenate samples with 0.32, 6.32, and 31.59 μg/g borneol were prepared with each in triplicate. The extractive recoveries were calculated by comparing with solutions without blank brain homogenate.
Intra-day and inter-day variability were studied to evaluate the precision of the method based on three replicate samples at each concentration (0.32, 6.32, and 31.59 μg/g) of borneol in blank brain homogenate. The inter-day reproducibility test was carried out on three different days.
To evaluate the brain samples stability, samples containing 0.32, 6.32, and 31.59 μg/g borneol with three duplicates were prepared. The samples were put in 20–25 °C environment for 0, 6, and 12 h and analyzed.
The limit of quantification was determined by establishing the minimum concentration of borneol in brain homogenate that could be quantified with satisfactory accuracy and precision under the experimental conditions.
2.8. Statistical analysis
SPSS Version 16.0 was used for statistical analysis. The data were presented as mean±standard deviation (SD). Differences between group means were evaluated by paired t-test. A significance level P<0.05 was applied.
3. Results
3.1. Method validation of plasma samples
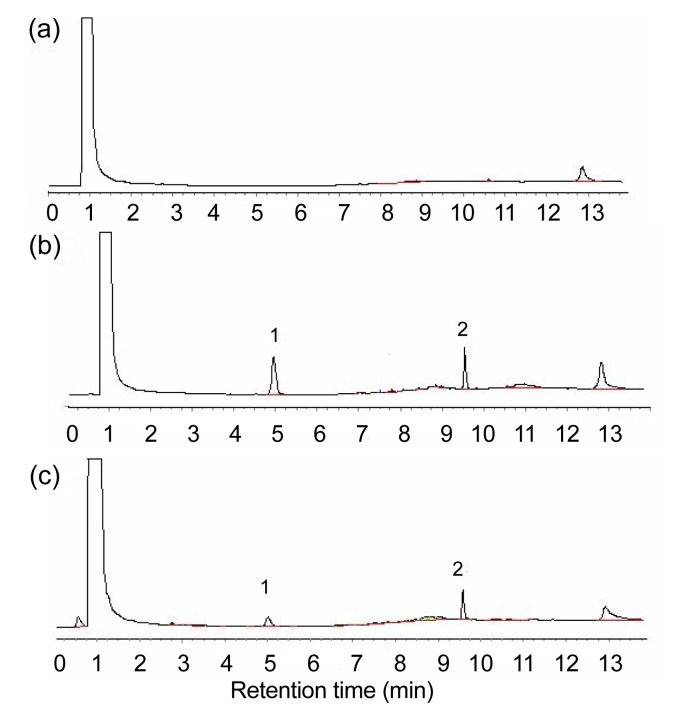
In the analytical conditions mentioned above, borneol, octadecane, and intrinsic substances were separated well, and the resolutions of the main peaks were all higher than 1.5 (Fig. 1). Under the chromatographic conditions described above, the retention times of borneol and octadecane were found at 4.9 min and 9.6 min, respectively.
Fig. 1.
Typical gas chromatographic (GC) chromatograms
(a) Blank mouse plasma; (b) Blank mouse plasma spiked with borneol and octadecane; (c) Plasma sample. 1: borneol; 2: octadecane
The calibration graphs were plotted by peak areas ratio of borneol and internal standard (Y) versus the concentration (X, μg/ml) of borneol. The regression equation was Y=0.1638X+0.0434 (correlation coefficient r=0.9999). Good linearity was obtained in the range of 0.11–84.24 μg/ml of borneol in plasma. The methodological recoveries of low, medium, and high concentrations were 102.05%, 96.92%, and 101.94%. The extractive recoveries were 99.20%, 97.62%, and 93.28%, respectively. The intra-day relative standard deviation (RSD) values of low, medium, and high concentrations were 4.74%, 3.33%, and 4.47%. And the inter-day RSD values were 2.62%, 4.35%, and 0.66%, respectively. The RSD values of low, medium, and high concentrations in 12 h were less than 5.00%. The plasma samples were stable in 12 h. The limit of quantification was 58 ng/ml.
3.2. Method validation of brain samples
In the analytical conditions mentioned above, borneol, octadecane, and intrinsic substances were separated well, and the blank brain homogenate had no interference to the main components’ analysis (Fig. 2).
Fig. 2.
Typical GC chromatograms
(a) Blank brain homogenate; (b) Blank brain homogenate spiked with borneol and octadecane; (c) Brain sample. 1: borneol; 2: octadecane
The calibration graphs were plotted by peak areas of the ratio of borneol and internal standard (Y) versus the borneol concentration in brain (X, μg/g). The regression equation was Y=0.1003X+0.0395 (r=0.9999). Good linearity was obtained in the range of 0.16–63.18 μg/g of borneol in brain. The methodological recoveries of low, medium and high concentrations were 100.08%, 99.10%, and 101.72%, respectively. The extractive recoveries were 97.97%, 93.46%, and 86.30%, respectively. The intra-day RSD values of low, medium, and high concentrations were 5.00%, 2.95%, and 0.79%, respectively. The inter-day RSD values were 4.91%, 3.28%, and 1.64%, respectively. The RSD values of low, medium, and high concentrations in 12 h were less than 5.00%. The brain samples were stable in 12 h. The limit of quantification was 80 ng/g.
3.3. Statistical analysis and pharmacokinetics
The concentration-time profiles of borneol in the plasma and brain after intravenous, intranasal, and oral administrations are shown in Figs. 3 and 4. Borneol was rapidly absorbed into the blood and brain and could be determined at 1 min after administration. The data of intravenous, intranasal, and oral administrations were fitted to a non-compartmental model. The main pharmacokinetic parameters of the non-compartmental model were calculated using Kinetica 4.4 software as shown in Tables 1 and 2. The absolute bioavailability was F, F=AUCblood(i.n./p.o.)/AUCblood(i.v.)×100%. The relative brain targeted coefficient was Re, Re=AUCbrain(i.n./p.o.)/AUCbrain(i.v.)×100%. The brain/blood drug ratio was Te, Te=AUCbrain/AUCblood×100%. So the drug target index was DTI, DTI=Te (i.n./p.o.)/Te (i.v.). The statistical differences were carried out by paired t-tests. (AUC: area under curve; i.v.: intravenous administration; i.n.: intranasal administration; p.o.: oral administration).
Fig. 3.
Mean plasma concentration-time curve of borneol in mice after intravenous, intranasal, or oral administration of 0.6 mg borneol
Data are expressed as mean±SD (n=5)
Fig. 4.
Mean brain concentration-time curve of borneol in mice after intravenous, intranasal, or oral administration of 0.6 mg borneol
Data are expressed as mean±SD (n=5)
Table 1.
Main plasma pharmacokinetic parameters of the non-compartmental model in mice after intravenous, intranasal, or oral administration
| Group | C max (μg/ml) | T max (min) | AUC0–120 min (µg∙ml−1·min) | MRT0–120 min (min) | F (%) |
| i.v. | 68.02±8.42 | 1 | 632.34±50.76 | 12.21±1.62 | 100 |
| i.n. | 25.94±4.95** | 3 | 573.42±77.71 | 28.17±1.38** | 90.68 |
| p.o. | 15.56±2.33** | 10 | 271.81±37.43** | 17.88±2.14** | 42.99 |
C max: calculated maximum concentration; T max: time corresponding to C max; AUC0–120 min: partial area under the curve from 0 to 120 min; MRT0–120 min: mean residence time from 0 to 120 min; F: absolute bioavailability; i.v.: intravenous administration; i.n.: intranasal administration; p.o.: oral administration. Data are expressed as mean±SD (n=5). * P<0.05
P<0.01 compared with the intravenous group
Table 2.
Main brain pharmacokinetic parameters of the non-compartmental model in mice after intravenous, intranasal, or oral administration
| Group | C max (μg/g) | T max (min) | AUC0–120 min (μg∙g−1·min) | MRT0–120 min (min) | Re (%) | Te (%) | DTI |
| i.v. | 43.02±5.11 | 1 | 505.34±82.91 | 20.47±8.83 | 100 | 79.92 | 1 |
| i.n. | 8.96±1.37** | 3.4 | 345.48±69.94* | 49.00±8.27** | 68.37 | 60.25 | 0.75 |
| p.o. | 8.54±1.96** | 10 | 194.03±23.46** | 43.63±17.07** | 38.40 | 71.38 | 0.89 |
C max: calculated maximum concentration; T max: time corresponding to C max; AUC0–120 min: partial area under the curve from 0 to 120 min; MRT0–120 min: mean residence time from 0 to 120 min; Re: relative brain targeted coefficient; Te: brain/blood drug ratio; DTI: the drug target index; i.v.: intravenous administration; i.n.: intranasal administration; p.o.: oral administration. Data are expressed as mean±SD (n=5)
P<0.05 compared with the intravenous group
P<0.01 compared with the intravenous group
4. Discussion
Based on the theory of traditional Chinese medicine, borneol is widely used as an assistive drug to medical prescriptions, which may contribute to help the other drugs enhance their pharmacological effects (Elshafeey et al., 2009; Lu et al., 2010; Li et al., 2012). Borneol has been mainly used to treat cardiovascular system diseases and encephalopathy in clinical therapy (Garcia-Rodriguez and Sosa-Teste, 2009). Traditional medicine prescriptions are administered mostly by oral route; some effective components are absorbed through gastroenterological mucous membrane, and some enter the blood circulation system and permeated BBB to perform their functions. Thus the components were distributed slowly and had a lower bioavailability. The effective components are absorbed quickly through intravenous route, but it brings many limitations, such as higher risk, inconvenient use, and higher price. With the development of administration technology, intranasal administration has recently become a research hotspot. In contrast, intranasal administration avoids degradation of the drug in the gastrointestinal tract from acidic or enzymatic degradation, avoids degradation of drug resulting from hepatic first pass metabolism, results in rapid absorption and onset of effect and higher bioavailability (Costantino et al., 2007; Pillion et al., 2010). The purpose of this study was to investigate the different effects of borneol in mice via intravenous, intranasal, or oral administration and to study the superiority and feasibility of intranasal administration.
Undoubtedly, the maximum concentrations of borneol in blood and brain were highest via intravenous administration; there was no absorption process and there was rapid onset of effect. However, the drug was eliminated faster than that in the other two groups. Intranasal administration plasma peak concentration was up to 2/5 of the injection, but oral administration was equivalent to 1/5. Intranasal and oral administrations brain peak concentration was up to 1/5 of the injection. T max values of borneol in plasma and brain of intranasal administration were 3.0 and 3.4 min, oral administration T max was 10 min, showing that the absorption rate of drugs in the nasal cavity of mice was higher than that of oral absorption with no difference between levels in blood or in brain. The AUC values of drug in plasma of intravenous, intranasal, and oral administrations were (632.34±50.76), (573.42±77.71), and (271.81±37.43) μg·ml−1·min, respectively. Although the peak plasma concentration after intranasal administration was lower than the injection, the corresponding AUC value was similar to the injection. Therefore, the absolute bioavailabilities F of intranasal and oral administration were 90.68% and 42.99%. The brain AUC values of intravenous, intranasal and oral administrations were (505.34±82.91), (345.48±69.94), and (194.03±23.46) μg·g−1·min, respectively. The Re values of intranasal and oral administrations were 68.37% and 38.40%. Therefore, the Te values of intravenous, intranasal, and oral administrations were 79.92%, 60.25%, and 71.38%, respectively. The DTI values of intranasal and oral administrations were 0.75 and 0.89. The three key pharmacokinetic parameters (calculated maximum concentration (C max), T max, and AUC) could explain the superiority of the intranasal administration. Due to the absorption process, the plasma and brain mean residence time (MRT) of nasal and oral administration was significantly longer than the injection.
This study observed that borneol showed the double chromatography peaks in brain tissue in mice, and reached the first peak at about 3.4 min and the second peak at 20 min. This phenomenon may be attributed to intranasal administration. Several studies (Arora et al., 2002; Born et al., 2002; Costantino et al., 2007) have shown that a drug to reach the CNS from the nasal cavity will have to cross the olfactory membrane and the arachnoid membrane surrounding the arachnoid space containing the cerebro-spinal fluid. The drug can also be absorbed across the nasal mucosa and reach the systemic circulation to reach the brain by crossing the BBB. Borneol is a small lipophilic molecule, it can cross mucous membrane and BBB rapidly because borneol can cross the olfactory membrane after absorption and form the first peak. At the same time, a quantity of borneol can cross mucous membrane to reach the systemic circulation to reach the brain. This creates the second chromatographic peak. Previous study (Dhuria et al., 2009) has shown that inclusion of a vasoconstrictor in nasal formulations can enhance intranasal drug targeting to multiple brain areas, which may hold relevance for the treatment of various neurological disorders. Whether the borneol formulation had the vasoconstrictive function, the mechanism was complicated and left to be solved in our future study.
From this study, it is demonstrated that nasal administration has the superiority of rapid absorption into blood and brain over oral administration, and has similar bioavailability compared to intravenous administration which could play a quick emergency role and therefore may become a promising administration route for encephalopathy treatment. Also, the methods which ethyl acetate extracted the drug in plasma and brain have higher recovery and simpler preparation. In order to enhance the analytical method’s veracity, we added octadecane as the internal standard in the preparation process. The GC-FID method was accurate and had satisfactory efficacy; it could determine the borneol in mice and provide evidence for borneol’s variation in vivo.
Footnotes
Project supported by the Key New Drug Creation and Development Programme of China (No. 2009ZX09502-008), the National Natural Science Foundation of China (No. 81073057), and the Innovation Team Development Program of Beijing University of Chinese Medicine (No. 2011-CXTD-13)
References
- 1.Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug discov Today. 2002;7(18):967–975. doi: 10.1016/S1359-6446(02)02452-2. [DOI] [PubMed] [Google Scholar]
- 2.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi: 10.1038/nn0602-849. [DOI] [PubMed] [Google Scholar]
- 3.Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S, Pu J. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16(2):178–184. doi: 10.1080/10611860701794395. [DOI] [PubMed] [Google Scholar]
- 4.Costantino HR, Illum L, Brandt G. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1-2):1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Dai JP, Chen J, Bei YF, Han BX, Wang S. Influence of borneol on primary mice oral fibroblasts: a penetration enhancer may be used in oral submucous fibrosis. J Oral Pathol Med. 2009;38(3):276–281. doi: 10.1111/j.1600-0714.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 6.Dhuria SV, Hanson LR, Frey WH. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther. 2009;328(1):312–320. doi: 10.1124/jpet.108.145565. [DOI] [PubMed] [Google Scholar]
- 7.Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 8.Elshafeey AH, Bendas ER, Mohamed OH. Intranasal microemulsion of sildenafil citrate: in vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS PharmSciTech. 2009;10(2):361–367. doi: 10.1208/s12249-009-9213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Rodriguez JC, Sosa-Teste I. The nasal route as a potential pathway for delivery of erythropoietin in the treatment of acute ischemic stroke in humans. Sci World J. 2009;9:970–981. doi: 10.1100/tsw.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(S3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH, II, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330(3):679–686. doi: 10.1124/jpet.108.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang P, Jiang XF, Zou JL, Yuan YM, Yao MC, Lu YS. A novel GC-MS bioanalytical method for natural borneol and its application in investigating natural borneol distribution in mice. Mode Tradit Chin Med Mater Med. 2009;11(6):821–827. doi: 10.1016/s1876-3553(10)60038-5. [DOI] [Google Scholar]
- 13.Li F, Feng J, Cheng Q, Zhu W, Jin Y. Delivery of 125I-cobrotoxin after intranasal administration to the brain: a microdialysis study in freely moving rats. Int J Pharm. 2007;328(2):161–167. doi: 10.1016/j.ijpharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Li WR, Chen RY, Yang L. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur J Drug Metab Pharmacokinet. 2012;37:39–44. doi: 10.1007/s13318-011-0058-5. [DOI] [PubMed] [Google Scholar]
- 15.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deli Rev. 2012;64(7):614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Chen XL, Du SY, Wu Q, Yao ZL, Zhai YS. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of geniposide in rats. Arch Pharm Res. 2010;33(5):691–696. doi: 10.1007/s12272-010-0507-8. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Du SY, Chen XL, Li PY, Zhai YS, Wu Q, Li DX. Study on pharmacokinetics of borneol in rats injected with novel-Xingnaojing by GC-FID. China J Chin Mat Med. 2011;36(16):2200–2202. doi: 10.4268/cjcmm20111610. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Du SY, Chen XL, Wu Q, Song X, Xu B, Zhai YS. Enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(2):143–148. doi: 10.1631/jzus.B1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Du SY, Song X, Lu Y, Li DX. Study on the intestinal absorption kinetics of aipian in rats. China Pharm. 2011;22(39):3651–3653. (in Chinese) [Google Scholar]
- 20.Pillion DJ, Fyrberg MD, Meezan E. Nasal absorption of mixtures of fast-acting and long-acting insulins. Int J Pharm. 2010;388(1-2):202–208. doi: 10.1016/j.ijpharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song X, Du SY, Lu Y, Ma Y, Chen XL, Wang Y, Zhang HX. Study on rat nasal absorption in situ of borneol based on single pass perfusion method. China J Chin Mat Med. 2011;36(18):2489–2492. doi: 10.4268/cjcmm20111808. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 22.State Pharmacopoeia Committee of People’s Republic of China. Chinese Pharmacopoeia. Beijing, China: The Medicine Science and Technology Press of China; 2010. p. 82. (in Chinese) [Google Scholar]