Abstract
The advent of new technologies such as high definition optical coherence tomography (OCT) has not only provided unprecedented imaging capabilities, but also raised the need to define concepts not yet settled and often confusing such as the vitreomacular traction (VMT) syndrome. While technological advances drive us into the future by clarifying the pathophysiology of many diseases and enabling novel therapeutic options, it is at the same time necessary to review basic disease concepts in addition to definitions and classifications. VMT syndrome is implicated in the pathophysiology of a number of macular disorders, translating into a variety of anatomical and functional consequences underscoring the complexity of the condition. These macular changes are closely related to the VMT configuration and have led to proposing classification of this syndrome based on OCT findings. The size and severity of the remaining vitreomacular attachment may define the specific maculopathy. Focal VMT usually leads to macular hole formation, tractional cystoid macular edema and foveal retinal detachment, while broad VMT is associated with epiretinal membranes, diffuse retinal thickening and impaired foveal depression recovery. Despite similar postoperative visual acuity (VA) in focal and broad VMT subgroups, visual improvement is greater with focal VMT because preoperative VA is frequently lower. Surgical procedures are effective to relieve VMT and improve VA in most eyes; outcomes vary with VMT morphology and the duration of symptoms.
Keywords: Cystoid Macular Edema, Epiretinal Membranes, HD-OCT, Macular Hole, Optical Coherence Tomography, Posterior Vitreous Detachment, Vitreomacular Traction Syndrome, Vitreoretinal Interface
INTRODUCTION
In 1970, Reese et al1 described an unusual macular condition in which an incomplete posterior vitreous detachment (PVD) exerted traction on the macula and was accompanied by decreased visual acuity (VA). This condition was confirmed, not by imaging studies, such as optical coherence tomography (OCT) which was not available at that time, but through the use of histological studies. Hence, the term vitreomacular traction (VMT) syndrome was coined.
VMT was assumed to be an uncommon entity and not associated with other macular disorders. In the classic form of VMT syndrome, as initially described1, the vitreous is separated from the retina throughout the peripheral fundus but remains adherent posteriorly, engendering anteroposterior traction on a broad, often dumbbell-shaped region encompassing the macular area and optic nerve several disc areas in size.2-4
Spectral domain high definition OCT (HD-OCT) has provided new insight into the understanding of VMT syndrome by providing better evaluation of tractional forces at the vitreoretinal interface, as well as comprehending its relationship with particular macular conditions.5-7
Among vitreoretinal interface abnormalities, VMT syndrome is probably one of the conditions which has been significantly improved in terms of pathophysiologic concepts. Nowadays, VMT is believed to be associated with a broad spectrum of maculopathies, including cystoid macular edema (CME), epiretinal membrane (ERM), and macular hole (MH) formation, all attributed to a common etiology.4,7-9
It remains unclear why patients with VMT have distinct maculopathies; a question that like many others is the focus of several recently published studies.8,10-15 Although the pathogenesis of these disorders is not completely understood,7,9 OCT has implicated tractional forces as a plausible cause. The recognition of the role of VMT in these macular abnormalities is imperative for diagnosis and appropriate management of affected patients.
THE Vitreoretinal interface
In order to better understand vitreoretinal interface disorders, it is first essential to comprehend the normal sequence of events during the evolution of PVD.
PVD is characterized by a separation between the posterior vitreous cortex and the internal limiting membrane (ILM) of the retina as a result of a normal physiologic process that occurs invariably with age. Agerelated vitreal changes, such as liquefaction and syneresis, contribute to the development of PVD. It is believed that age-related PVD begins as a shallow, localized separation of the vitreous from the perifoveal retina and slowly progresses over months or years before its completion at the time of vitreopapillary separation.
In many instances, the evolution of PVD occurs spontaneously without the patient ever noting any symptoms. When this process is completed and the posterior vitreous cortex is fully separated from the ILM, symptoms such as flashes and floaters can be the sole complaint. However, in a subset of patients, the tractional effect of early stages of PVD produce or exacerbate a variety of pathologic macular and optic disc features, determined in part by the size and strength of residual vitreoretinal adhesions.8
Changes associated with aging in vitreous biochemistry
There are significant structural changes in the aging vitreous that may result in PVD. Total collagen content in the vitreous body does not change after the age of 20 to 30 years. Nevertheless, collagen concentration in the vitreous gel at 70 to 90 years of age, is significantly greater as compared to younger ages.16 Since total collagen content does not alter, the higher collagen concentration can be explained by a decrease in gel vitreous volume that occurs with aging. In old age, there is advanced liquefaction, thickening and tortuosity of vitreous fibers and collapse of the vitreous body, known as syneresis.
Vitreous syneresis predisposes the eye to PVD, which is a prerequisite but not sufficient alone to precipitate complete PVD. Ultimately progressive vitreous liquefaction in conjunction with progressive age-related weakening of adhesions between the posterior vitreous cortex and the ILM, allow liquid vitreous to dissect a plane between the vitreoretinal interface.8,17
Complications of PVD are more likely to arise in eyes where accelerated vitreous liquefaction occurs prior to adequate weakening of vitreoretinal adhesions; for instance, ocular inflammatory diseases, trauma, hereditary vitreoretinal conditions (such as Stickler and Marfan syndromes), retinal vascular diseases, myopia, aphakia, and vitreous hemorrhage, the majority of which are more common in younger patients.18-20
When an abnormal PVD process exists, most likely as a result of premature vitreous liquefaction associated with insufficiently weakened vitreoretinal adhesion, vitreoretinal traction exerts force in regions where the posterior hyaloid membrane is firmly attached to the retina, due to extracellular matrix “glue” effect.21 This stronger adhesion is more significant at several points throughout the fundus where the ILM is thinnest; i.e. at the peripapillary area, along major vessels, at the location of lattice degeneration, enclosed ora bays, retinal tufts, at points of degenerative remodeling or areas of acquired changes such as post inflammatory lesions, at the vitreous base, and mainly at the 500μm foveolar zone and the margin of the 1500μm foveal zone.8
Persistent vitreoretinal adhesion at such points allows static and dynamic traction forces to act over time. As a consequence, it may induce retinal tags, retinal folds, cystic degeneration, retinoschisis, tractional retinal detachment, avulsion of retinal vessels, vitreous hemorrhage and retinal tears. At the posterior pole, continuous vitreoretinal traction may induce macular lesions, leading to VMT syndrome.9,22,23
HistoPathology
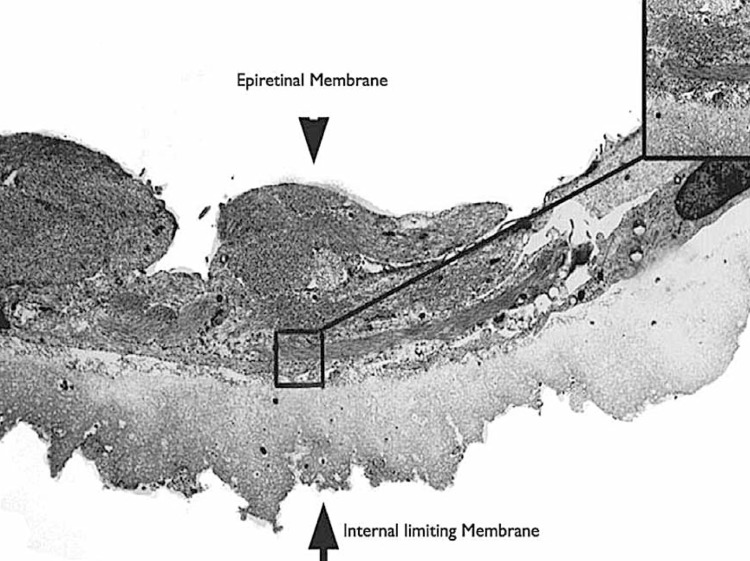
Since Reese first described and demonstrated vitreomacular adhesions by histopathology,1 VMT syndrome and idiopathic ERM have often been considered as distinct entities. Although they seem to share some features like epiretinal fibrocellular proliferation, the relationship between these conditions is incompletely defined (Fig. 1).
Figure 1.
Epiretinal membrane (ERM, arrow head) and internal limiting membrane (ILM, arrow) analyzed by transmission electron microscopy (TEM, ×3800). The matrix around the surface is composed of moderate amounts of native collagen fibrils (top right inset, TEM, ×24,000).
Histopathologic examination of VMT specimens obtained during surgery demonstrate a variety of cell types such as fibrous astrocytes, myofibroblasts and fibrocytes.22,23 In a similar manner, idiopathic ERM exhibits the growth of comparable cells such as fibrous astrocytes, myofibroblasts, and fibrocytes as well as retinal pigment epithelial (RPE) cells on the surface of the retina mostly following PVD.23 Astrocytes, myofibroblasts, and fibrocytes predominate in VMT, while RPE cells are commonly found in ERM. These glial cells contribute to the contractile forces in both entities.9,24
Recent studies have demonstrated similarities in the anatomical features of VMT and ERM.10,22 Chang and co-workers evaluated 6 prominent VMT cases by ultrastructural analysis and found, in all cases, several cell types not usually present in the vitreous cavity, including RPE cells, myofibroblasts and fibrocytes.10 Most striking was the presence of RPE cells in vitreous specimens, a typical feature of ERM. This is in contrast to previous histopathological studies on VMT showing absence of RPE cells in vitreous samples. The authors explained that previous studies had examined specimens removed from the surface of the retina at the site of vitreoretinal attachment but had not concentrated on the vitreous cone above the retina.
The ophthalmoscopic appearance of some VMT cases can share similarities with idiopathic ERM. Histologically, these eyes have fibrocellular proliferation on the posterior vitreous surface with a composition that may also be found on the retinal surface in idiopathic ERM. The fibrocellular proliferation along retinal and vitreal interfaces in VMT may contribute to the tenacity of vitreoretinal adhesion.10
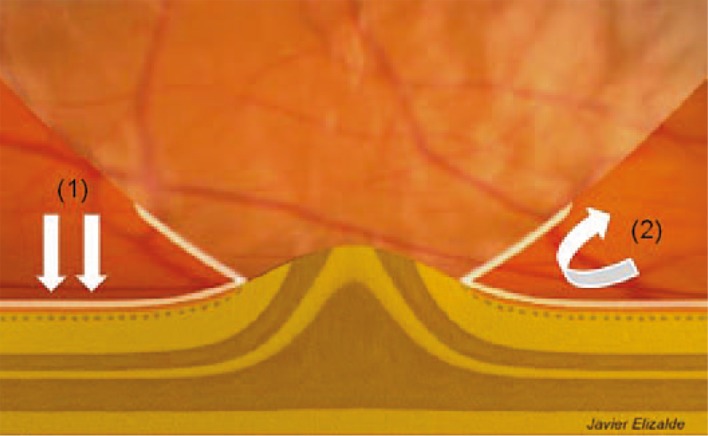
PVD is believed to play an important role in the pathogenesis of ERM. Although the majority of ERM cases are associated with complete PVD, ERM has also been observed in conjunction with partial PVD.25 It has been hypothesized that PVD can create dehiscences in the ILM through which cells can migrate and proliferate on the retinal surface.23 These cells include fibrous astrocytes, fibrocytes, myofibroblasts and curiously, RPE cells.23 In addition, ERM outgrowth may be stimulated or facilitated by cortical vitreous remnants on the retinal surface after PVD. These remnants appear to act as a scaffold for cellular proliferation. The corresponding side of the split vitreous cortex forms the outer surface of the cone of detached vitreous. This portion of the vitreoretinal interface may proliferate onto the detached posterior hyaloid face and may also serve as a scaffold for proliferation of cells. These cells and their associated extracellular matrix may enhance the strength of the vitreoretinal adhesion to the fovea, helping to prevent the ordinarily expected complete separation of the vitreous from the macular surface (Fig. 2).12
Figure 2.
Proposed mechanism of ERM proliferation in VMT syndrome based on Chang et al.10 After partial posterior vitreous detachment, small splits within the ILM may form which allow glial cells to gain access to the superficial retina which acts as a scaffold for ERM proliferation.2 These cells may also proliferate onto the detached hyaloid face, stongly anchoring the vitreous to the macula.
Small mechanical defects in the ILM have been proposed as a means of access for proliferating cells, but this defect does not seem to explain the presence of RPE cells.10 RPE cells have been shown to undergo epithelial-tomesenchymal transition in vitro, with changes in epithelial cell markers which is one possible explanation for this occurrence. Another interesting possibility involves bone marrowderived progenitor cells; following RPE injury, bone marrow–derived cells migrate into the eye and adopt RPE characteristics.26-28 This raises the possibility that cells which are proliferating along the surface of the retina and the back surface of the vitreous cone may also have originated, in part, from bone marrow-derived progenitor cells.
Clinical Features
Idiopathic VMT syndrome can occur in either sex, at any age, and has no racial predilection.4 The incidence seems to be discretely higher in women (up to 65%), which can be attributed to premature vitreous liquefaction and therefore earlier-onset PVD, probably associated with declining estrogen levels in the postmenopausal state.29
Patients with VMT may experience decreased vision, photopsia, micropsia and some degrees of metamorphopsia.2,7 Symptoms are usually mild and develop slowly with progressive vision loss due to chronic traction effects. A discrepancy commonly exists among symptoms and clinical findings; patients with extensive macular traction may present with excellent visual acuity (VA). However, some VMT cases may suffer from acute visual loss with a small central scotoma, usually as a result of severe anteroposterior VMT with subretinal detachment.9
Diagnosis and Ancillary examinations
The diagnosis of VMT syndrome is often difficult to establish clinically. Even with thorough fundus contact lens examination, firm translucent adhesions of the vitreous at the macula may be imperceptible which explains why this condition was considered rare and may remain underdiagnosed.7
Distinct clinical presentations of VMT have already been described and include macular surface wrinkling, similar in appearance to ERM.2 Although this syndrome was previously considered infrequent and not correlated with other maculopathies, nowadays VMT is known to be associated with ERM in most cases. In addition, a thickened and taut posterior hyaloid membrane may also be noted in VMT.10
Tractional CME is a subtle variant of VMT syndrome. It may be present in cases with unifocal vitreo-foveal traction arising from partial PVD. Clinically, it can easily be confused with pseudophakic or uveitic CME. The tractional etiology may manifest with metamorphopsia, however leakage may be absence or minimal on fluorescein angiography.11 Chronic cystoid macular changes may also be found in broad vitreomacular adhesion, usually associated with ERM and macular thickening. In these cases, capillary leakage may be present, however this is less common than with inflammatory edema.13
VMT syndrome may clinically resemble MH due to the tractional schisis phenomenon causing focal CME, foveal distortion and subretinal detachment. These cases can be considered as a variant of VMT syndrome, linking it to the progression of MH.3,11 OCT and ultrasound data demonstrate that perifoveal vitreous detachment is commonly associated with the earliest stages of idiopathic MH.30,31
Dynamic B-scan ultrasonic examination of VMT syndrome may demonstrate the peripherally detached hyaloid with attached hyaloid over the posterior pole, differentiating this syndrome from idiopathic macular pucker in which a complete PVD and Weiss ring is usually present. Ultrasonic B-scan has higher sensitivity and specificity for evaluating the vitreoretinal relationship as compared to stereoscopic slit lamp biomicroscopy, and can be useful in eyes with opaque media, where OCT is not possible, or when OCT devices are not available.32 In these conditions, combined ultrasonographic studies using 10 and 20 MHz frequencies provide a valid diagnosis of the vitreoretinal interface.
Optical coherence tomography
OCT was a revolutionary imaging modality in understanding the vitreomacular interface and its advent has led to better comprehending the VMT syndrome.4,9,11 This technique has allowed investigators to study disease processes previously unrecognizable by biomicroscopy in a noninvasive manner. Therefore, OCT has permitted a huge amount of papers to be published that clarify and introduce novel staging schemes for these processes.8,10-15 Although VMT syndrome was considered an isolated pathology in the past, it is now believed to play a contributory role in a wide spectrum of macular disorders including CME, MH, and ERM.7
In VMT syndrome, the posterior hyaloid space usually appears hyperreflective and thickened on OCT, firmly adherent to the foveal region, causing morphological alterations.
Based on OCT, the pattern of vitreomacular adhesion can be divided into different categories, each with specific macular findings.33,34 However, no major classification of VMT syndrome has yet been defined and generally accepted.
Yamada and Kishi34 classified VMT configuration into two patterns; the first group had incomplete vitreous detachment nasally and temporally causing a V-shaped pattern due to persistent attachment at the fovea, the second group had persistent nasal attachment with detachment temporal to the fovea and incomplete posterior hyaloid separation resulting in a J-shaped configuration. These authors also suggested that the specific preoperative OCT pattern of VMT may be useful in predicting postoperative outcomes. The former group fared better postoperatively, with restoration of retinal architecture or improved vision as compared to the latter group.34
By means of 3-dimensional (3-D) imaging techniques and HD-OCT measurements, there are two main categories for the VMT configuration: focal VMT, where the maximum diameter of vitreomacular attachment is 1500μm or less; and broad VMT, where the maximum diameter of vitreomacular attachment is more than 1500μm.12
Johnson has recently graded macular complications of the early-stages of PVD into those with adhesions within the 500μm, or around the 1500μm zones.8 According to this author, VMT syndrome would encompass macular adhesions around 1500μm, while vitreofoveolar traction would be considered a distinct entity with macular adhesions smaller than 500μm.
Although VMT syndrome has distinct but not yet firmly established classification schemes, a basic concept proposed by Spaide and colleagues14 has been widely accepted; the diameter of the vitreomacular attachment is inversely correlated with macular morbidity and foveal deformation. The narrower the vitreomacular attachment, the greater the force exerted upon the macula, whereas a diffuse vitreomacular adhesion may distribute the tractional force beyond the border of the foveal region.12
A recent study (Bottós et al, unpublished data) on 36 eyes with idiopathic VMT syndrome proposed high correlation between focal and V-shaped VMT, and also broad and J-shaped forms, except in 3 cases that despite being V-shaped, had broad adhesions. All these 3 cases had common characteristics and evolved in a similar way to eyes with broad vitreal attachment regarding duration of symptoms, associated maculopathies, and initial and final visual functions. Based on these findings, the authors suggest that the classification of VMT syndrome considering the diameter of adhesion, and not its pattern, might better predict anatomical lesions and functional outcomes.
While the amount of tractional forces on the fovea cannot be measured by OCT, the stress acting on it would be expected to increase with decreasing attachment area. Not only excessive strain, but also permanent deformation and plasticity of the foveal structure, beyond its elastic range, may lead to internal damage and mechanical disruption of the macula. With removal of stress, there is complete strain recovery, but anatomical and functional improvement cannot always be achieved.14
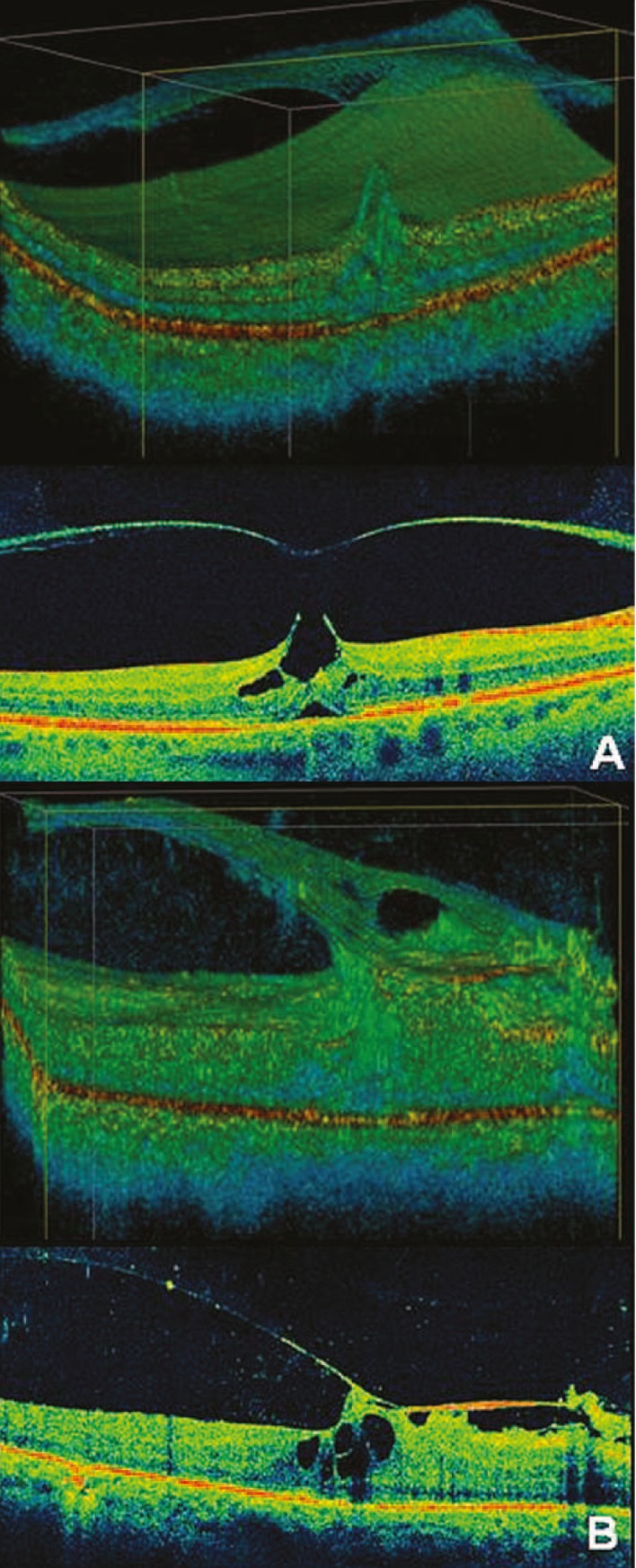
The manifestation of a particular maculopathy may depend on structural integrity of the macula as well as the size and strength of remaining vitreomacular attachments. This explains why some specific macular entities are associated with a particular type of VMT. Bottós et al (unpublished data) found that in focal VMT, CME was the predominant macular change (88.9%), followed by impending and full-thickness MHs (61.1%) and subfoveal detachments (16.6%). On the contrary, in broad VMT cases, diffuse retinal thickening (72.2%) prevailed, largely associated with ERM (94.4%) while only one case showed MH (5.5%). These results suggest that certain types of adhesions are more prevalent in certain disease processes (Fig. 3).
Figure 3.
Macular changes associated with specific types of vitreomacular traction syndrome (VMT). (A) High definition optical coherence tomography (HDOCT) in “focal” VMT shows intraretinal cysts and subfoveal retinal detachment. (B) HD-OCT in “broad” VMT demonstrates degenerative retinal changes, an associated epiretinal membrane and retinal thickening
As the diameter of persistent foveal attachment (associated with perifoveal detachment) is correlated with changes in foveal anatomy, the smaller the area of foveal attachment, the greater the force exerted and the more severe the pathology and anatomical macular abnormalities.14 This condition of focal adhesion is also known as vitreofoveal traction syndrome, and implies MH and CME formation.
Nowadays, vitreofoveal traction is believed to be important in the pathogenesis of idiopathic MH. Since Gass described the early stages of MH, the development of this condition has been attributed to tangential traction, not to anteroposterior forces.35,36 However, this theory is now changing.
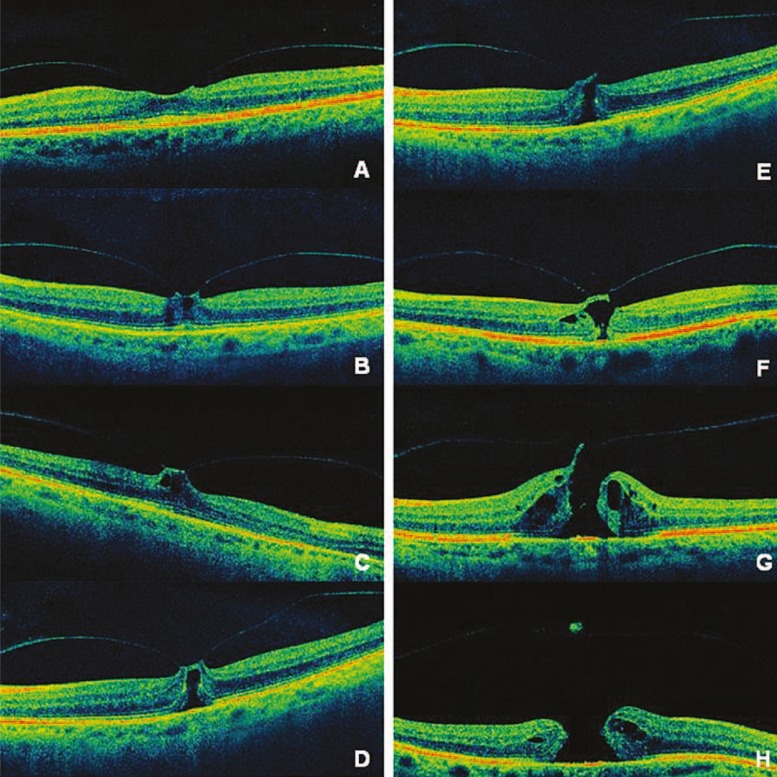
In some susceptible patients, there is abnormal and strong vitreomacular adhesion, causing persistent foveal traction with perifoveal detachment associated with the earliest stages of MH. Afterwards, the detachment extends superiorly, increasing tractional forces. A pseudocyst can be formed and may extend through the entire foveal thickness, disturbing outer retinal layers. If this pseudocyst loses its roof (while the posterior hyaloid is still attached), a full-thickness MH is formed, progressing to stage II holes. In addition, defects of inner retinal layers, caused by persistent TVM, allow infiltration of fluid vitreous into the retina, leading to intraretinal cyst formation and enlargement of the hole. When PVD extends temporally and inferiorly, it is associated with stage III holes. Finally, when the posterior hyaloid detaches from the optic disc, the vitreous detachment extends to the extreme periphery and characterizes stage IV MH (Fig. 4).37,38
Figure 4.
High definition optical coherence tomography images of patients in distinct stages of vitreomacular traction (VMT) syndrome. (A) In some susceptible patients, there is abnormal and strong vitreomacular adhesion causing persistent foveal traction with perifoveal detachment, which is universally associated with the earliest stages of macular hole (MH). (B, C, D) If tractional forces persist, a pseudocyst may form and extend through the entire foveal thickness. (E, F) If this pseudocyst loses its roof while the posterior hyaloid is still attached, a full-thickness MH is formed, progressing to stage II holes. (G) In addition, defects of inner retinal layers, caused by persistent VMT, allow infiltration of fluid vitreous into the retina leading to intraretinal cysts and enlargement of the MH (stage III). (H) Finally when the posterior hyaloid detaches from the optic disc, the vitreous detachment extends to the extreme periphery characterizing stage IV MH.
Hikichi et al39 reported that complete PVD can prevent MH formation or further deterioration of VA in eyes with full-thickness MHs. This finding also confirms the importance of the anteroposterior traction theory in MH formation.39
The vitreoretinal relationship in eyes with tractional CME is similar to that seen in eyes with early stages of idiopathic MH.11 Prominent CME is most commonly associated with focal VMT (88.9%), this is in contrast to broad VMT cases in which CME occurs in only 44.4% of cases and is more diffuse in nature. These observations corroborate the profound influence of narrow adhesions in this subtle variant of VMT which is also called tractional CME (Fig. 5). This type of CME must be distinguished from that associated with inflammatory disorders, post cataract surgery, retinal vascular diseases or uveitis, which generally exhibit considerable capillary leakage on fluorescein angiography. In distinction, tractional CME (not linked with inflammation) shows only minimal leakage, if any at all.11
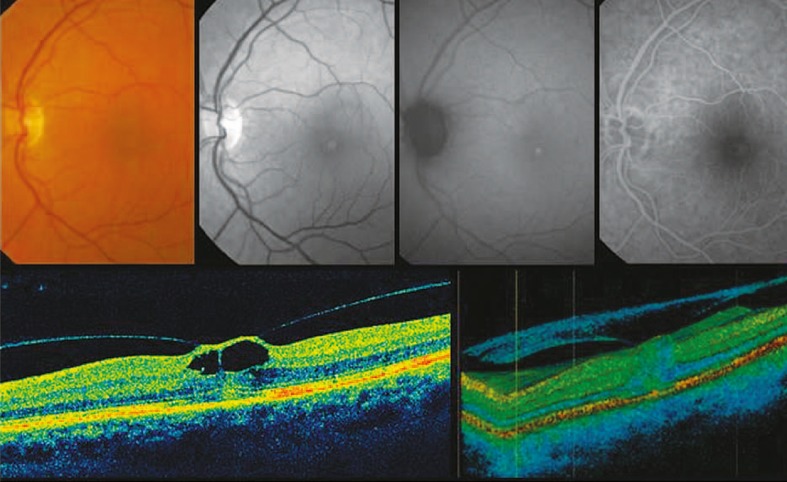
Figure 5.
Fundus photograph, red free image, fundus autofluorescence and fluorescein angiogram (top row images, from left to right) in a patient with tractional cystoid macular edema. Fundus photograph shows a yellow spot which corresponds to the hyper-autofluorescent pattern observed on the autofluorescence image. Corresponding mid-phase fluorescein angiogram reveals minimal leakage from retinal capillaries. Optical coherence tomography (bottom images) demonstrates focal vitreomacular adhesion with perifoveal vitreous detachment causing tractional cystic foveal edema.
It remains unclear why some eyes with vitreofoveal traction progress to MH formation, whereas others exhibit tractional CME without hole formation, even with prolonged follow-up.
Treatment
Many cases of VMT syndrome maintain good VA and mild metamorphopsia, and do not require treatment. Some complete PVD cases can resolve spontaneously, generally with favorable anatomic and functional outcomes comparable to surgical treatment.40,41
However, other cases manifest poor VA and progressive macular traction necessitating surgical intervention. Several investigators have reported surgical outcomes in VMT syndrome with improvement of VA occurring in 44% to 78% of cases.2,4,15,33,34,42 Melberg and associates42 obtained visual improvement in only 44% of eyes, and attributed this limited visual improvement to chronic retinal detachment, premacular fibrosis, CME and macular schisis.
Most authors have not addressed the relationship between surgical outcomes and the type of vitreous adhesion.2,4,42 Nevertheless, recent studies have shown that specific preoperative OCT patterns in VMT may predict postoperative visual improvement. Shorter duration of symptoms, lower preoperative macular thickness and focal VMT configuration (V-shaped) were associated with better visual outcomes after surgery.33,34 On the other hand, partial posterior vitreous detachment temporal to the fovea (J-shaped configuration), in which prominent CME has developed, may result in MH or macular atrophy postoperatively.34
Despite the successful release of posterior hyaloid traction, signs and symptoms may not always be improved. Generally, patients with focal VMT adhesion and a V-shaped configuration have lower preoperative VA than those with broad adhesions and a J-shaped pattern. It is important to consider that final VA results are similar in both groups, therefore it may be concluded that VA improvement is greater in focal cases (Bottós et al, unpublished data). This may also be a consequence of degenerative macular changes due to the chronic nature of broad VMT leading to a longer duration of symptoms and prolonged macular thickening.
Vitreous surgical techniques include release of both anteroposterior and tangential traction. A “double layer” of preretinal proliferation can be found in VMT. The anterior layer may simply represent a thickened posterior hyaloid. In addition, a double layer of posterior hyaloid may sometimes be present, an anatomical abnormality known as hyaloidoschisis which is more frequently observed in high myopic and diabetic eyes. Deeper, on the surface of the retina, an ERM may be present. Generally, in these cases, the vitreous is tightly anchored to the foveal center, and its separation is extremely difficult, requiring peeling of the ERM prior to vitreofoveal separation.
The use of vital dyes to stain preretinal tissues during vitreoretinal surgery, i.e. chromovitrectomy, allows visualization of thin transparent tissues in the vitreoretinal interface including the ILM, ERM and the posterior vitreous surface.43,44
Triamcinolone acetonide (TA) is the gold standard for identification of vitreous. Indocyanine green (ICG), infracyanine green (IFCG) and brilliant blue stains (BriB) are alternative choices for identification of the ILM while trypan blue (TB) is ideal for ERM (Table 1).43,45
Table 1.
Comparison of substances currently used in chromovitrectomy.
| Substance | DilutionOsmolarity | Affinityfor Intraocular Structures | Avoiding RPE/Retinal Toxicity | High Cost | Chemical Properties |
|---|---|---|---|---|---|
| Triamcinolone acetonide 40 mg/ml4% | Nodilution | Vitreous | Use apreservative-free solution | + | Triamcinolone is a synthetic nonsoluble steroid (C24H31F06; 434 daltons) |
| Trypanblue 1.2 mg/ml0.12% | Nodilution or mix with glucose 1.2 mg/ml (0.12%)/310 mOsm | ERM | Usewith no dilution or mix 0.3 ml with 0.1 ml glucose 5% for better ERMidentification | + | Trypanblue is an anionic hydrophilic azo dye (C34H24N6Na4014S4; 960 daltons) |
| Patentblue 2.5 mg/ml0.25% | Nodilution or mix with glucose 2.5mg/ml (0.25%)/290 mOsm | ERM | Usewith no dilution or mix 0.3 ml with 0.1 ml glucose 5% for better ERMidentification | ++ | Patentblue is a triarylmethane dye (C27H31N2Na06S2; 582 daltons) |
| Brilliant blue 0.25 mg/ ml0.025% | Nodilution/280 mOsm | ILM | Usewith dilution | +++ | Brilliant blue is a blue anionic aminotriarylmethane compound(C47H48N3S207Na; 854 daltons) |
| Indocyanine green 5 mg, 0.5%; 25mg, 2.5%; 50mg, 5.0% | Lessthan 0.5 mg/ml (0.05%) dissolve in small amount of distilled water.Dilution: use large amount of BSS | ILM | Add 1ml distilled water to 1 vial 5 mg. Take 0.1 ml of the solution and mixwith 0.9 ml BSS | ++++ | Indocyanine green is a tricarbocyanine dye (C43H47N2Na06S2; 775 daltons)and contains 3% to 5% iodine |
| Infracynine green 5 mg, 0.5%, 25mg, 2.5% | Lessthan 0.5 mg/ ml (0.05%) dissolve in glucose 5%/290 mOsm | ILM | Add 1or 2 ml glucose 5% to 1 vial of 5 mg | +++++ | Infracynine green has the same chemical formula as ICG but contains nosodium iodine |
BSS, balanced salt solution; ERM, epiretinal membrane; ICG, indocyanine green;
ILM, internal limiting membrane; RPE, retinal pigment epithelium
TA particles deposit on the vitreous surface. Crystals of this steroid compound adhere to the acellular tissue, creating clear contrast between the empty vitreous cavity and areas where vitreous fibers are still present.46,47
When VMT is associated with ERM, chromovitrectomy can facilitate its removal. TB exhibits a strong affinity for ERM because of presence of many dead glial cells within the membrane. TB staining of ERM may minimize mechanical trauma to the retina during ERM removal and allows recognizing the extent of the ERM.48
It is postulated that ILM removal can minimize the recurrence of ERMs and completely relieve tractional forces on the macular area.
In humans, BriB provides satisfactory ILM staining in an iso-osmolar solution when used during surgery for idiopathic ERM and MH. BriB is emerging as a good alternative to ICG and IFCG in chromovitrectomy because of its remarkable affinity for the ILM, and lack of retinal toxicity as demonstrated by multifocal electroretinograms (ERGs) (Figures 6 and 7).45
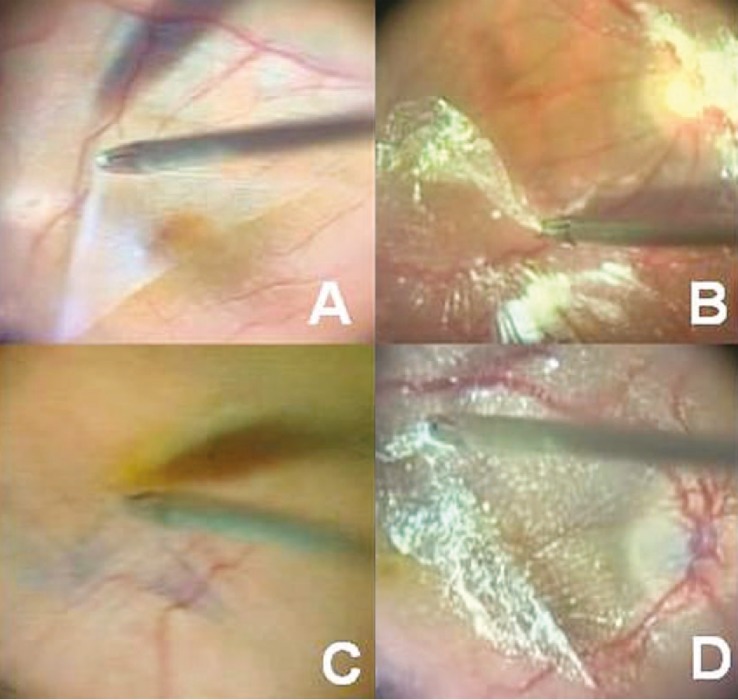
Figure 6.
Surgical technique for epiretinal membrane (ERM) peeling. (A) ERM peeling using no dye. (B) ERM peeling using 0.2 ml of 40 mg/ml triamcinolone acetonide (TA). (C) ERM peeling using trypan blue (TB). (D) ERM peeling using TA and TB, the so-called “double staining technique”. Staining was performed with 0.2 ml of 40 mg/ml TA along with 0.2 ml of 0.05% TB.
[Re-printed with permission from: Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332-340.]
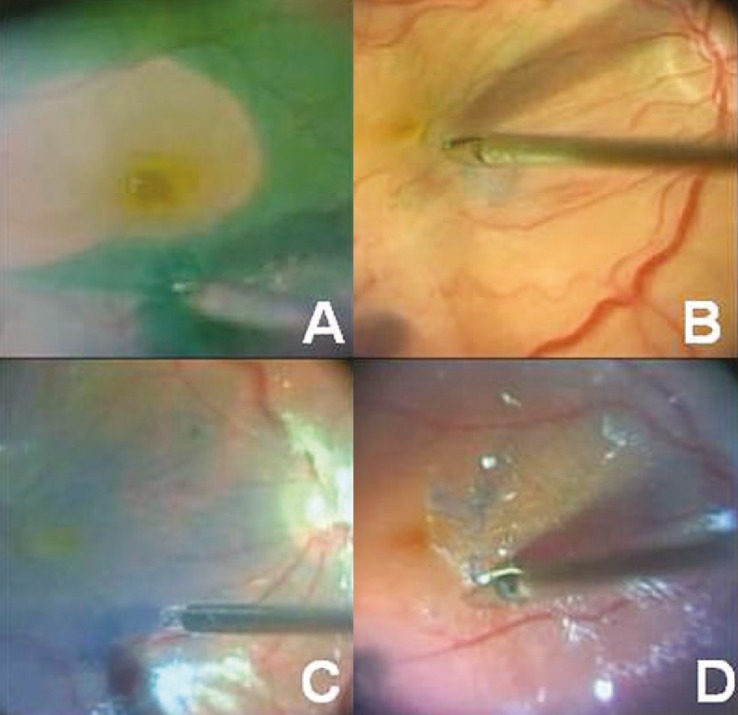
Figure 7.
Surgical technique for internal limiting membrane (ILM) peeling. (A) ILM peeling guided by ICG staining in macular hole (MH) surgery. (B) ILM peeling guided by brilliant blue (BriB) staining in MH surgery. (C) Technique of “double staining“ using BriB and triamcinolone acetonide (TA) injected over the retinal surface using a soft tip cannula while the infusion port remains closed. (D) ILM peeling guided by the double staining technique after epiretinal membrane (ERM) removal. Staining was performed with 0.2 ml of 40 mg/ml TA along with 0.2 ml of 0.25% BriB.
[Re-printed with permission from: Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332-340.]
However, as is often the case in surgical interventions, inherent limitations including cost, complexity, and complications of surgery, can limit its safety, efficacy, and usefulness.
Possible complications associated with vitrectomy for treatment of VMT syndrome include cataract formation, inadvertent ruptures and retinal detachment, iatrogenic macular hole formation, visual field defects, RPE damage and other complications such as glaucoma, endophthalmitis and hypotony which are inherent to many ophthalmic procedures.
A pharmacologic agent that could facilitate the induction of PVD may enable safer and more effective surgical management for many conditions.
Recent studies on “enzymatic vitrectomy” have shown improvement in VA and release of retinal traction without the need for pars plana vitrectomy.49 Plasmin (a serine protease enzyme) induces PVD, avoids surgically induced trauma, and provides a more uniform retinal surface. This enzyme hydrolyzes laminin and fibronectin, which are both present at the vitreoretinal interface. Moreover, plasmin does not degrade collagen type IV, a major component present in the basement membrane of the ILM. Thus, it potentially allows separation of the posterior hyaloid without damaging retinal tissue.
An alternative approach to autologous plasmin is a recombinant product named microplasmin, which shares the catalytic properties of human plasmin and seems to possess a promising activity profile.50
Conclusions
Introduction and widespread availability of modern technology, HD-OCT for instance, not only promote unprecedented imaging but also raise the need to redefine concepts which are not yet settled and often confusing such as the VMT syndrome.
While technological advances drive us into the future by clarifying the pathophysiology of many diseases and enabling therapeutic progress, it is at the same time necessary to review the basis of these entities in terms of definition and classification.
VMT syndrome is implicated in the pathophysiology of a number of macular disorders, with variable anatomical and functional outcomes underscoring the complexity of the disease. These macular changes are intimately related to the VMT configuration and have led to proposals for classification of this syndrome based on OCT findings.
Moreover, the size and strength of remaining vitreomacular attachment may define the specific maculopathy. Focal VMT usually leads to MH formation, tractional CME and foveal retinal detachment. In contrast broad VMT is widely associated with ERM, diffuse retinal thickening and worse foveal depression recovery.
Despite similar postoperative VA in VMT subgroups, improvement is greater in focal cases, since their preoperative VA is usually lower. Surgical procedures are effective in relieving VMT syndrome and improving VA in most eyes. Postoperative outcomes vary with VMT morphology and duration of symptoms.
Acknowledgments
Supported in part by FAPESP (Fundação de Apoio a Pesquisa do Estado de São Paulo); CNPq (Conselho Nacional de Pesquisa).
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Reese AB, Jones IS, Cooper WC. Vitreomacular traction syndrome confirmed histologically. Am J Ophthalmol. 1970;69:975–977. doi: 10.1016/0002-9394(70)91041-x. [DOI] [PubMed] [Google Scholar]
- 2.Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106:624–628. doi: 10.1001/archopht.1988.01060130678025. [DOI] [PubMed] [Google Scholar]
- 3.Smiddy WE, Flynn HW. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137:525–537. doi: 10.1016/j.ajo.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 4.McDonald HR, Johnson RN, Schatz H. Surgical results in the vitreomacular traction syndrome. Ophthalmology. 1994;101:1397–1402. doi: 10.1016/s0161-6420(94)31158-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen TC, Cense B, Pierce MC, Nassif N, Park BH, Yun SH, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123:1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 6.Nassif N, Cense B, Park B, Pierce M, Yun S, Bouma B, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12:367–376. doi: 10.1364/opex.12.000367. [DOI] [PubMed] [Google Scholar]
- 7.Shechtman DL, Dunbar MT. The expanding spectrum of vitreomacular traction. Optometry. 2009;80:681–687. doi: 10.1016/j.optm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371–382. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Smiddy WE, Michels RG, Green WR. Morphology, pathology, and surgery of idiopathic vitreoretinal macular disorders. A review. Retina. 1990;10:288–296. doi: 10.1097/00006982-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Chang LK, Fine HF, Spaide RF, Koizumi H, Grossniklaus HE. Ultrastructural correlation of spectral-domain optical coherence tomographic findings in vitreomacular traction syndrome. Am J Ophthalmol. 2008;146:121–127. doi: 10.1016/j.ajo.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MW. Tractional cystoid macular edema: a subtle variant of the vitreomacular traction syndrome. Am J Ophthalmol. 2005;140:184–192. doi: 10.1016/j.ajo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi H, Spaide RF, Fisher YL, Freund KB, Klancnik JM, Yannuzzi LA. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145:509–517. doi: 10.1016/j.ajo.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Mirza RG, Johnson MW, Jampol LM. Optical coherence tomography use in evaluation of the vitreoretinal interface: a review. Surv Ophthalmol. 2007;52:397–421. doi: 10.1016/j.survophthal.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Spaide RF, Wong D, Fisher Y, Goldbaum M. Correlation of vitreous attachment and foveal deformation in early macular hole states. Am J Ophthalmol. 2002;133:226–229. doi: 10.1016/s0002-9394(01)01377-0. [DOI] [PubMed] [Google Scholar]
- 15.Witkin AJ, Patron ME, Castro LC, Reichel E, Rogers AH, Baumal CR, et al. Anatomic and visual outcomes of vitrectomy for vitreomacular traction syndrome. Ophthalmic Surg Lasers Imaging. 2010;41:425–431. doi: 10.3928/15428877-20100525-07. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann H. Senile changes of the lens and the vitreous. The Arthur J. Bedell lecture. Am J Ophthalmol. 1964;57:1–13. doi: 10.1016/0002-9394(64)92026-4. [DOI] [PubMed] [Google Scholar]
- 17.Miller B, Miller H, Ryan SJ. Experimental vitreous syneresis. Arch Ophthalmol. 1985;103:1385–1388. doi: 10.1001/archopht.1985.01050090137049. [DOI] [PubMed] [Google Scholar]
- 18.Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:690–698. doi: 10.1007/s00417-004-0980-1. [DOI] [PubMed] [Google Scholar]
- 19.Murphy AI, Irvine AR. Spontaneous release of retinal traction due to subretinal strands. Retina. 1983;3:273–276. doi: 10.1097/00006982-198300340-00008. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Jalkh A, Hoskins J, Trempe CL, Schepens CL. Biomicroscopic evaluation and photography of liquefied vitreous in some vitreoretinal disorders. Arch Ophthalmol. 1981;99:1555–1559. doi: 10.1001/archopht.1981.03930020429003. [DOI] [PubMed] [Google Scholar]
- 21.Sebag J. Anatomy and pathology of the vitreo-retinal interface. Eye (Lond) 1992;6:541–552. doi: 10.1038/eye.1992.119. [DOI] [PubMed] [Google Scholar]
- 22.Smiddy WE, Green WR, Michels RG, de la. Ultrastructural studies of vitreomacular traction syndrome. Am J Ophthalmol. 1989;107:177–185. doi: 10.1016/0002-9394(89)90219-5. [DOI] [PubMed] [Google Scholar]
- 23.Smiddy WE, Maguire AM, Green WR, Michels RG, de la, Enger C, et al. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology. 1989;96:811–820. doi: 10.1016/s0161-6420(89)32811-9. [DOI] [PubMed] [Google Scholar]
- 24.Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86:902–909. doi: 10.1136/bjo.86.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appiah AP, Hirose T, Kado M. A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol. 1988;106:533–535. doi: 10.1016/0002-9394(88)90581-8. [DOI] [PubMed] [Google Scholar]
- 26.Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405. [PubMed] [Google Scholar]
- 27.Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990;31:14–28. [PubMed] [Google Scholar]
- 28.Harris JR, Brown GA, Jorgensen M, Kaushal S, Ellis EA, Grant MB, et al. Bone marrow-derived cells home to and regenerate retinal pigment epithelium after injury. Invest Ophthalmol Vis Sci. 2006;47:2108–2113. doi: 10.1167/iovs.05-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonemoto J, Ideta H, Sasaki K, Tanaka S, Hirose A, Oka C. The age of onset of posterior vitreous detachment. Graefes Arch Clin Exp Ophthalmol. 1994;232:67–70. doi: 10.1007/BF00171665. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan DS, Antcliff RJ, Rai PA, Williamson TH, Marshall J. Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol. 2000;118:32–38. doi: 10.1001/archopht.118.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Johnson MW, Van Newkirk, Meyer KA. Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol. 2001;119:215–222. [PubMed] [Google Scholar]
- 32.Van Newkirk, Johnson MW, Hughes JR, Meyer KA, Byrne SF. B-scan ultrasonographic findings in the stages of idiopathic macular hole. Trans Am Ophthalmol Soc. 2000;98:163–169. [PMC free article] [PubMed] [Google Scholar]
- 33.Sonmez K, Capone A, Trese MT, Williams GA. Vitreomacular traction syndrome: impact of anatomical configuration on anatomical and visual outcomes. Retina. 2008;28:1207–1214. doi: 10.1097/IAE.0b013e31817b6b0f. [DOI] [PubMed] [Google Scholar]
- 34.Yamada N, Kishi S. Tomographic features and surgical outcomes of vitreomacular traction syndrome. Am J Ophthalmol. 2005;139:112–117. doi: 10.1016/j.ajo.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 36.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–759. doi: 10.1016/s0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 37.Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erginay A. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999;117:744–751. doi: 10.1001/archopht.117.6.744. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Terasaki H, Suzuki T, Kojima T, Mori M, Ishikawa K, et al. Mapping posterior vitreous detachment by optical coherence tomography in eyes with idiopathic macular hole. Am J Ophthalmol. 2003;135:351–355. doi: 10.1016/s0002-9394(02)01944-x. [DOI] [PubMed] [Google Scholar]
- 39.Hikichi T, Akiba J, Trempe CL. Effect of the vitreous on the prognosis of full-thickness idiopathic macular hole. Am J Ophthalmol. 1993;116:273–278. doi: 10.1016/s0002-9394(14)71343-1. [DOI] [PubMed] [Google Scholar]
- 40.Kusaka S, Saito Y, Okada AA, Sasamoto M, Hayashi A, Ohji M, et al. Optical coherence tomography in spontaneously resolving vitreomacular traction syndrome. Ophthalmologica. 2001;215:139–141. doi: 10.1159/000050847. [DOI] [PubMed] [Google Scholar]
- 41.Sulkes DJ, Ip MS, Baumal CR, Wu HK, Puliafito CA. Spontaneous resolution of vitreomacular traction documented by optical coherence tomography. Arch Ophthalmol. 2000;118:286–287. [PubMed] [Google Scholar]
- 42.Melberg NS, Williams DF, Balles MW, Jaffe GJ, Meredith TA, Sneed SR, et al. Vitrectomy for vitreomacular traction syndrome with macular detachment. Retina. 1995;15:192–197. doi: 10.1097/00006982-199515030-00002. [DOI] [PubMed] [Google Scholar]
- 43.Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332–340. doi: 10.1016/j.ajo.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues EB, Maia M, Meyer CH, Penha FM, Dib E, Farah ME. Vital dyes for chromovitrectomy. Curr Opin Ophthalmol. 2007;18:179–187. doi: 10.1097/ICU.0b013e32811080b5. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues EB, Costa EF, Penha FM, Melo GB, Bottós J, Dib E, et al. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009;54:576–617. doi: 10.1016/j.survophthal.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Maia M, Farah ME, Belfort RN, Penha FM, Lima Filho, Aggio FB, et al. Effects of intravitreal triamcinolone acetonide injection with and without preservative. Br J Ophthalmol. 2007;91:1122–1124. doi: 10.1136/bjo.2007.115386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maia M, Penha FM, Farah ME, Dib E, Príncipe A, Lima Filho, et al. Subretinal injection of preservative-free triamcinolone acetonide and supernatant vehicle in rabbits: an electron microscopy study. Graefes Arch Clin Exp Ophthalmol. 2008;246:379–388. doi: 10.1007/s00417-007-0718-y. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues EB, Penha FM, de Paula, Maia M, Dib E, Moraes M, et al. Ability of new vital dyes to stain intraocular membranes and tissues in ocular surgery. Am J Ophthalmol. 2010;149:265–277. doi: 10.1016/j.ajo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Díaz-Llopis M, Udaondo P, Cervera E, García-Delpech S, Salom D, Quijada A, et al. [Enzymatic vitrectomy by intravitreal autologous plasmin injection as initial treatment for macular epiretinal membranes and vitreomacular traction syndrome]. Arch Soc Esp Oftalmol. 2009;84:91–100. doi: 10.4321/s0365-66912009000200007. [DOI] [PubMed] [Google Scholar]
- 50.de Smet, Gandorfer A, Stalmans P, Veckeneer M, Feron E, Pakola S, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology. 2009;116:1349–1355. doi: 10.1016/j.ophtha.2009.03.051. [DOI] [PubMed] [Google Scholar]