Abstract
Purpose
To report the clinical, confocal scan, and histopathologic features of nocardia keratitis in a patient who developed bilateral infection following photorefractive keratectomy (PRK).
Case Report
A 23-year-old woman underwent bilateral PRK for low myopia. On postoperative day 3, dense central stromal infiltrates were noticed in both eyes. Empirical antibiotic therapy was initiated which was converted into specific therapy after a definite diagnosis was made based on clinical features and confirmed by confocal scan and histopathologic findings. Clinical and confocal scan features were consistent with the diagnosis of Nocardia keratitis, and topical 2% amikacin eye drops were started. Because of poor response to medical therapy, lamellar keratectomy was performed in both eyes which shortened the treatment course. Histopathologic examination reconfirmed the initial diagnosis.
Conclusion
Familiarity with clinical and confocal scan features facilitates early diagnosis of Nocardia keratitis leading to proper management and hence a rapid therapeutic response.
Keywords: Nocardia Keratitis, Photorefractive Keratectomy, Confocal Scan
INTRODUCTION
Infectious keratitis is a potentially devastating complication following corneal refractive surgery and may be caused by bacteria, viruses, fungi, or amoebae. Although the majority of the cases are due to normal flora of the eyelids, eyelashes, and conjunctiva, (including Streptococcus and Staphylococcus species), unusual causes such as atypical Mycobacteria and Nocardia species have also been reported.1-4 Factors predisposing to infectious keratitis following keratorefractive surgery consist of exposed corneal stroma, breach in operative field or instrument sterility, extended-wear bandage soft contact lenses, and use of topical steroids.5
Herein, we describe a patient who developed bilateral Nocardia keratitis following PRK. A correct diagnosis was made upon initial presentation to our center based on clinical and confocal scan features. Rapid diagnosis led to timely initiation of specific and appropriate therapy and therefore a favourable clinical response.
Case report
A 23-year-old woman was referred to our clinic with bilateral corneal ulcers 3 days after photorefractive keratectomy which had been performed simultaneously on both eyes. According to the surgeon’s report, preoperative refraction had been -1.5-1.75×65º and -2.25- 0.5×85º in the right and left eyes respectively with best spectacle corrected visual acuity of 20/20 in both eyes. There were no ocular or systemic predisposing factors and the operation had been uneventful. During surgery, the corneal epithelium was mechanically removed using a hockey spatula but mitomycin-C was not applied. Soft bandage contact lenses were fitted and chloramphenicol, betamethasone 0.1%, artificial tears and diclofenac eye drops had been administered every 6 hours. On postoperative day 3, the patient presented to her surgeon complaining of bilateral decreased vision, redness, and photophobia. With a diagnosis of bacterial keratitis, the contact lenses were immediately removed and ciprofloxacin 0.5% eye drops and fortified vancomycin 50 mg/ ml were initiated every 30 minutes. Because of inadequate response to the prescribed medications, she was referred to our center on postoperative day 8.
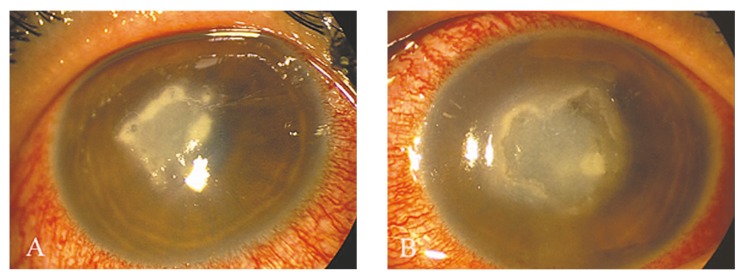
Upon referral, visual acuity was hand motions in both eyes (OU) and gross examination revealed severe lid edema and conjunctival injection OU. Slit lamp examination revealed corneal epithelial defects measuring 3.0×4.5 mm in the right eye (OD) and 3.0×5.0 mm in the left eye (OS) together with dense anterior stromal infiltrates and some areas of stromal melting in both eyes (Fig. 1). Multiple discrete and superficial yellow-white pinhead-sized infiltrates forming a characteristic wreath pattern at the borders of the main lesion were observed in both eyes (Fig. 1). Other findings included stromal edema, Descemet’s membrane foldings, diffuse keratic precipitates, and moderate anterior chamber reaction. The pattern of corneal involvement raised the diagnosis of nocardia keratitis, and based on our previous experience,3 2.0% amikacin eye drops were started every 30 minutes while other topical antibiotics were discontinued. Samples were taken from both eyes using a #15 surgical blade and sent for culture which failed to grow any colonies after 10 days.
Figure 1.
Corneal ulcer with a wreath-like pattern and feathery borders involving the central anterior stroma in the right (A) and left eye (B).
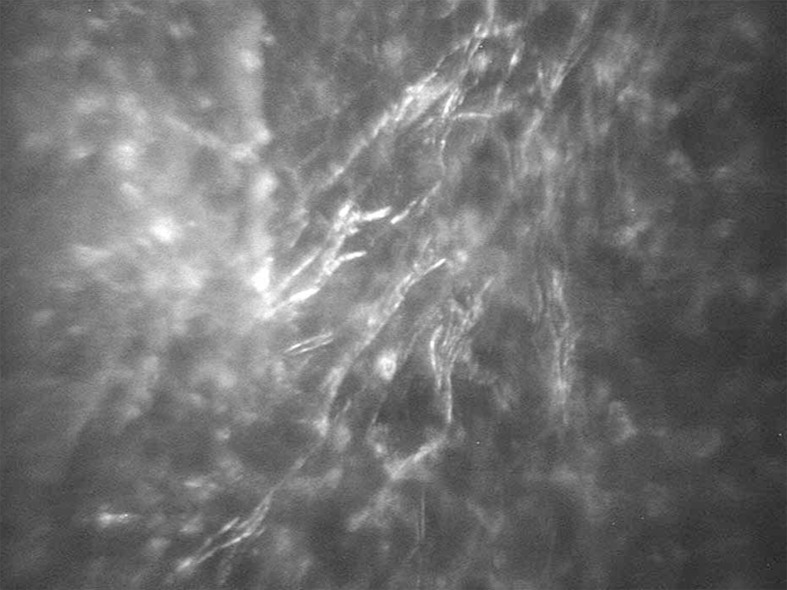
Two days after referral (i.e. on postoperative day 10) confocal microscopy (Confoscan 3.4, Nidek Technologies, Padova, Italy) was requested which disclosed multifocal areas of severe hyperreflectivity in each eye containing inflammatory cells in the anterior and middle stroma adjacent to which fine curved branches of thin filamentlike structures measuring 9.0 μm in length and 1.5 μm in width were present; these features supported the clinical diagnosis (Fig. 2).
Figure 2.
Chains of high contrast thin and beaded filament-like structures with a branching appearance on confocal scan imaging of the right cornea (magnification×500).
Six days later and due to inadequate response to therapy, lamellar keratectomy was performed in both eyes to debulk the involved stroma and obtain specimens for histopathologic evaluation while the patient was still receiving amikacin eye drops at the same dose. Three days after debulking, significant improvement was noted with dramatic reduction in corneal infiltrates and complete healing of the epithelial defects OU. The frequency of topical amikacin was reduced to every 3 hours and the patient was discharged.
At the most recent follow-up (i.e. two months after lamellar keratectomy), visual acuity was 20/100 OD and 20/600 OS; slit lamp examination revealed an avascular central corneal scar OU without any epithelial defect or active infiltration.
Histopathologic examination
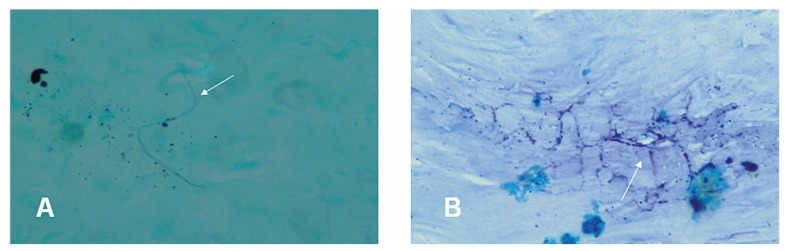
Histopathologic examination demonstrated foci of intrastromal necrosis with scattered acute and chronic inflammatory cell infiltrates. On Gram-Twort staining, small clusters of grampositive filaments were found in the necrotic areas which were acid-fast on modified Ziehl- Neelsen staining (Fig. 3).
Figure 3.
A) A fine branch of gram-positive filament (arrow) in the stroma (Gram-Twort stain; magnification ×1000). B) Acid-fast branches of thin filaments (arrow) suggestive of Nocardia species (modified Ziehl-Neelsen stain; magnification ×1000).
Discussion
Nocardia species are aerobic, branching, beaded filamentous bacilli with Gram stain variability and are often acid-fast positive. These species cause a wide variety of diseases and have variable drug susceptibilities.6 Usual predisposing factors for Nocardia keratitis are trauma related to agricultural work, corneal refractive surgery, corticosteroid use and contact lens wear.3,4,6,7
The onset of symptoms after corneal refractive procedures may vary from a few days to a few weeks. In our previous report, Nocardia keratitis developed 21 to 40 days after PRK.3 In contrast, in the patient presented herein, symptoms developed as early as postoperative day 3 which is in line with the time interval of 3 to 5 days observed in a Nocardia outbreak after LASIK.4 The infection usually has a prolonged course; presenting symptoms usually include pain which can be out of proportion to clinical findings, photophobia, and blepharospasm. On slit lamp examination, keratitis may present as patchy infiltrates chiefly involving the anterior stroma and overlying epithelial and subepithelial tissues resembling keratitis caused by fungi or atypical Mycobacteria.
The infiltrates may have margins studded with discrete yellow- white pinhead-sized superficial infiltrates or be arranged in a ring-like fashion, forming the characteristic “wreath” pattern which is considered pathognomonic for Nocardia keratitis.8 This typical pattern is usually observed when the patient presents within 15 days of the onset of symptoms.9 After this period, the lesions coalesce and the picture may resemble mycotic keratitis or keratitis caused by atypical Mycobacteria.8 Confocal microscopy obtains real-time images with lateral resolution of 1 μm and depth of field of 10 μm, depicting cellular structures in normal and abnormal corneas. Among various types of infectious keratitis, fungal (both yeast and filamentous) and Acanthamoeba keratitis can be diagnosed with this technique. On the contrary, it is difficult to detect bacterial keratitis using in vivo confocal microscopy.10 The only exception is the Nocardia species which are filamentous bacteria with a certain size and structure that produce a unique morphology on confocal scan. Performing confocal microscopy in microbiologically proven cases of Nocardia asteroides keratitis, Vaddavalli et al11 reported thin (<1 μm), short, beaded filamentous structures that demonstrated right-angled branching. These filamentous structures, which were best seen at the edge of the infiltrate against a dark background, seemed to be clumped together. Similarly, we observed hyper-reflective and slender, fibril-like structures with right-angled branching about 9.0 μm in length and 1.5 μm in width. However, in the previous report from our center,3 the confocal features of Nocardia keratitis were slightly different; it is possible that different species have different confocal features. Unfortunately, we were not able to determine the species as none of the cultures taken from the patient’s eyes grew any microorganisms.
Treatment of Nocardia infection ranges from antibiotic therapy to surgical intervention such as debulking, therapeutic lamellar keratectomy, penetrating keratoplasty, and conjunctival flap procedures, depending on the stage and severity of the infection. The majority of cases in whom a correct diagnosis and hence appropriate treatment are established early, are quiet amenable to medical treatment. Visual outcomes of treatment for Nocardia keratitis were described by Lalitha et al.9 Based on their observations, patients who present within 15 days of the onset of the infection show the highest recovery rate and are more likely to respond to topical antibiotics. Any delay in initiating appropriate therapy can lead to severe complications including progressive corneal thinning, resulting in perforation, endophthalmitis, and extension to the adjacent sclera.12
Different Nocardia strains vary in antibiotic sensitivity. Most isolates are sensitive to sulfonamides such as sulfamethoxazole, trimethoprim–sulfamethoxazole, doxycycline and amikacin.6 Because of its low minimum inhibitory concentration, amikacin is considered the treatment of choice for Nocardia keratitis. Nevertheless, a case of resistance to this antibiotic has been reported in the literature.13
The patient described herein was on inappropriate medications for 8 days; upon referral to our clinic, a correct diagnosis was made based on clinical features and supported by confocal scan findings, and the medication of choice was started. However, lamellar keratectomy was required to debulk the infection and facilitate drug penetration into the corneal stroma which was deeply involved at the time of presentation. This surgical intervention hastened the treatment course and obviated the need for tectonic keratoplasty which has a poor prognosis in such eyes.
In summary, the characteristic clinical appearance together with the use of confocal scan imaging can facilitate early diagnosis of Nocardia keratitis leading to timely management and a favorable response to therapy for this rare form of infectious keratitis.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Donnenfeld ED, O’Brien TP, Solomon R, Perry HD, Speaker MG, Wittpenn J. Infectious keratitis after photorefractive keratectomy. Ophthalmology. 2003;110:743–747. doi: 10.1016/S0161-6420(02)01936-X. [DOI] [PubMed] [Google Scholar]
- 2.Chandra NS, Torres MF, Winthrop KL, Bruckner DA, Heidemann DG, Calvet HM, et al. Cluster of Mycobacterium chelonae keratitis cases following laser in-situ keratomileusis. Am J Ophthalmol. 2001;132:819–830. doi: 10.1016/s0002-9394(01)01267-3. [DOI] [PubMed] [Google Scholar]
- 3.Javadi MA, Kanavi MR, Zarei-Ghanavati S, Mirbabaei F, Jamali H, Shoja M, et al. Outbreak of Nocardia keratitis after photorefractive keratectomy: clinical, microbiological, histopathological, and confocal scan study. J Cataract Refract Surg. 2009;35:393–398. doi: 10.1016/j.jcrs.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 4.Garg P, Sharma S, Vemuganti GK, Ramamurthy B. A cluster of Nocardia keratitis after LASIK. J Refract Surg. 2007;23:309–312. doi: 10.3928/1081-597X-20070301-17. [DOI] [PubMed] [Google Scholar]
- 5.Feizi S, Jadidi K, Naderi M, Shahverdi S. Corneal interface contamination during laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:1734–1737. doi: 10.1016/j.jcrs.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridhar MS, Sharma S, Reddy MK, Mruthyunjay P, Rao GN. Clinicomicrobiological review of Nocardia keratitis. Cornea. 1998;17:17–22. doi: 10.1097/00003226-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lalitha P. Nocardia keratitis. Curr Opin Ophthalmol. 2009;20:318–323. doi: 10.1097/ICU.0b013e32832c3bcc. [DOI] [PubMed] [Google Scholar]
- 9.Lalitha P, Tiwari M, Prajna NV, Gilpin C, Prakash K, Srinivasan M. Nocardia keratitis: species, drug sensitivities, and clinical correlation. Cornea. 2007;26:255–259. doi: 10.1097/ICO.0b013e318033d853. [DOI] [PubMed] [Google Scholar]
- 10.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007;26:782–786. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]
- 11.Vaddavalli PK, Garg P, Sharma S, Thomas R, Rao GN. Confocal microscopy for Nocardia keratitis. Ophthalmology. 2006;113:1645–1650. doi: 10.1016/j.ophtha.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar MS, Gopinathan U, Garg P, Sharma S, Rao GN. Ocular nocardia infections with special emphasis on the cornea. Surv Ophthalmol. 2001;45:361–378. doi: 10.1016/s0039-6257(00)00207-1. [DOI] [PubMed] [Google Scholar]
- 13.Pandya VB, Petsoglou C. Nocardia transvalensis resistant to amikacin: an unusual cause of microbial keratitis. Cornea. 2008;27:1082–1085. doi: 10.1097/ICO.0b013e3181783a20. [DOI] [PubMed] [Google Scholar]