Abstract
Background
Acquired hemophilia A is rarely found in association with myeloproliferative neoplasms, such as the JAK2 kinase V617F mutation-positive chronic neutrophilic leukemia (CNL).
Case report
An 80-year-old Japanese male was diagnosed with acquired hemophilia A. He had compartment-like symptoms due to soft tissue hemorrhage in his left forearm and right lower extremity. A blood examination showed neutrophilia with a white blood cell count of 31,900/μL (91.9% neutrophils), an activated partial thromboplastin time of 69.0 seconds, coagulation factor VIII (FVIII) < 1.0%, and anti-FVIII inhibitor, 190 BU/mL. The bleeding episodes were controlled with intravenous activated prothrombin complex concentrate (FEIBA®) followed by recombinant factor VIIa (NovoSeven®). In addition, oral prednisolone (maximum dose, 30 mg/day) plus four doses of rituximab effectively suppressed anti-FVIII inhibitor levels while simultaneously reducing the neutrophil count. CNL with the JAK2 kinase V617F mutation was identified as the underlying disease.
Conclusion
This report describes the effectiveness of a combination of prednisolone and rituximab in managing acquired hemophilia A in an elderly man with a rare case of JAK2 kinase V617F mutation-positive CNL.
Keywords: acquired hemophilia A, chronic neutrophilic leukemia, JAK2 kinase, V617 mutation, rituximab
Introduction
Inhibition of coagulation factor VIII (FVIII) by anti-FVIII inhibitor causes acquired hemophilia A (AHA). While AHA is rare, it can be a life-threatening disease in elderly people.1 Autoimmune disease, neoplasia, pregnancy, and drug reactions frequently underlie the development of AHA.2–4 In particular, about 15% of AHA cases are associated with malignant disease, such as malignant lymphoma or solid neoplasia.4,5 The hemato-oncology review by Franchini et al3 indicates that chronic lymphocytic leukemia is most frequently associated with AHA, followed by lymphoma. Although Franchini’s list did not include cases of AHA associated with myeloproliferative neoplasms (MPNs), a more recent report observed this association.6 Here, we report the case of an elderly patient with both AHA and an MPN; namely, chronic neutrophilic leukemia (CNL)7 harboring a rare JAK2 kinase V617 mutation. To our knowledge, this is the first report of CNL-associated AHA. In addition, we found that rituximab and prednisolone effectively reduced the neutrophil counts and suppressed anti-FVIII inhibitor levels in this patient.
Case presentation
An 80-year-old Japanese male (height, 173 cm; weight, 53 kg) was referred to us because of a severe hemorrhagic tendency. He had been a healthy farmer with an Eastern Cooperative Oncology Group/World Health Organization performance score of 0. However, in the preceding 2 years, he visited the clinic near his residence 13 times and was noted to have leukocytosis with mostly mature neutrophils (white blood cells [WBC]; median 15,500/μL with a range of 10,000/μL to 35,100/μL). The reason for the leukocytosis was unknown. In particular, the WBC counts increased in the year before his admission (median WBC, 25,000/μL; range, 18,000–35,100/ μL). During this period, his hemoglobin (Hb) and platelet counts remained within the normal range. On admission, his left forearm and right lower extremity were tender, tight, and swollen, with superficial bruising and compartment-like symptoms due to extensive soft tissue hemorrhage. There were also ecchymoses on the right arm and the left lower extremity. Over time, this induced swelling of the left dorsum of the foot. An abdominal computed tomography scan revealed no significant splenomegaly. The laboratory data were as follows: WBC, 31,900/μL (91.9% neutrophils); Hb, 8.0 g/dL; platelet count, 357,000/μL; aspartate aminotransferase (AST), 30 U/L; alanine aminotransferase (ALT), 16 U/L; total protein, 5.8 g/dL; blood urea nitrogen, 23.1 mg/dL; creatinine, 0.88 mg/ dL; and C-reactive protein, 0.15 mg/dL. The coagulation data were: prothrombin time (international normalized ratio), 0.89 (reference values, 0.84–1.14); activated partial thromboplastin time (APTT), 69.0 (25.2–40.0) seconds; fibrinogen, 181 (146–380) mg/dL; and D-Dimer, 16.9 (<1.0) μg/mL. The blood coagulation factors and inhibitors were assessed using the APTT and Bethesda methods, respectively, which revealed the following: FVIII, <1.0%; FVIII inhibitor, 190 BU/mL; FIX, 74%; FIX inhibitor, 0%; von Willebrand factor, >201%; and anti-thrombin (AT)-III, 88%. This indicated that the patient had AHA. The patient was negative for antinuclear antibodies (<40). Oral prednisolone (30 mg/day) was started immediately. He was also treated with intravenous activated prothrombin complex concentrate (aPCC; FEIBA®) (Baxter, USA)8,9; 3000 U × 2 followed by 5000 U × 10 doses) on a twice-daily schedule. Treatment by aPCC was stopped, but had to be reinstated 2 days later due to rebleeding. Thus, five more doses of aPCC were given. After completing aPCC treatment, recombinant activated factor VIIa (rFVIIa, NovoSeven®) (Novo Nondisk, Denmark)10,11 were required on two separate occasions (5 mg × 3 and 1 dose, respectively), when the patient complained of a new subcutaneous hemorrhage on the dorsum of the right hand. The hemorrhaging then stopped. Given the advanced age and impaired glucose tolerance of the patient, coupled with the risk of osteoporosis, we decided not to increase the dose of prednisolone. Instead, the patient was given two doses of rituximab (375 mg/m2/dose). However, 1 month after admission, his coagulation data still showed FVIII levels of <1.0% and anti-FVIII inhibitor levels of 47.8 BU/mL. Two and a half months after admission (and after two more doses of rituximab), the APTT normalized. Bone marrow aspiration was then performed (Figure 1). Thereafter, the FVIII recovered up to 28.0% with a significant decline of anti-FVIII inhibitor levels to 0.1–0.2 BU/mL (Table 1). However, the disease relapsed by week 20, with a FVIII of 6% and anti-FVIII inhibitor levels of 5.3 BU/mL. Two weeks later, these values worsened with 2% and 91.5 BU/mL, respectively. Thus, treatment with prednisolone and rituximab was reinstated for suppressing anti-FVIII inhibitor, along with hydroxyurea (1,000 mg/day) for the control of CNL. Four weeks after reinstitution of treatment at 22nd week with prednisolone, 2 additional doses of rituximab and hydroxyurea, the patient has attained a remission, with full recovery (up to 55%) of FVIII activity and undetectable anti-FVIII inhibitor.
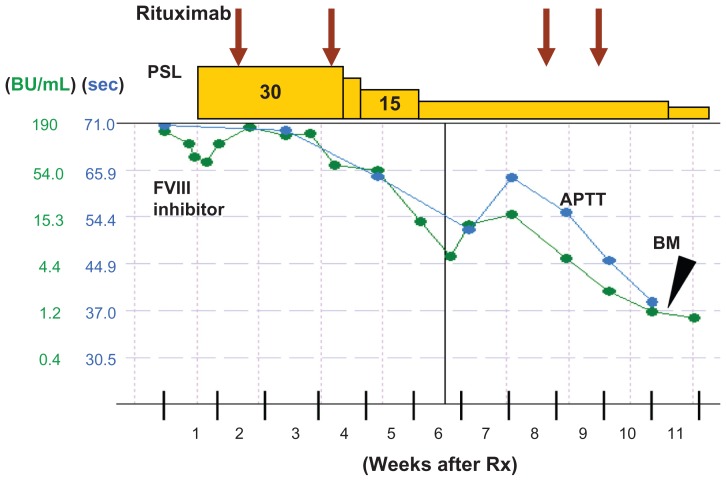
Figure 1.
Treatment responses to APTT and anti-FVIII inhibitors.
Notes: Oral prednisolone (PSL; starting dose 30 mg/day) and four doses of rituximab (375 mg/m2) were administered. Two and a half months after starting the treatment, the APTT normalized and the anti-FVIII inhibitor levels dropped from 190 BU/mL to 1.6 BU/mL. A bone marrow (BM) study was then performed.
Abbreviations: APTT, activated partial thromboplastin time; FVIII, coagulation factor VIII.
Table 1.
The effect of prednisolone/rituximab on the FVIII activity, anti-FVIII inhibitor and neutrophil counts in a patient with AHA in association with CNL
| Time after first treatment* (weeks) | FVIII activity (%) | Anti-FVIII inhibitor (BU/mL) | Absolute neutrophil count (ANC/μL) | P*** | |
|---|---|---|---|---|---|
| Before treatment | – | <1.0 | 190 | 25,943 ± 5,567** | – |
| After two doses of rituximab | 4–8 | 2.0 | 4.9 | 16,393 ± 5,330 | 0.04 |
| After two more doses of rituximab | 10–11 | from 5.0 to 28.0 | 1.2 | 7,689 ± 7,472 | 0.004 |
| BM tested | 11th | – | 1.2 | –# | – |
| Thereafter | 12–16 | from 28.0 to 6.0 | 0.2–0.1 | 11,850 ± 1,404 | 0.016 |
| Relapse | 20th | 6.0 | 5.3 | 6,332 | – |
| 22nd## | 2.0 | 91.5 | 7,678 |
Notes:
Prednisolone and rituximab;
mean ± SD;
Student’s t-test (ANC compared with the prerituximab ANC);
the bone marrow was moderately cellular with an M/E ratio of 2.2.
treatment and response after relapse (see text).
Abbreviations: AHA, acquired hemophilia A; ANC, absolute neutrophil count; BM, bone marrow; BU, Bethesda unit; CNL, chronic neutrophilic leukemia; FVIII, coagulation factor VIII; M/E, myeloid:erythroid; SD, standard deviation.
Regarding the patient’s persistent leukocytosis, which had been noted for the past 2 years, no causative infectious diseases were found. A granulocyte colony-stimulating factor (G-CSF)-producing tumor was ruled out on the basis of a normal plasma G-CSF level of 4.69 (reference values, 10.5–57.5) pg/mL. The neutrophil alkaline phosphatase score of 359% was within the normal range (reference values, 170%–367%). Initially, a bone marrow aspiration/biopsy was deferred due to the hemorrhagic tendency of the patient, which meant that bone marrow findings were not available until the APTT normalized (at 2.5 months after admission). The post-treatment bone marrow smear showed moderately cellular marrow with an myeloid:erythroid ratio of 2.2, myeloblasts at <1.0%, promyelocytes at 1.2%, myelocytes at 8.4%, metamyelocytes at 3.2%, band-form neutrophils at 2.9%, segmented neutrophils at 33.7%, eosinophils at 2.0%, basophils at 0.2%, lymphocytes at 17.6%, monocytes at 5.8%, and plasma cells at 1.5%. Megakaryocyte numbers were slightly increased, but with no atypia. These morphological findings not characteristic of CNL were thought to be due to therapy-related bone marrow suppression (Table 1). Both the peripheral blood and bone marrow showed a normal karyotype of 46, XY (20/20). Chronic myeloid leukemia (CML) was ruled out because the BCR/ ABL fusion gene was not detected. However, the JAK2 kinase V617F mutation was detected by allele-specific polymerase chain reaction.12 The high allele burden (~80%) was also confirmed by sequencing performed as described previously (data not shown).13 These clinical and molecular findings led to the exclusion of polycythemia vera (PV) or essential thrombocythemia (ET). The final diagnosis was JAK2 kinase V617F-positive CNL.7
Discussion
To control bleeding episodes, AHA patients are first treated with aPCC followed by rFVIIa. There is currently no consensus regarding which of these two drugs is better, although You et al11 suggested that rFVIIa should be considered as the first-line treatment. Another report noted that the greater initial efficacy of rFVIIa could reduce the predicted total medical costs.10 However, the parallel use of both agents has also been recommended2,14 and is effective. AHA cases can be classified on the basis of anti-FVIII inhibitor titers as either low titer cases (<10 BU/mL) or high titer cases (>10 BU/mL).15 The current patient showed significantly high titers (190 BU/mL). A previous case report shows that corticosteroids effectively reduce anti-FVIII inhibitor levels.4 Another case report shows that when treatment with prednisolone and cyclophosphamide fails, rituximab is effective.5 Currently, the recommended inhibitor eradication therapy is the combined use of rituximab and prednisolone.2 In the current case, the patient was not only treated with measures to stop the bleeding (aPCC and rFVIIa), he also received moderate doses of oral prednisolone and a total of four doses of rituximab. It took 2.5 months for the APTT to normalize, with the anti-FVIII inhibitor titer dropping rapidly to 1.2 BU/mL and then to 0.1–0.2 MU/mL (Table 1).
When making a differential diagnosis of leukocytosis or neutrophilia in the current case, we ruled out a G-CSF-producing neoplasm and CML. Instead, the patient was thought to fulfill the diagnostic criteria for CNL.7 Previously, a few cases of JAK2 kinase V617F mutation positive-CNL has been described.16–18 The patient was clearly positive for the JAK2 kinase V617F mutation, although the bone marrow morphology was not entirely compatible with a diagnosis of an MPN.19 This discrepancy may reflect the effects of the treatment on the bone marrow; a bone marrow biopsy could not be taken before starting treatment because of the hemorrhagic diathesis of the patient. The fact that the patient did not show the levels of Hb and platelet counts suggesting PV or ET, but significant levels of triggerunknown leukocytosis (median WBC, 25,000/μL; range, 18,000–35,100/μL, with >85.0% mature neutrophils, no immature granulocytes, and <1,000 monocytes/μL) in the preceding years prior to the development of AHA, supports a diagnosis of CNL.
Surprisingly, while treating the AHA with a combination of rituximab and intermediate doses of prednisolone, the patient’s neutrophil counts dropped significantly in parallel with a decline in anti-FVIII inhibitor levels (summarized in Table 1). Rituximab, a monoclonal anti-CD20 antibody, suppresses anti-FVIII inhibitor levels by affecting B cells. However, the present case suggests the novel possibility that rituximab may also have a significant neutrophil-reducing effect in CNL. Supporting this notion are several reports that describe the phenomenon of rituximab-associated late onset neutropenia.20–22 In the current patient, the combination of rituximab and prednisolone effectively (and fairly rapidly) suppressed both CNL and anti-FVIII inhibitor levels. However, the AHA relapsed 9 weeks later (Table 1). After relapse, the patient eventually has attained a remission with prednisolone, 2 additional doses of rituximab and hyroxyurea.
Conclusion
A case of CNL-associated AHA was successfully managed with oral prednisolone and rituximab infusions, without directly treating the CNL. However, the AHA relapsed. At present, this patient is being well controlled with a combination prednisolone, rituximab, and hydroxyurea. If the AHA becomes refractory, other anti-MPN agents may be required, including a recently approved JAK2 kinase inhibitor.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Series Editor of this journal.
Acknowledgments
We thank Dr Tohru Inaba, Department of Infection Control and Laboratory Medicine, Kyoto Prefectural University of Medicine, Kyoto, for his advice regarding the management of the patient.
Footnotes
Contributions
SI, NK, and KS managed the patient. KK undertook bone marrow studies. NO and KT were involved in the molecular study of JAK2 kinase. SI and KT prepared the manuscript. All authors have read and approved the final manuscript.
Disclosure
The authors declare that they have no conflicts of interests in this work.
References
- 1.Shetty S, Bhave M, Ghosh K. Acquired hemophilia a: diagnosis, aetiology, clinical spectrum and treatment options. Autoimmun Rev. 2011;10(6):311–316. doi: 10.1016/j.autrev.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Sborov DW, Rodgers GM. Acquired hemophilia a: a current review of autoantibody disease. Clin Adv Hematol Oncol. 2012;10(1):19–27. [PubMed] [Google Scholar]
- 3.Franchini M, Targher G, Manzato F, Lippi G. Acquired factor VIII inhibitors in oncohematology; a systematic review. Crit Rev Oncol Hematol. 2008;66(3):194–199. doi: 10.1016/j.critrevonc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Ferre A, Arlet JB, Darnige L, et al. Acquired hemophilia as the presenting manifestation of neoplasia: diagnostic workup and monitoring. Rev Med Interne. 2009;30(7):630–633. doi: 10.1016/j.revmed.2008.09.001. Article in French. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa S, Kohata K, Okitsu Y, et al. Acquired hemophilia A with sigmoid colon cancer: successful treatment with rituximab followed by sigmoidectomy. Int J Hematol. 2009;90(1):33–36. doi: 10.1007/s12185-009-0347-9. [DOI] [PubMed] [Google Scholar]
- 6.Kremyanskaya M, Aledort L. Acquired hemophilia: we now see it with myeloproliferative neoplasms. Am J Hematol. 2011;86(3):329–330. doi: 10.1002/ajh.21949. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point- of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 8.Tjønnfjord GE, Brinch L, Gedde-Dahl T, Brosstad FR. Activated prothrombin complex concentrate (FEIBA) treatment during surgery in patients with inhibitors to FVIII/IX. Haemophilia. 2004;10(2):174–178. doi: 10.1046/j.1365-2516.2003.00857.x. [DOI] [PubMed] [Google Scholar]
- 9.Holmström M, Tran HT, Holme PA. Combined treatment with APCC (FEIBA®) and tranexamic acid in patients with haemophilia A with inhibitors and in patients with acquired haemophilia A – a two-centre experience. Haemophilia. 2012;18(4):544–549. doi: 10.1111/j.1365-2516.2012.02748.x. [DOI] [PubMed] [Google Scholar]
- 10.Lyseng-Williamson KA, Plosker GL. Recombinant factor VIIa ( eptacogalfa): a pharmacoeconomic review of its use in haemophilia in patients with inhibitors to clotting factors VIII or IX. Pharmacoeconomics. 2007;25(12):1007–1029. doi: 10.2165/00019053-200725120-00004. [DOI] [PubMed] [Google Scholar]
- 11.You CW, Lee SY, Park SK. Cost and effectiveness of treatments for mild-to-moderate bleeding episodes in haemophilia patients with inhibitors in Korea. Haemophilia. 2009;15(1):217–226. doi: 10.1111/j.1365-2516.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 12.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Okuno N, Naruse H, et al. Validation of the revised 2008 WHO diagnostic criteria in 75 suspected cases of myeloproliferative neoplasm. Leuk Lymphoma. 2008;49(9):1784–1791. doi: 10.1080/10428190802258972. [DOI] [PubMed] [Google Scholar]
- 14.Ingerslev J, Sørensen B. Parallel use of by-passing agents in haemophilia with inhibitors: a critical review. Br J Haematol. 2011;155(2):256–262. doi: 10.1111/j.1365-2141.2011.08854.x. [DOI] [PubMed] [Google Scholar]
- 15.Lak M, Sharifian RA, Karimi K, Mansouritorghabeh H. Acquired hemophilia A: clinical features, surgery and treatment of 34 cases, and experience of using recombinant factor VIIa. Clin Appl Thromb Hemost. 2010;16(3):294–300. doi: 10.1177/1076029608331227. [DOI] [PubMed] [Google Scholar]
- 16.Steensma DP, Dewald GW, Lasho TL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106(4):1207–1209. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mc Lornan DP, Percy MJ, Jones AV, et al. Chronic neutrophilic leukemia with an associated V617F JAK2 tyrosine kinase mutation. Haematologica. 2005;90(12):1696–1697. [PubMed] [Google Scholar]
- 18.Kako S, Kanda Y, Sato T, et al. Early relapse of JAK2 V617F-positive chronic neutrophilic leukemia with central nervous system infiltration after unrelated bone marrow transplantation. Am J Hematol. 2007;82(5):386–390. doi: 10.1002/ajh.20805. [DOI] [PubMed] [Google Scholar]
- 19.Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group. Semin Thromb Hemost. 1997;23(4):339–347. doi: 10.1055/s-2007-996107. [DOI] [PubMed] [Google Scholar]
- 20.Cattaneo C, Spedini P, Casari S, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47(6):1013–1017. doi: 10.1080/10428190500473113. [DOI] [PubMed] [Google Scholar]
- 21.Tesfa D, Gelius T, Sander B, et al. Late-onset neutropenia associated with rituximab therapy: evidence for a maturation arrest at the (pro) myelocyte stage of granulopoiesis. Med Oncol. 2008;25(4):374–379. doi: 10.1007/s12032-008-9049-z. [DOI] [PubMed] [Google Scholar]
- 22.Wolach O, Shpilberg O, Lahav M. Neutropenia after rituximab treatment: new insights on a late complication. Curr Opin Hematol. 2012;19(1):32–38. doi: 10.1097/MOH.0b013e32834da987. [DOI] [PubMed] [Google Scholar]